Abstract
We present a unique case of locally advanced, inflammatory hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer in a young woman. A MammaPrint gene signature performed on the core biopsy indicated a very high risk of recurrence (MammaPrint high risk 2, MPH2). BluePrint 80-gene signature was used for the molecular subtyping and identified the tumor as a basal subtype, resembling triple-negative breast cancer (TNBC) despite the strong estrogen receptor (ER) expression on immunohistochemical (IHC) staining. We treated the patient with a TNBC protocol, incorporating carboplatin and immunotherapy into the anthracycline-based chemotherapy backbone in the neoadjuvant setting. The patient achieved a complete response and remains to be disease-free after 2 years, with ongoing follow-up.
Similar content being viewed by others
Introduction
Inflammatory breast cancer (IBC) constitutes 1–5% of all breast cancer cases. It is notorious for its aggressive clinical behavior and poor prognosis1,2. It is characterized by rapid onset of symptoms and is defined by the presence of erythema and edema (peau d’orange) involving at least one-third of the skin of the breast, often associated with warmth and tenderness. The skin changes are related to the frequent involvement of dermal lymphatics by tumor emboli. The diagnosis is primarily clinical, with no need for confirmatory skin punch biopsies3.
Conventional breast cancer treatment is guided by immunohistochemistry (IHC) classification, which divides breast cancer into three main subtypes based on the expression of estrogen (ER) receptor, progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) protein on the breast cancer cells. More recently, multiple RNA-based assays are available to categorize heterogeneous breast cancer into more “functional” molecular subtypes with distinct biological characteristics and outcomes4. The molecular classification takes into consideration the downstream active functional pathways in the breast cancer cells rather than the mere expression of receptors and proteins via IHC staining.
The vast majority of hormone receptor (HR)-positive, HER2-negative breast cancers have “luminal” molecular subtypes and are highly dependent on estrogen signaling5,6. However, there is accumulating evidence from multiple reports showing that a small subset of HR-positive breast cancers defined by IHC were classified as basal molecular subtype by genomic testing, behaving more similarly to triple-negative breast cancer (TNBC) regarding biological aggressiveness and outcomes. For instance, the BluePrint 80-gene signature molecular classifier4 reclassified 15% and 29% of ER-positive tumors into a basal molecular subtype (ER+/basal) in the NBRST trial7 and the I-SPY2 trial8, respectively. These ER+/Basal tumors do not exhibit functional hormone signaling pathways despite ER positivity by IHC, likely due to a non-functioning ER splice variant that blocks any downstream estrogen-dependent signaling9. This raises the question of when to leverage molecular data to guide the treatment decisions for these unique tumors, which could otherwise be undertreated or denied the benefit of immunotherapy if we were to follow the conventional treatment paradigms based on their “misleading” IHC classification.
In this report, we present a case of inflammatory ER-positive, HER2-negative breast cancer with a basal molecular subtype (ER+/Basal), treated with a TNBC regimen incorporating immunotherapy, achieving an excellent response.
Results
Clinical case
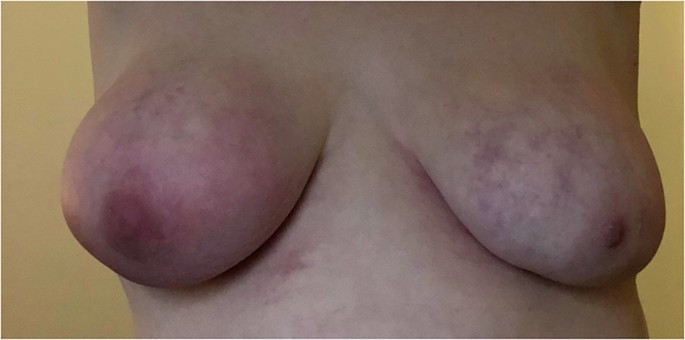
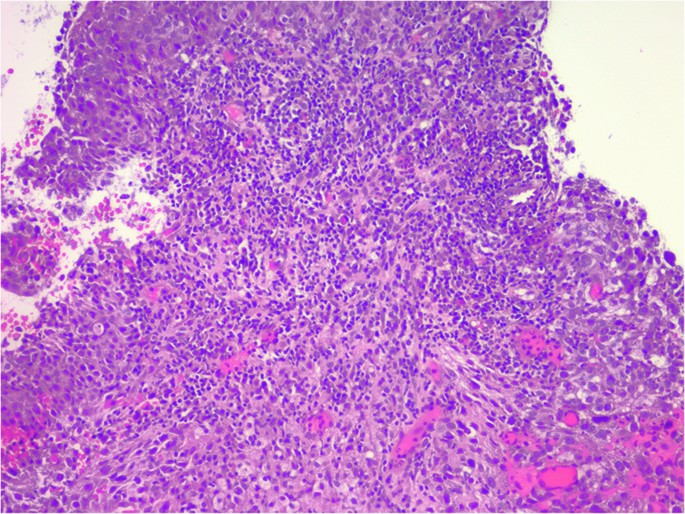
A 30-year-old woman presented to our department with a very painful, rapidly growing right breast mass and skin discoloration (Fig. 1) over a period of 3 months. Diagnostic ultrasound showed a 6 cm hypoechoic mass and several enlarged right axillary lymph nodes with cortical thickening. A core biopsy of the primary tumor showed grade 3 infiltrative ductal carcinoma. ER expression by Allred score was 5/8 (35%, 1+), PR was 0/8; HER2 was 1+. Ki-67 was 90%, while programmed death-ligand 1 (PD-L1) by combined positive score (CPS) score was 70. Tumor-infiltrating lymphocytes (TILs) were 40% (Fig. 2). The lymph node biopsy was negative for cancer metastasis, but it was discordant. Staging computed tomography (CT) scan of the chest, abdomen, and pelvis showed a partially necrotic mass in the right breast compatible with malignancy; enlarged right axillary and subpectoral lymph nodes suggestive of metastases; otherwise, no evidence of distant metastasis. Magnetic resonance imaging (MRI) of the breast showed a 6.4 × 6 × 4.9 cm tumor at 6 o’clock position with a necrotic center. Irregular non-mass enhancement extended anteriorly towards the nipple and posteriorly towards the chest wall abutting the pectoralis muscle with no evidence of chest invasion. Diffuse skin thickening along the antero-inferior right breast with maximum thickness of up to 0.5 cm was also noted. Axillary and subpectoral lymph nodes were again demonstrated, the largest measuring 1.6 × 1.6 cm.
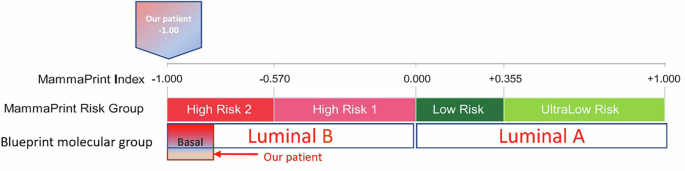
MammaPrint signature score on the tumor core biopsy was (−1.00), which was the highest possible score, indicating an extremely high risk of recurrence without chemotherapy (MammaPrint high risk 2, MPH2). BluePrint test confirmed a basal subtype (+0.52) (Fig. 3). Given the aggressive presentation of her tumor and the basal subtype confirmation by BluePrint, we opted for a TNBC treatment regimen. She received neoadjuvant Adriamycin-Cyclophosphamide for 4 cycles, followed by triweekly carboplatin area under the curve (AUC) 5 for 4 cycles and weekly paclitaxel for 9 weeks, though the last 3 doses were omitted due to progressive neuropathy. We successfully obtained compassionate use of pembrolizumab from our institution per the KEYNOTE-522 trial. It was given every 3 weeks only in the neoadjuvant setting. Following bilateral mastectomy and sentinel lymph node biopsy without reconstruction, pathology revealed a complete response (pCR) in the right breast and lymph nodes. The patient completed adjuvant radiation to her chest wall and chose to take tamoxifen without ovarian suppression, understanding that her tumor might not be truly hormone-dependent. She remains disease-free 2 years after her surgery, with semi-annual surveillance.
Discussion
We report a case of locally advanced HR-positive, HER2-negative invasive ductal carcinoma manifesting as inflammatory breast cancer, which achieved an excellent outcome following a tailored neoadjuvant regimen. The treatment incorporated immunotherapy into a chemotherapy backbone, guided by the tumor’s very high MammaPrint score, basal molecular subtype identified through BluePrint, very high PD-L1 expression, and abundant TILs.
The addition of immunotherapy to the neoadjuvant chemotherapy backbone in stage II and III TNBC became the new standard-of-care in 2022 based on the KEYNOTE-522 trial, where a significant 7% increase in the pCR rate was seen in the chemo-immunotherapy group compared to the chemotherapy only group (63% vs 55.6%, p = 0.00055). This led to an increase in event-free survival (EFS) and a trend towards a favorable overall survival (OS)10. Interestingly, the improved EFS was seen across the entire study cohort regardless of PD-L1 expression11 and therefore, immunotherapy use in the neoadjuvant setting is being endorsed by the American Society of Clinical Oncology (ASCO) guidelines without the need for PD-L1 testing12.
The same concept of adding immunotherapy to the chemotherapy backbone was tested in stage II and III HR-positive breast cancer in two large phase III trials. In order to enrich for more aggressive/potentially immunologically active tumors, the enrollment focused mostly on grade 3 tumors. In the Checkmate 7FL trial13, it was shown that the addition of nivolumab significantly improved the pCR rate in patients with high PD-L1 signal (20.2 vs 44.3%, OR 3.1) compared to PD-L1 negative tumors (14.2 vs 10.7%). The KEYNOTE-756 trial showed that the addition of pembrolizumab to the chemotherapy backbone increased pCR rate in the PD-L1 positive tumors (29.7% vs 19.6%) vs PD-L1 negative tumors (7.2 vs 2.6%)14. However, in both trials, some grade 3 tumors had low Oncotype DX recurrence scores15 and some Oncotype low-risk tumors still displayed positive PD-L1 expression16. Therefore, in hindsight, neither the grade nor the PD-L1 expression could perfectly and reliably capture all the tumors that are likely to respond to immunotherapy.
Upon further capitalization on the same immunotherapy concept, but while incorporating the modern molecular profiling in the selection criteria for HR+ tumors, a strong signal was identified in the window of opportunity I-SPY trial, which used the MammaPrint genomic classifier for risk stratification in the neoadjuvant setting. The pCR rate was shown to be selectively increased in MPH2 tumors but not MPH1 tumors in the Durvalumab-Olaparib-Taxol arm vs standard-of-care paclitaxel arm—the pCR was (64% vs 22%) in MPH2 compared to (9% vs 10%) in MPH117. The response signal in MPH2 tumors has been the basis of the ongoing SWOG 2206 trial (NCT06058377), in which patients with MPH2 tumor will be randomized to standard-of-care chemotherapy vs chemotherapy plus durvalumab in the neoadjuvant setting.
Our patient’s tumor had several predictive factors for her favorable treatment response. First, her tumor was immunologically active displaying high PD-L1 expression. In fact, in a meta-analysis by Azim et al. including 2403 patients with non-metastatic TNBC treated with chemotherapy alone, PD-L1 positive tumors were more likely to achieve pCR, compared to PD-L1 negative tumors. The authors concluded that PD-L1 expression is a surrogate marker for chemosensitivity18. Second, it had an “ultra-high risk” or in other words, MPH2 score, which is believed to be a surrogate for response to immunotherapy. Third, the patient’s tumor also displayed a high TIL percentage (40%). TILs have been shown to consistently predict a favorable response to immunotherapy for breast cancer in the neoadjuvant setting19 as well as in the metastatic setting20. Fourth, the basal molecular subtype is indicative of its equivalent chemosensitivity to TNBC. In fact, in the NBRST trial7, the ER+/basal tumors showed high pCR rates approaching that of ER-/Basal tumors in response to neoadjuvant chemotherapy (34% vs 38%). Additionally, ER+/Basal tumors display higher chemosensitivity than ER+/luminal tumors in a pooled meta-analysis of 36 public databases (32% vs 9%)21. ER+/Basal tumors were also shown to demonstrate upregulation of immune genes and less expression of ESR1 mRNA differentiating them from luminal tumors and making them more similar to TNBC/Basal tumors21. It is very important to highlight that although the incidence of basal tumors is expectedly higher among ER low expressing tumors (ER 1–9% positive), which were around 18% in some reports22. PAM50 molecular classifier frequently identifies some tumors with ER expression >10% as basal subtype. Therefore, relying on the percentage of ER expression to predict the molecular subtype of breast cancer is not reliable. Last but not least, the concept of adding immunotherapy to neoadjuvant chemotherapy is being tested in two ongoing IBC pilot studies with encouraging preliminary results23,24. However, due to the limited number of IBC patients in landmark breast cancer studies, the general ASCO guidelines for breast cancer treatment are still applied to the inflammatory category through extrapolation12 and are consistent with the international consensus25.
In conclusion, this case highlights the importance of incorporating molecular profiling in treatment decisions, as ER expression level alone does always reliably and precisely predict the molecular subtype.
Methods
The patient provided written informed consent for both participation and publication of the study. She also provided informed consent to publish the photograph in Fig. 1. Separate IRB/FDA approval is not required for off-label use of the checkpoint inhibitor or reporting of the case.
ER and PR testing
The Allred scoring system for ER and PR detection via IHC staining is a semi-quantitative method that combines the proportion of positive-staining cells and the intensity of staining to provide a comprehensive score. This system is designed to standardize the assessment of ER status in breast cancer tissues.
The Allred score consists of two components:
-
Proportion Score: This score ranges from 0 to 5 and is based on the percentage of tumor cells that show positive staining: 0: No staining, 1: <1% of cells, 2: 1–10% of cells, 3: 11–33% of cells, 4: 34–66% of cells, 5: 67–100% of cells.
-
Intensity Score: This score ranges from 0 to 3 and is based on the average intensity of the positive staining: 0: No staining, 1: Weak staining, 2: Moderate staining, 3: Strong staining.
The final Allred score is the sum of the proportion and intensity scores, resulting in a total score ranging from 0 to 8. A score of 0 indicates no ER expression, while scores of 3–8 indicate varying degrees of ER positivity. This scoring system helps to clearly distinguish ER-negative from ER-positive breast cancers, improving the accuracy and consistency of ER status assessment26,27,28.
HER2 testing
HER2 staining by IHC is a method used to evaluate the overexpression of the HER2 protein in breast cancer tissues. According to the ASCO and the College of American Pathologists (CAP) guidelines, HER2 IHC results are scored on a scale from 0 to 3+ based on the intensity and completeness of membrane staining in tumor cells29.
-
0: No staining or membrane staining in <10% of tumor cells. This is considered HER2-negative.
-
1+: Faint or barely perceptible membrane staining in >10% of tumor cells. This is also considered HER2-negative.
-
2+: Weak to moderate complete membrane staining observed in >10% of tumor cells. This is considered equivocal and requires further testing with in situ hybridization (ISH) to determine HER2 gene amplification status.
-
3+: Strong complete membrane staining in >10% of tumor cells. This is considered HER2-positive.
TILs evaluation
Stromal TILs were enumerated following the methods established by the International TILs Working Group30,31 and reported as a percentage by the pathologist (ES). TILs were assessed within the boundaries of the invasive tumor using the core biopsy.
MammaPrint test
MammaPrint is a genomic assay that evaluates the expression of 70 genes in tumor cells to assess the risk of distant recurrence in early-stage hormone-positive, HER2-negative breast cancer. It stratifies patients into low-risk and high-risk categories for distant metastasis, aiding in personalized treatment decisions regarding the utilization or omission of chemotherapy according to the designated risk score32.
BluePrint test
BluePrint is an 80-gene molecular subtyping assay that classifies breast tumors into three primary molecular subtypes: Luminal, HER2 enriched and Basal. This classification relies on the expression of 80 genes on the breast cancer cells and provides a more precise molecular characterization compared to traditional IHC and fluorescence in situ hybridization (FISH) methods32.
Data availability
The authors declare that data supporting the findings of this study are available within the paper.
References
Abraham, H. G., Xia, Y., Mukherjee, B. & Merajver, S. D. Incidence and survival of inflammatory breast cancer between 1973 and 2015 in the SEER database. Breast Cancer Res. Treat. 185, 229–238 (2021).
Menta, A. et al. Inflammatory breast cancer: what to know about this unique, aggressive breast cancer. Surg. Clin. North Am. 98, 787–800 (2018).
Hester, R. H., Hortobagyi, G. N. & Lim, B. Inflammatory breast cancer: early recognition and diagnosis is critical. Am. J. Obstet. Gynecol. 225, 392–396 (2021).
Mittempergher, L. et al. Performance characteristics of the BluePrint® breast cancer diagnostic test. Transl. Oncol. 13, 100756 (2020).
Sebastian, W. et al. Genetics, treatment, and new technologies of hormone receptor-positive breast cancer. Cancers 15, 1303 (2023).
Habashy, H. O. et al. A review of the biological and clinical characteristics of luminal‐like oestrogen receptor‐positive breast cancer. Histopathology 60, 854–863 (2012).
Whitworth, P. W. et al. Distinct neoadjuvant chemotherapy response and 5-year outcome in patients with estrogen receptor–positive, human epidermal growth factor receptor 2–negative breast tumors that reclassify as basal-type by the 80-gene signature. JCO Precis. Oncol. 6, e2100463 (2022).
van’t Veer, L. J. et al. BluePrint basal subtype predicts neoadjuvant therapy response in similar to 400 HR+ HER2-patients across 8 arms in the I-SPY 2 TRIAL. Eur. J. Cancer 103, E15–E16 (2018).
Groenendijk, F. H. et al. Estrogen receptor variants in ER-positive basal-type breast cancers responding to therapy like ER-negative breast cancers. NPJ Breast Cancer 5, 15 (2019).
Schmid, P. et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med. 386, 556–567 (2022).
Pusztai, L. et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: exploratory analysis from KEYNOTE-522. Ann. Oncol. 35, 429–436 (2024).
Korde, L. A. et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J. Clin. Oncol. 39, 1485–1505 (2021).
Loi, S. et al. LBA20 A randomized, double-blind trial of nivolumab (NIVO) vs placebo (PBO) with neoadjuvant chemotherapy (NACT) followed by adjuvant endocrine therapy (ET) ± NIVO in patients (pts) with high-risk, ER+ HER2− primary breast cancer (BC). Ann. Oncol. 34, S1259–S1260 (2023).
Cardoso, F. et al. LBA21 KEYNOTE-756: phase III study of neoadjuvant pembrolizumab (pembro) or placebo (pbo) + chemotherapy (chemo), followed by adjuvant pembro or pbo + endocrine therapy (ET) for early-stage high-risk ER+/HER2− breast cancer. Ann. Oncol. 34, S1260–S1261 (2023).
Singh, K. et al. Relationship of histologic grade and histologic subtype with oncotype Dx recurrence score; retrospective review of 863 breast cancer oncotype Dx results. Breast Cancer Res. Treat. 168, 29–34 (2018).
Rozenblit, M. et al. PD-L1 protein expression in relation to recurrence score values in early-stage ER + breast cancer. Breast Cancer Res. Treat. 196, 221–227 (2022).
Pusztai, L. et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: results from the adaptively randomized I-SPY2 trial. Cancer Cell 39, 989–998.e5 (2021).
Azim, H. A. et al. Programmed death-ligand 1 (PD-L1) expression predicts response to neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Biomarkers 27, 764–772 (2022).
Wood, S. J. et al. High tumor infiltrating lymphocytes are significantly associated with pathological complete response in triple negative breast cancer treated with neoadjuvant KEYNOTE-522 chemoimmunotherapy. Breast Cancer Res. Treat. 205, 193–199 (2024).
Emens, L. A. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. J. Natl Cancer Inst. 113, 1005–1016 (2021).
Bertucci, F., Finetti, P., Goncalves, A. & Birnbaum, D. The therapeutic response of ER+/HER2− breast cancers differs according to the molecular Basal or Luminal subtype. NPJ Breast Cancer 6, 8 (2020).
Cejalvo, J. M. et al. Clinical implications of the non-luminal intrinsic subtypes in hormone receptor-positive breast cancer. Cancer Treat. Rev. 67, 63–70 (2018).
Bertucci, A. et al. PELICAN-IPC 2015-016/Oncodistinct-003: a prospective, multicenter, open-label, randomized, non-comparative, phase II study of pembrolizumab in combination with neo adjuvant EC-Paclitaxel regimen in HER2-negative inflammatory breast cancer. Front. Oncol. 10, 575978 (2020).
Kwa, M. J. et al. Nivolumab with chemotherapy as neoadjuvant treatment for inflammatory breast cancer. J. Clin. Oncol. 40, e12633 (2022).
Ueno, N. T. et al. International consensus on the clinical management of inflammatory breast cancer from the Morgan Welch Inflammatory Breast Cancer Research Program 10th anniversary conference. J. Cancer 9, 1437–1447 (2018).
Ahmad Fauzi, M. F. et al. Allred scoring of ER-IHC stained whole-slide images for hormone receptor status in breast carcinoma. Diagnostics 12, 3093 (2022).
Collins, L. C., Botero, M. L. & Schnitt, S. J. Bimodal frequency distribution of estrogen receptor immunohistochemical staining results in breast cancer: an analysis of 825 cases. Am. J. Clin. Pathol. 123, 16–20 (2005).
Qureshi, A. & Pervez, S. Allred scoring for ER reporting and it’s impact in clearly distinguishing ER negative from ER positive breast cancers. J. Pak. Med. Assoc. 60, 350–353 (2010).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO–College of American Pathologists guideline update. J. Clin. Oncol. 41, 3867–3872 (2023).
Salgado, R. et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 26, 259–271 (2015).
Loi, S. et al. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: clinical utility in an era of checkpoint inhibition. Ann. Oncol. 32, 1236–1244 (2021).
Haan, J. C. et al. MammaPrint and BluePrint comprehensively capture the cancer hallmarks in early-stage breast cancer patients. Genes. Chromosomes Cancer 61, 148–160 (2022).
Acknowledgements
We are grateful to the patient who generously contributed to this study.
Author information
Authors and Affiliations
Contributions
J.E. is the patient’s primary oncologist. J.E. conceived the idea, drafted the initial draft and did a comprehensive literature review. Y.Z. revised the draft before an additional round of revisions by all authors and provided an additional literature review. J.E. prepared Figs. 1 and 3. E.S. provided pathology information and prepared Fig. 2. This was approved by all authors before submission to the journal. J.E. and Y.Z. revised the paper again, and all authors again approved of the final changes before resubmission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Elayoubi, J., Zong, Y. & Schwartz, E.J. Successful unconventional precision treatment of inflammatory hormone receptor-positive breast cancer guided by molecular profiling. npj Precis. Onc. 9, 63 (2025). https://doi.org/10.1038/s41698-025-00845-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-00845-5
This article is cited by
-
Antineoplastics
Reactions Weekly (2025)