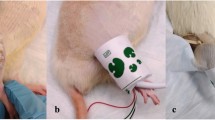
Abstract
Rhabdomyolysis following revascularization of the ischemic upper extremity can lead to life- and limb-threatening sequelae. In the context of replantations and vascularized composite allografting, a reconstructive procedure usually reserved for upper limb amputees, prolonged tissue ischemia is detrimental to extremity functional recovery. Currently, validated survival small animal models of extremity reperfusion injury that permit longitudinal assessment of limb function are lacking. So far, studies that evaluated reperfusion injury-induced neuromuscular impairment have relied on terminal ex vivo procedures and did not provide clinically translatable measurements. Here we present a reliable rat model of extremity post-reperfusion syndrome (PRS) that comprehensively recapitulates the biochemical hallmarks of rhabdomyolysis secondary to upper-extremity reperfusion injury and allows the monitoring of in vivo upper limb function using clinically relevant electrodiagnostic and kinematic metrics. In addition to inducing severe metabolic derangements, our forelimb PRS model provided insights on gross motor and electrophysiological alterations following upper-extremity reperfusion injury. We identify gait coordination parameters—such as stride frequency and the forelimb–hindlimb coordination index—and electrophysiological metrics, including compound muscle action potential amplitude, as objective and noninvasive outcome measures for assessing limb function in small animal models of extremity PRS. This comprehensive, validated functional model can serve as an invaluable tool to evaluate therapeutics or preconditioning regimens to attenuate PRS and mitigate resulting neuromuscular dysfunction.
This is a preview of subscription content, access via your institution
Access options






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Apichartpiyakul, P., Shinlapawittayatorn, K., Rerkasem, K., Chattipakorn, S. C. & Chattipakorn, N. Mechanisms and interventions on acute lower limb ischemia/reperfusion injury: a review and insights from cell to clinical investigations. Ann. Vasc. Surg. 86, 452–481 (2022).
Carroll, W. R. & Esclamado, R. M. Ischemia/reperfusion injury in microvascular surgery. Head Neck 22, 700–713 (2000).
He, J., Khan, U. Z., Qing, L., Wu, P. & Tang, J. Improving the ischemia–reperfusion injury in vascularized composite allotransplantation: clinical experience and experimental implications. Front. Immunol. 13, 998952 (2022).
Walker, P. M. Ischemia/reperfusion injury in skeletal muscle. Ann. Vasc. Surg. 5, 399–402 (1991).
Ton, C. et al. Methods of ex vivo analysis of tissue status in vascularized composite allografts. J. Transl. Med. 21, 609 (2023).
Žargi, T., Drobnič, M., Stražar, K. & Kacin, A. Short-term preconditioning with blood flow restricted exercise preserves quadriceps muscle endurance in patients after anterior cruciate ligament reconstruction. Front. Physiol. 9, 1150 (2018).
Kubo, Y. et al. Association between serum n-3 polyunsaturated fatty acids and quadriceps weakness immediately after total knee arthroplasty. PLoS ONE 15, e0228460 (2020).
Longchamp, A. & Deglise, S. The strength of reperfusion: the dark side of ischaemia. Eur. J. Vasc. Endovasc. Surg. 58, 257 (2019).
Tu, H. et al. A comparison of acute mouse hindlimb injuries between tourniquet- and femoral artery ligation-induced ischemia-reperfusion. Injury 52, 3217–3226 (2021).
Gok, E. et al. Single muscle fibre contractility testing in rats to quantify ischaemic muscle damage during reperfusion injury. Eur. J. Vasc. Endovasc. Surg. 58, 249–256 (2019).
Kern, B. et al. A novel rodent orthotopic forelimb transplantation model that allows for reliable assessment of functional recovery resulting from nerve regeneration. Am. J. Transplant. 17, 622–634 (2017).
Pendexter, C. A. et al. Development of a rat forelimb vascularized composite allograft (VCA) perfusion protocol. PLoS ONE 18, e0266207 (2023).
Yi, H., Kim, M. A., Back, S. K., Eun, J. S. & Na, H. S. A novel rat forelimb model of neuropathic pain produced by partial injury of the median and ulnar nerves. Eur. J. Pain 15, 459–466 (2011).
Chen, X. K., Rathbone, C. R. & Walters, T. J. Treatment of tourniquet-induced ischemia reperfusion injury with muscle progenitor cells. J. Surg. Res. 170, e65–e73 (2011).
Corrick, R. M. et al. Dexamethasone protects against tourniquet-induced acute ischemia–reperfusion injury in mouse hindlimb. Front. Physiol. 9, 244 (2018).
de Carvalho, E. G., Corsini, W. & Hermes, T. A. Severe muscle damage after a short period of ischemia and reperfusion in an animal model. Surgery 174, 363–368 (2023).
Cearra, I., Herrero De La Parte, B., Moreno-Franco, D. I. & García-Alonso, I. A reproducible method for biochemical, histological and functional assessment of the effects of ischaemia–reperfusion syndrome in the lower limbs. Sci. Rep. 11, 19325 (2021).
Petrasek, P. F., Homer-Vanniasinkam, S. & Walker, P. M. Determinants of ischemic injury to skeletal muscle. J. Vasc. Surg. 19, 623–631 (1994).
Charles, A.-L. et al. Muscles susceptibility to ischemia–reperfusion injuries depends on fiber type specific antioxidant level. Front. Physiol. 8, 52 (2017).
Utagi, B., Kumar, R. & Bhagavan, K. R. Endovascular management of two uncommon cases of acute upper limb ischemia in young. J. Health Allied Sci. NU 13, 431–435 (2023).
Weibrecht, K., Dayno, M., Darling, C. & Bird, S. B. Liver aminotransferases are elevated with rhabdomyolysis in the absence of significant liver injury. J. Med. Toxicol. 6, 294–300 (2010).
Kodadek, L. et al. Rhabdomyolysis: an American Association for the Surgery of Trauma Critical Care Committee Clinical Consensus Document. Trauma Surg. Acute Care Open 7, e000836 (2022).
Herrero de la Parte, B. et al. The prevention of ischemia–reperfusion injury in elderly rats after lower limb tourniquet use. Antioxidants 11, 1936 (2022).
Kuroda, Y. et al. Oxidative stress evaluation of skeletal muscle in ischemia–reperfusion injury using enhanced magnetic resonance imaging. Sci. Rep. 10, 10863 (2020).
Torres, P. A., Helmstetter, J. A., Kaye, A. M. & Kaye, A. D. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 15, 58–69 (2015).
Nance, J. R. & Mammen, A. L. Diagnostic evaluation of rhabdomyolysis. Muscle Nerve 51, 793–810 (2015).
Brow, T. D., Kakkar, V. V. & Das, S. K. The significance of creatine kinase in cardiac patients with acute limb ischaemia. J. Cardiovasc. Surg. 40, 637–644 (1999).
Clemens, M. S. et al. Extracorporeal filtration of potassium in a swine model of bilateral hindlimb ischemia–reperfusion injury with severe acute hyperkalemia. Mil. Med. 183, e335–e340 (2018).
Tricarico, D., Capriulo, R. & Camerino, D. C. Involvement of K(Ca2+) channels in the local abnormalities and hyperkalemia following the ischemia–reperfusion injury of rat skeletal muscle. Neuromuscul. Disord. 12, 258–265 (2002).
Cearra, I. et al. Effects of folinic acid administration on lower limb ischemia/reperfusion injury in rats. Antioxidants 10, 1887 (2021).
Korei, C. et al. Hematological, micro-rheological, and metabolic changes modulated by local ischemic pre- and post-conditioning in rat limb ischemia–reperfusion. Metabolites 11, 776 (2021).
Deune, E. G. et al. Prevention of ischemia–reperfusion injury with a synthetic metalloprotein superoxide dismutase mimic, SC52608. Plast. Reconstr. Surg. 98, 711–718 (1996).
Yassin, M. M. I., Harkin, D. W., Barros D’Sa, A. A. B., Halliday, M. I. & Rowlands, B. J. Lower limb ischemia–reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J. Surg. 26, 115–121 (2002).
Ege, T., Us, M. H., Sungun, M. & Duran, E. Cytokine response in lower extremity ischaemia/reperfusion. J. Int. Med. Res. 32, 124–131 (2004).
Ferreira, J. et al. Higher levels of cytokines in patients with chronic limb-threatening ischemia. Ann. Vasc. Surg. 106, 255–263 (2024).
Orfany, A. et al. Mitochondrial transplantation ameliorates acute limb ischemia. J. Vasc. Surg. 71, 1014–1026 (2020).
Islam, M. N., Bradley, B. A. & Ceredig, R. Sterile post-traumatic immunosuppression. Clin. Transl. Immunol. 5, e77 (2016).
Horner, E., Lord, J. M. & Hazeldine, J. The immune suppressive properties of damage associated molecular patterns in the setting of sterile traumatic injury. Front. Immunol. 14, 1239683 (2023).
Lai, Y.-R. et al. Clinical disease severity mediates the relationship between stride length and speed and the risk of falling in Parkinson’s disease. J. Pers. Med. 12, 192 (2022).
Krizsan-Agbas, D. et al. Gait analysis at multiple speeds reveals differential functional and structural outcomes in response to graded spinal cord injury. J. Neurotrauma 31, 846–856 (2014).
Lakes, E. H. & Allen, K. D. Gait analysis methods for rodent models of arthritic disorders: reviews and recommendations. Osteoarthritis Cartilage 24, 1837–1849 (2016).
Schoen, M. et al. Ischemic preconditioning prevents skeletal muscle tissue injury, but not nerve lesion upon tourniquet-induced ischemia. J. Trauma 63, 788–797 (2007).
Juel, V. C. in Handbook of Clinical Neurology Vol. 160 (eds Levin, K. H. & Chauvel, P.) 303–310 (Elsevier, 2019).
Barkhaus, P. E., Nandedkar, S. D., de Carvalho, M., Swash, M. & Stålberg, E. V. Revisiting the compound muscle action potential (CMAP). Clin. Neurophysiol. Pract. 9, 176–200 (2024).
Parry, G. J., Cornblath, D. R. & Brown, M. J. Transient conduction block following acute peripheral nerve ischemia. Muscle Nerve 8, 409–412 (1985).
Stecker, M. M., Baylor, K. & Chan, Y. M. Acute nerve compression and the compound muscle action potential. J. Brachial Plex Peripher. Nerve Inj. 3, 1 (2008).
Iida, H., Schmelzer, J. D., Schmeichel, A. M., Wang, Y. & Low, P. A. Peripheral nerve ischemia: reperfusion injury and fiber regeneration. Exp. Neurol. 184, 997–1002 (2003).
Wilson, R. J. et al. Voluntary running protects against neuromuscular dysfunction following hindlimb ischemia-reperfusion in mice. J. Appl. Physiol. 126, 193–201 (2019).
Tömböl, T., Pataki, G., Németh, A. & Hamar, J. Ultrastructural changes of the neuromuscular junction in reperfusion injury. Cells Tissues Organs 170, 139–150 (2002).
Harrigan, M. E. et al. Assessing rat forelimb and hindlimb motor unit connectivity as objective and robust biomarkers of spinal motor neuron function. Sci. Rep. 9, 16699 (2019).
Sanders, D. B. et al. Guidelines for single fiber EMG. Clin. Neurophysiol. 130, 1417–1439 (2019).
Acknowledgements
O.A.S. gratefully acknowledges support as a trainee on the NIAMS NIH T32 AR56950 program (PI: Jennifer J. Westendorf, Ph. D.). This work was also supported in part by the Obaid Reconstructive Transplant Award.
Author information
Authors and Affiliations
Contributions
O.A.S. and S.L.M. conceptualized and designed the study. O.A.S. developed the methodology and visualized data presentation. O.A.S. and A.S. performed the in vivo experiments. O.A.S. performed in vivo electrophysiology. M.T. assisted in vivo work. O.A.S. wrote the manuscript. O.A.S., C.Z. and S.L.M. analyzed and interpreted the data. O.A.S. and S.L.M supervised the research. C.Z. and S.L.M provided resources. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Lab Animal thanks David Hercher and Huiyin Tu for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Table 1.
Supplementary Video 1
Representative pre-injury rat gait.
Supplementary Video 2
Gait analysis 3 h after PRS.
Supplementary Video 3
Gait analysis 14 days after PRS.
Supplementary Data 1
ARRIVE guidelines checklist.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Selim, O.A., Sarcon, A., Tunaboylu, M. et al. A longitudinal rat forelimb model for assessing in vivo neuromuscular function following extremity reperfusion injury. Lab Anim 54, 259–269 (2025). https://doi.org/10.1038/s41684-025-01601-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41684-025-01601-9