Abstract
Molecular Tumor Boards (MTBs) are pivotal in personalized cancer care. This systematic review and meta-analysis included 34 studies out of 576 articles (2020–January 2024) involving 12,176 patients across 26 major cancer entities. Of these, 20.8% (2,532 patients) received MTB-recommended therapies, with 178 outcome measures reported, achieving a median overall survival (OS) of 13.5 months, progression-free survival (PFS) of 4.5 months, and an objective response rate (ORR) of 5–57%. A pooled PFS2/PFS1 ratio ≥ 1.3 from 14 reports was observed in 38% (33–44%) of cases. Comparative data showed improved outcomes for MTB-treated patients, with hazard ratios of 0.46 (0.28–0.76, p < 0.001) for OS in 19 and 0.65 (0.52–0.80, p < 0.001) for PFS in 3 studies. These results highlight the benefits of MTB evaluations in improving outcomes for patients with solid tumors but also emphasize the need for standardized evaluation criteria to enable robust comparisons across studies.
Similar content being viewed by others
Introduction
In recent decades, advances in cancer research have illuminated the genetic foundations driving its initiation and progression. This understanding has paved the way for groundbreaking advancements in cancer treatment and the emergence of personalized medicine. With the invention of multi-omics approaches, major advances in cancer genomic analysis and molecular profiling have been accomplished, expanding the range of available targeted therapies for cancer patients, especially those who have exhausted their conventional treatment options1,2,3,4. Personalized medicine in oncology requires multiple processes. This includes identifying particular biomarkers or alterations with next-generation sequencing (NGS), searching vast databases or literature, and discussing appropriate drugs or drug combinations for each patient. These complex procedures stimulated the widespread establishment of interdisciplinary molecular tumor boards (MTB).
The MTB team broadly reviews each patient’s unique characteristics, complex molecular profiling, pathology, imaging, and clinical history to identify targeted therapies by matching the drugs to the molecular alterations or biomarkers detected, resulting in recommendations for molecular-guided personalized cancer treatments2. Though complex and time-consuming, an MTB referral allows cancer patients and their attending physicians to receive molecular cancer treatments outside established therapies based on the latest scientific evidence, for example, Bitzer et al.5, Hoefflin et al.6, and Luchini et al.7. MTB recommendations typically include the suggestions of clinical studies, in-label, off-label, or matched experimental treatments.
Clinical networks in precision oncology allow a constant improvement of these complex procedures by sharing expertize and accelerating the process so that individual hospitals may benefit from expert-agreed, consistent decision-making and structured data capture8,9,10,11,12. Despite establishing all the complex procedures along with its need for vast human and technological resources, there is no consensus on standardized, structured assessments of benefits of MTB recommendations and their assumed improvement over time. A systematic review of clinical outcomes of MTBs by Larson et al.13 identified 14 studies done until early 2020, pointing out the need for better quality data and recommended standardization of approaches and outcomes. The review focused on clinical outcome measures with partial, complete, and overall response rates among patients referred to MTBs but did not try to quantify the overall effect of the recommended treatments on the patients.
An essential prerequisite to accessing reliable outcome data of MTBs is to ensure the capture of high-quality, real-world data of the course of treated patients9, which is not readily available in most published reports describing MTB procedures and diagnostic results. With this background, we performed a systematic review and meta-analysis with the primary objective of assessing the effectiveness of MTB recommendations for cancer treatment strategies in terms of improvement of clinical outcomes among cancer patients. The secondary objectives were to describe all outcome measures reported and to identify gaps in the assessment of the effectiveness of MTBs.
Results
Study characteristics
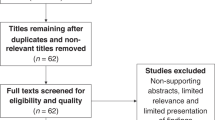
The search identified 576 articles and 340 articles were screened after removing duplicates to identify 34 MTB studies. Primary data on patient outcomes for 12.176 patients referred to the MTB and 2.532 patients (20.8%) who received MTB-recommended therapy (Fig. 1). More than half of the studies were retrospective cohort or register based studies (18/34, 52.9%), while the others were either data from clinical trials or prospective cohorts. The majority of studies (12, 35%) were from Germany followed by France (6, 18%) and USA (7, 21%). 28 studies (82%) collected their data for a period of at least 3 years. The main characteristics of the included studies are presented in Table 1 and Supplementary Table 1. Patients with any advanced cancer were included in 21 (62%) studies, while the others focused on specific cancer types (breast/gynecological cancer, non-small cell lung cancer (NSCLC), specific gastrointestinal and nervous system related cancers) and involved between 69 and 1.772 cancer patients. Overall, there were 26 major tumor entities mentioned (Supplementary Fig. 1), the most frequent being breast (1.516, 14%), lung (1.273, 12%), upper gastro-intestinal (GI) (928, 9%), lower GI (900, 8%), bone & soft tissue (876, 8%), biliary tract (691, 6%), gynecologic (658, 6%), urinary tract and kidney (559, 5%), pancreatic (511, 5%), neuroendocrine (468, 4%), and brain (462, 4%) cancers.
Detailed information on the inclusion criteria for MTB presentation was available in 29 of the 34 studies. These criteria, which guide the use of next-generation sequencing (NGS) diagnostics and subsequent MTB evaluation, were as follows: advanced solid tumors irrespective of treatment line (13/29), lack of further established therapeutic options (10/29), rare cancers (7/29), disease progression during at least one line of prior therapy (5/29), patient age ≤50 years (2/29), relapse following initial remission (1/29), and recurrent glioma (1/29). Regarding the participants and their pivotal roles in the MTB, 21 out of the 34 studies (62%) provided information on the professional disciplines involved. Clinical and/or medical oncologists were included in all 21 studies. Other key disciplines represented were pathology (19/21), human or clinical genetics (15/21), bioinformatics and data science (13/21), molecular biology or molecular pathology (13/21), basic or translational science (9/21), radiology (5/21), clinical pharmacology (4/21), clinical trial coordination (4/21), and structural biology (1/21).
The median number of patients referred to MTB per study were 216 patients (range 69 to 1772 with IQR: 104–516; N = 34), of which a median of 99 (range 32–1138 with IQR: 57–255; N = 28) patients received treatment recommendations by MTB per study and a median of 54 (range 9–362 with IQR: 24–86; N = 34) patients were treated according to MTB recommendations. The median age of patients referred to MTBs ranged between 45–64 years with the lower and upper limits between 1–54 years and 64–95 years respectively. The patients were followed up at a median interval of 8 (range 6–26; N = 20) weeks from the start of MTB-recommended therapy, with 20 (59%) studies using the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria for assessment14. The median duration of patient follow-up was 11 months (IQR: 9–15; N = 10) ranging between 7 and 36 months. Individual data for all patients receiving MTB-recommended treatment was provided in 26 (77%) studies, however, only 12 (35%) provided outcome data for all patients and 9 (27%) only for a subset of patients.
19 (56%) of the 34 MTBs utilized prespecified actionability scales to classify recommendations; 14 used the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT)15 alone or in combination with other scales, such as NCT/DKTK from the National Center for Tumor Diseases (NCT) and the German Cancer Consortium (DKTK)16, while 5 used various other actionability scales (Supplementary Table 1). Data on performance status was provided in 17 (50%) studies, with ECOG being the most frequently used scale (13, 38%). The turn-around time (TAT) with varying definitions of this period was reported in 13 (38%) studies. Among 9 MTB studies using similar definitions, the median TAT varied between 12–115 days.
Descriptive characteristics of included studies are presented in Supplementary Table 1.
Outcomes among patients on MTB-recommended therapy
Overall, 186 outcome measures were reported with 42 (23%) related to PFS, 21 (11%) to OS, 23 (12%) to DCR, and 20 (11%) to ORR among 26, 22, 23 and 20 studies, respectively. All outcome measures are listed in Table 2 and Supplementary Table 2. Of note, PFS was the most frequently studied outcome investigated in 26 of the 34 studies (77%). A high variation was noticed in the required period to fulfill the criteria to define SD, ranging between 6 weeks and 6 months (Table 2). Patients with MTB-recommended therapies had a median OS of 13.5 (10.9–19.5) months and a median PFS of 4.5 (2.8–6.5) months. The ORR ranged from 5% to 57% and DCR from 29% to 84%, with a median partial response rate of 18% (range: 3%–57%) and complete response rate of 1% (range: 0%–8%). The PFS at 6 months reported in 3 studies were 2%, 28% and 79% respectively.
Figure 2 shows the CR-, PR-, and SD-rates along with the respective cancer types, sample size and the starting year of the data collection. The pooled estimates of the CR-, PR-, and SD-rates were 1% (95%CI: 0%–3%), 19% (95%CI: 15%–24%), and 25% (95%CI: 21%–30%) respectively. The pooled estimates of ORR and DCR were 21% (95%CI: 16%–26%) and 45% (95%CI: 39%–52%) as shown in Fig. 3.The ratio of PFS on a MTB-recommended treatment (PFS2) to the PFS on the last previous line of therapy (PFS1) to assess whether patients benefitted from the treatment, introduced by Von Hoff et al.17, was reported in 14 studies. A ratio of PFS2/PFS1 ≥ 1.3 to 1.5 is considered as clinical benefit18,19 and proportion of patients with PFS2/PFS1 ratio ≥1.3 (pPFSR ≥ 1.3) was found to vary between 25 and 68% with a pooled estimate of 38% (33–44%) as seen in Fig. 4.
Red circle refers to cross cancer, purple circle refers to GI cancer, green circle refers to breast cancer, blue circle refers to CNS cancer, turquoise circle refers to NSCLC. The size of the circle represents the number of patients on MTB recommended therapy. GI Gastro-intestinal, CNS Central Nervous system, NSCLC Non-small-cell lung cancer. Panel (A) presents partial response rates, (B) presents complete response rates and (C) presents stable disease rates.
Panel (A) presents objective response rates and (B) presents disease control rates. The point and the horizontal line represent the observed study estimate and its confidence interval. The size of the gray square box varies according to the weightage given to the estimate. The gray diamond represents the pooled estimate, and its length symbolizes its confidence interval. The vertical reference line indicates no effect. The red line represents the prediction interval.
The point and the horizontal line represent the observed study estimate and its confidence interval. The size of the gray square box varies according to the weightage given to the estimate. The gray diamond represents the pooled estimate, and its length symbolizes its confidence interval. The vertical reference line indicates no effect. The red line represents the prediction interval.
An association of predefined actionability scales to clinical outcomes was investigated in 7 (21%) studies (details are shown in Supplementary Table 3). All 5 MTBs using ESCAT or NCT/DKTK scales showed a larger benefit in survival or response rates among patients with the highest level of evidence (ESCAT tier I/II or NCT/DKTK m1A-C), a significant association of outcome parameters and evidence levels was found in 4 of these studies. MTBs applying the OncoKB therapeutic level of evidence (N = 1) or a so-called University of Kentucky grading of evidence scale (N = 1) could not establish an association of the outcomes with the actionability scale. The effect of the performance status on clinical outcomes was only studied in 3 studies. While multivariate analyses by Gambardella et al.20 found an independent positive association of ECOG performance status on PFS2, Repetto and Crimini et al.21 did not find any effect. An unadjusted positive effect of WHO status on clinical outcome was reported by Reda et al.22.
Effect measures in comparison to control groups
A comparison between patients with MTB-recommended treatments and any control group was performed in 19 (56%) studies, and 3 studies had 2 comparison groups each. Overall, 5 different kinds of control groups were reported: (i) patients who were not treated in accordance with the MTB recommendations / standard of care therapies (15 studies, 44%), (ii) patients with no actionable driver / did not receive MTB recommendation (2 studies, 6%), (iii) patients who received a recommended treatment with a low study-defined matching score (2 studies, 6%), (iv) MTB referred patients who were not given any treatment (2 studies, 6%) or (v) patients not referred to the MTB (1 study, 3%). The definitions of matching scores or matched therapies varied slightly, though overlapping (details provided in Supplementary Table 4).
In total, 38 separate comparisons were reported, including 12 hazard ratios (4 PFS, 8 OS), 2 odds ratios (DCR) and 2 ratios (1 PFS, 1 OS) as effect measures. The remaining 22 comparisons did not provide any effect measure but rather assessed statistical significance using standard tests: 6 for median PFS, 3 for PFS-rate at 6 months, 7 for median OS, 1 for OS-rate at 6 months, 1 for median PFS2/PFS1ratio, 2 for pPFSR≥1.3, 1 for DCR, and 1 for CRR. Patients on MTB-recommended therapy numerically had a better outcome in 36/38 (95%) comparisons. Of note, 24 (66.7%) of these comparisons reached statistical significance.
Meta-analysis of the HRs for OS estimated a pooled HR of 0.46 (0.28 to 0.76, p < 0.001) with an I2 of 73.2% (p = 0.001; Fig. 5A). Similarly, patients receiving MTB recommended treatment had a significant better progression free survival (pooled HR of 0.65 (0.52 to 0.80, p < 0.001) with an I2 < 1% (p = 0.38; Fig. 5B)).
Panel (A) presents overall survival and (B) presents progression free survival. The point and the horizontal line represent the observed study estimate and its confidence interval. The size of the gray square box varies according to the weightage given to the estimate. The gray diamond represents the pooled estimate, and its length symbolizes its confidence interval. The vertical reference line indicates no effect. The red line represents the prediction interval.
The pPFSR≥1.3 was significantly higher among the MTB patients as compared to control groups with a rate ratio of 1.7 and 4.2 in 2 studies as shown in Fig. 6. There were 2 studies reporting HR of PFS among patients with advanced or metastatic malignant disease and patients with advanced colorectal cancer, both among US patients, reporting an unadjusted HR of 0.68 (0.51 to 0.90) and an adjusted HR of 0.41(0.21 to 0.81) respectively. The median PFS was more than twice longer among the MTB patients as compared to the control patients in 5 studies based in Italy, France, Spain, Germany and USA (14.2 vs. 5 months, 8.5 vs. 5.7 months, 6.5 vs. 2.8 months, 6.4 vs. 3 months, and 4.3 vs. 1.9 months), while it was similar in data from advanced French cancer patients between 2016–2018 (2.5 vs. 2.4 months). Bertucci et al compared MTB and control groups of advanced cancer patients from 2014–2019 in France according to best response assessment. That study found a higher PFS at 6 months with 79% vs. 41% among patients with disease control (CR, PR or SD), 2% vs. 1% among those with progressive disease (PD), and 28% vs. 16% overall23.
Meta-analysis for DCR showed a significant benefit among patients receiving MTB recommended treatment with an odds ratio of 2.97 (1.44–6.09), p with an I2 < 1% (p = 0.001; Fig. 7). Patients with diverse advanced or metastatic malignancy in the US provided an adjusted odds ratio of 0.40 (0.24 to 0.67) while comparing those with a study-defined matching score ≥50% versus <50%. Similarly, an adjusted odds ratio of 0.21 (0.04 to 1.06) for disease control of at least 6 months among US patients was reported for advanced colorectal cancer on unmatched therapy (n = 17) in comparison with matched therapy (n = 34). Further comparisons reported a significantly higher DCR among MTB patients (53% vs. 21%, p = 0.019) from 2012–2018 in the US with a high (≥50%) versus low (<50%) matching score and a 2% vs. 1% complete response rate among MTB patients (p = 0.249) with a study-defined matched versus non-matched therapy from 2014–2019 in France.
The point and the horizontal line represent the observed study estimate and its confidence interval. The size of the gray square box varies according to the weightage given to the estimate. The gray diamond represents the pooled estimate, and its length symbolizes its confidence interval. The vertical reference line indicates no effect. The red line represents the prediction interval.
Risk of bias assessment
Though all included studies provided relevant outcome measures of interest, the impact of MTB on patient outcomes was not the primary aim for some studies. With this background, 8 (24%) studies were assessed to be of good quality, 24 (71%) medium and 2 (6%) of low quality (Supplementary Fig. 2). Half the studies did not have a comparison group; those with comparison groups posed a high risk of bias in the area of detection of confounders, adjustment for confounding and statistical analysis. For questions regarding outcome assessment and follow-up which included outcome definition, length of follow-up, follow-up data, and incomplete follow-up, there was a low risk of bias for >70% of the studies (Supplementary Fig. 3).
Discussion
With the widespread implementation of MTBs in routine oncology practice2,24, there is a high unmet need to assess patients’ clinical benefit and compare these parameters among various patient populations and periods. To date, standardized parameters to validate these assessments are yet to be proposed, and a consensus on the specificities of data, which needs to be analyzed in this context, still needs to be defined.
Our investigation encountered highly heterogeneous estimates of effect and outcome measures, reflecting the diverse cancer types, molecular alterations, treatment protocols, hospital settings, and expertize availability across the included studies. Despite these limitations, our analyses revealed remarkable results. Patients with MTB-recommended therapies had a median OS of 13.5 (10.9–19.5) months and a median PFS of 4.5 (2.8–6.5) months. From 38 separate comparisons between patients treated according to MTB recommendations and any control group, 36 had a numerically better outcome, with 67% reaching statistical significance. Importantly, our systematic review and meta-analysis, evaluating the impact of molecular tumor boards on clinical outcomes of cancer patients compared to study-defined control groups, found a significant positive effect in terms of OS, PFS, and DCR. Addressing methodological concerns regarding the definition of relevant control groups, our meta-analysis of OS data revealed a 54% lower hazard of dying compared to those not treated according to MTB recommendations. Even the intra-patient comparison of MTB-guided therapies with the previous therapy, reflected by over a 30% increase of PFS2 over PFS1 (pPFSR≥1.3)18,19, showed a significant improvement compared to non-MTB-recommended therapies.
The investigated publications reflect an effort to quantify the benefit of precision medicine within the investigated period. However, a critical finding of our systematic review is a need for enhanced standardization in endpoints and reporting of outcome measures. Important aspects to be considered in outcome selection are the type of response to MTB-guided therapies regarding sensitivity or resistance and the clinical magnitude of benefits2. The currently available evidence included important survival estimates such as PFS and OS, with PFS reported more often than OS. There was a high variation in the statistics reported; for example, the authors reported PFS at 6 months, median PFS, or overall PFS. Outcome measures quantifying treatment responses such as DCR and ORR among patients receiving MTB-recommended treatments could be estimated only in 68% and 59% of the studies, respectively. Though we restricted our meta-analysis to predefined parameters, we documented all the reported clinical outcome measures and picked up subtle to considerable differences in the definitions of endpoints of interest, such as criteria used to define SD varying between - at least 6 weeks, 8 weeks, 3 months, or 6 months. Such discrepancies add to the background noise of an already heterogeneous multidimensional data. Furthermore, only 62% of the studies provided patient level data on outcomes. Standardization efforts of study designs, data collation, analytical techniques, and reporting are much needed now when increasing evidence is reported through available multiple data sources and advanced data collections.
Actionability scales that define clinical evidence-based criteria to prioritize alterations, in contrast to relying solely on expert opinions, were reported in 19 of the 34 analyzed studies. However, an association between evidence levels and outcome parameters was investigated in only 7 of these studies. Notably, all studies that analyzed ESCAT or NCT/DKTK evidence levels demonstrated a greater benefit in survival or response rates among patients with the highest evidence levels.
Considering all the studies we included in this meta-analysis, excluding patients from the analysis and selecting a relevant control group pose challenges in assessing the benefit without any bias. Prospective cohort studies with appropriate comparison groups represent the minimum requirement for proper evaluation of effectiveness. Among the 34 studies included in this analysis, only 19 reported control groups for their investigations6,20,21,22,23,25,26,27,28,29,30,31,32,33,34,35,36,37,38. Patients in these control groups were either not treated according to the MTB recommendation, MTB referred patients with no actionable driver, not referred to the MTB, or grouped according to a predefined matching score for the MTB-guided therapy. Disparate definitions of these control groups hinder inter-study comparison and pooling of results.
Potential control groups for the study of MTB effectiveness can be chosen at different levels (Supplementary Fig. 4); however, each choice comes with different challenges. A comparison with patients not referred to MTB or patients with MTB recommendations but treated with an alternative therapy or best supportive care (BSC) may have primarily induced a bias towards a worse performance status. Moreover, the therapy line to be used to compare patients not referred to the MTB needs to be clarified; maybe the most suitable comparison group to investigate a potential benefit for MTB presentations is patients without an MTB recommendation. However, even for this comparison, a clear baseline, e.g., the date of the first new therapy regime after the MTB recommendation, has to be thoroughly defined. Additionally, in heterogeneous patient groups such as “all comers” with advanced disease, selecting the right control group becomes critical. Potential control groups could include patients with similar histology but no actionable mutation, or patients with a similar alteration but without access to the recommended treatment. Each of these options carries inherent challenges, such as biological variability between tumors or differences in treatment access, which must be accounted for during study design. Furthermore, the absence of actionable alterations seems to influence the prognosis of patients at least in some tumor entities, as e.g. recently reported by Nakamura et al.39. External or synthetic control arms primarily derived from real-world data to supplement the comparison in oncological research40 are a new development considered in precision medicine and may also be applicable to MTB research as well41. Most of these study designs demand using propensity scores to adjust and minimize any confounding owing to control selection processes.
Standardization of patient flow and reimbursement for molecular profiling and MTB structures, as exemplified by the Centers for Personalized Medicine in Germany9, is imperative. Benefit assessments from care providers’ and patients’ perspectives, including quality-of-life endpoints, are warranted. This meta-analysis advances beyond Larson et al.13 by providing a more detailed summary and analysis of outcome measures, an overview of MTB inclusion criteria and actionability scales, as well as an analysis of control group outcomes. Altogether, our analysis should stimulate the discussion on defining parameters that should be reported in MTB-cohort analysis, e.g., by an interdisciplinary consensus conference.
Our systematic review may be limited by the inclusion criteria focusing on the large sample size and the number of genes studied, potentially leading to homogenized results. However, having a minimum sample size and a defined study period is crucial to reflect the standard functionality of MTBs. The concentration of studies in high-income countries potentially limits generalizability; however, it reflects the historical establishment of MTBs and advancements in precision oncology research in this context.
In conclusion, our systematic review and meta-analysis based on the evidence base between 2020 and early 2024 show that MTBs positively impact the course of patients with advanced solid tumors. Notably 67% of the reported clinical outcomes from various MTB studies showed significant benefits. Importantly, this study identifies a significant positive effect of MTBs on the clinical outcomes of cancer patients, including Progression-Free Survival (PFS), Overall Survival (OS) and disease control rates (DCR). Even in comparison with previous therapies, MTB-guided treatments exhibit significant improvement in PFS2 over PFS1 (pPFSR≥1.3). Our findings underline the need for a structured evaluation and reporting of MTB benefits, which requires particular focus. We recommend discussing a consensus for assessing relevant parameters that should be standardized between MTB groups. This approach could tremendously improve MTB research, including comparing MTB-guided therapies between patient cohorts, regional areas, or available drug regimens.
Methods
This review was performed following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for systematic reviews and the protocol registered in PROSPERO under CRD42023404806.
Literature search and selection process
An extensive literature search was conducted on February 1st, 2023, and later updated in October 2024 in PubMed and Web Of Science in accordance with the Cochrane Handbook of Systematic reviews using the search terms “molecular tumor board” OR “molecular tumor board” OR “precision medicine tumor board” OR “precision medicine tumor board” to identify all articles published between January 1st, 2020 and January 31st, 2024. Interventional or observational studies based on primary data on MTBs reporting outcomes of clinical, economic or any other impact published in English were included. Additionally, previous systematic reviews and references were combed through to identify any missed articles. Studies involving <50 patients, with molecular profiling based on <300 genes and focus on acute hematological cancer patients were excluded.
Title and abstract screening followed by full text screening were conducted by two reviewers (JB and PB) and discrepancies sorted out through discussion with MTB experts (PM, MB). The researchers were not blinded to study authors or location. Data from included articles were entered onto a standardized pre-formatted data sheet, cross-verified, and inconsistencies sorted out by consensus. Data related to publication, target population, study design, MTB-recommended therapy, follow-up, clinical outcomes and comparison of outcome measures were extracted.
Data synthesis and meta-analysis
Primary outcomes were objective response rate (ORR), disease control rate (DCR), overall survival (OS) and progression free survival (PFS). ORR was defined as percentage of patients who achieved partial (PR) or complete response (CR) among patients receiving MTB-directed therapy. DCR was defined as percentage of patients who achieved stable disease (SD), partial response (PR), or complete response (CR) among patients receiving MTB-directed therapy. Variance estimates were calculated for the outcome measures whenever possible. Pooled estimates of outcome measures were obtained using random effects meta-analysis and heterogeneity studied using I-squared statistic when outcome or effect measures were available for 3 or more studies. Adjusted and unadjusted hazard ratios (HR) were log transformed for meta-analysis. The rates are presented as median and inter quartile range (IQR). The effect measures in the form of HR, odds ratio (OR) or rate ratio (RR) are presented as the estimate with 95% confidence intervals (CI). Heterogeneity was assessed using I-squared (I2). One study was excluded from the analysis of effect measures in comparison to the control group since the comparison group included patients on existing standard therapies, which was excluded for the MTB cohort37. Quality of eligible studies were assessed using Joanna Briggs quality assessment criteria (JBI)42 adapted to studies reporting outcomes from MTB-based data. Ethical approval was not required as all data are based on published studies. All statistical analyses were carried out using Stata version 15.143.
Data availability
The datasets used in the current study as well as the codes used for meta-analysis are available from the corresponding author on reasonable request.
References
Hyman, D. M., Taylor, B. S. & Baselga, J. Implementing genome-driven oncology. Cell 168, 584–599 (2017).
Tsimberidou, A. M. et al. Molecular tumour boards—current and future considerations for precision oncology. Nat. Rev. Clin. Oncol. 20, 843–863 (2023).
Rosenquist, R., Frohling, S. & Stamatopoulos, K. Precision medicine in cancer: a paradigm shift. Semin. Cancer Biol. 84, 1–2 (2022).
Hoadley, K. A. et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 158, 929–944 (2014).
Bitzer, M. et al. Next-generation sequencing of advanced GI tumors reveals individual treatment options. JCO Precis. Oncol. 4, PO.19.00359 (2020).
Hoefflin, R. et al. Transitioning the molecular tumor board from proof of concept to clinical routine: a German single-center analysis. Cancers (Basel) 13, 1151 (2021).
Luchini, C., Lawlor, R. T., Milella, M. & Scarpa, A. Molecular tumor boards in clinical practice. Trends Cancer 6, 738–744 (2020).
Stenzinger, A. et al. Trailblazing precision medicine in Europe: a joint view by genomic medicine Sweden and the centers for personalized medicine, ZPM, in Germany. Semin. Cancer Biol. 84, 242–254 (2022).
Illert, A. L. et al. The German network for personalized medicine to enhance patient care and translational research. Nat. Med. 29, 1298–1301 (2023).
Fioretos, T. et al. Implementing precision medicine in a regionally organized healthcare system in Sweden. Nat. Med. 28, 1980–1982 (2022).
Tasken, K. et al. A national precision cancer medicine implementation initiative for Norway. Nat. Med. 28, 885–887 (2022).
Tamborero, D. et al. The molecular tumor board portal supports clinical decisions and automated reporting for precision oncology. Nat. Cancer 3, 251–261 (2022).
Larson, K. L. et al. Clinical outcomes of molecular tumor boards: a systematic review. JCO Precis. Oncol. 5, PO.20.00495 (2021).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Mateo, J. et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO scale for clinical actionability of molecular targets (ESCAT). Ann. Oncol. 29, 1895–1902 (2018).
Leichsenring, J. et al. Variant classification in precision oncology. Int. J. Cancer 145, 2996–3010 (2019).
Von Hoff, D. D. et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol. 28, 4877–4883 (2010).
Rodon, J. et al. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat. Med. 25, 751–758 (2019).
Sicklick, J. K. et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat. Med. 25, 744–750 (2019).
Gambardella, V. et al. Molecular profiling of advanced solid tumours. The impact of experimental molecular-matched therapies on cancer patient outcomes in early-phase trials: the MAST study. Br. J. Cancer 125, 1261–1269 (2021).
Repetto, M. et al. Molecular tumour board at European institute of oncology: report of the first three year activity of an Italian precision oncology experience. Eur. J. Cancer 183, 79–89 (2023).
Reda, M. et al. Implementation and use of whole exome sequencing for metastatic solid cancer. EBioMedicine 51, 102624 (2020).
Bertucci, F. et al. Prospective high-throughput genome profiling of advanced cancers: results of the PERMED-01 clinical trial. Genome Med. 13, 87 (2021).
Liu, A. et al. Molecular tumor boards: the next step towards precision therapy in cancer care. Hematol Rep 15, 244–255 (2023).
Boileve, A. et al. Molecular profiling and target actionability for precision medicine in neuroendocrine neoplasms: real-world data. Eur. J. Cancer 186, 122–132 (2023).
Debien, V. et al. Molecular analysis for refractory rare cancers: sequencing battle continues - learnings for the MOSCATO-01 study. Crit. Rev. Oncol. Hematol. 181, 103888 (2023).
El Helali, A. et al. The impact of the multi-disciplinary molecular tumour board and integrative next generation sequencing on clinical outcomes in advanced solid tumours. Lancet Reg. Health West Pac. 36, 100775 (2023).
Fukada, I. et al. Prognostic impact of cancer genomic profile testing for advanced or metastatic solid tumors in clinical practice. Cancer Sci. 114, 4632–4642 (2023).
Giacomini, P. et al. The molecular tumor board of the regina elena national cancer institute: from accrual to treatment in real-world. J. Transl. Med. 21, 725 (2023).
Huang, B. et al. Molecular tumor board review and improved overall survival in non-small-cell lung cancer. JCO Precis. Oncol. 5, PO.21.00210 (2021).
Kato, S. et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat. Commun. 11, 4965 (2020).
Lamping, M. et al. Support of a molecular tumour board by an evidence-based decision management system for precision oncology. Eur. J. Cancer 127, 41–51 (2020).
Louie, B. H. et al. Precision medicine-based therapies in advanced colorectal cancer: the University of California San Diego molecular tumor board experience. Mol. Oncol. 16, 2575–2584 (2022).
Louie, B. H. et al. Pan-cancer molecular tumor board experience with biomarker-driven precision immunotherapy. NPJ Precis. Oncol. 6, 67 (2022).
Mosteiro, M. et al. Molecular profiling and feasibility using a comprehensive hybrid capture panel on a consecutive series of non-small-cell lung cancer patients from a single centre. ESMO Open 8, 102197 (2023).
Pinet, S. et al. Clinical management of molecular alterations identified by high throughput sequencing in patients with advanced solid tumors in treatment failure: real-world data from a French hospital. Front Oncol. 13, 1104659 (2023).
Scheiter, A. et al. Critical evaluation of molecular tumour board outcomes following 2 years of clinical practice in a comprehensive cancer centre. Br. J. Cancer 128, 1134–1147 (2023).
Tarawneh, T. S. et al. Combined focused next-generation sequencing assays to guide precision oncology in solid tumors: a retrospective analysis from an institutional molecular tumor board. Cancers (Basel) 14, 4430 (2022).
Nakamura, Y. et al. Targeted therapy guided by circulating tumor DNA analysis in advanced gastrointestinal tumors. Nat. Med. 14, 165–175 (2024).
Mishra-Kalyani, P. S. et al. External control arms in oncology: current use and future directions. Ann. Oncol. 33, 376–383 (2022).
Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 29, 49–58 (2023).
Joanna Briggs Institute. Critical Appraisal Tools. https://jbi.global/critical-appraisal-tools (2024).
StataCorp L. L. C. Stata Statistical Software: v. 15.1 https://www.stata.com/ (2017).
Hlevnjak, M. et al. CATCH: A prospective precision oncology trial in metastatic breast cancer. JCO. Precis. Oncol. 5, PO.20.00248 (2021).
Horak, P. et al. Comprehensive genomic and transcriptomic analysis for guiding therapeutic decisions in patients with rare cancers. Cancer Discov. 11, 2780–2795 (2021).
Koopman, B. et al. Relevance and effectiveness of molecular tumor board recommendations for patients with non-small-cell lung cancer with rare or complex mutational profiles. JCO Precis. Oncol. 4, 393–410 (2020).
Martin-Romano, P. et al. Implementing the European society for medical oncology scale for clinical actionability of molecular targets in a comprehensive profiling program: impact on precision medicine oncology. JCO Precis. Oncol. 6, e2100484 (2022).
Miller, R. W. et al. Molecular tumor board-assisted care in an advanced cancer population: results of a phase II clinical trial. JCO Precis. Oncol. 6, e2100524 (2022).
Pleasance, E. et al. Whole-genome and transcriptome analysis enhances precision cancer treatment options. Ann. Oncol. 33, 939–949 (2022).
Sultova, E. et al. NGS-guided precision oncology in metastatic breast and gynecological cancer: first experiences at the CCC Munich LMU. Arch. Gynecol. Obstet. 303, 1331–1345 (2021).
Sultova, E. et al. Implementation of precision oncology for patients with metastatic breast cancer in an interdisciplinary MTB setting. Diagnostics (Basel) 11, 733 (2021).
Cobain, E. F. et al. Assessment of clinical benefit of integrative genomic profiling in advanced solid tumors. JAMA Oncol. 7, 525–533 (2021).
Bayle, A. et al. Clinical utility of circulating tumor DNA sequencing with a large panel: a national center for precision medicine (PRISM) study. Ann. Oncol. 34, 389–396 (2023).
Zhang, D. et al. A retrospective analysis of biliary tract cancer patients presented to the molecular tumor board at the comprehensive cancer center Munich. Target Oncol. 18, 767–776 (2023).
Ladekarl, M. et al. Feasibility and early clinical impact of precision medicine for late-stage cancer patients in a regional public academic hospital. Acta. Oncol. 62, 261–271 (2023).
Renovanz, M. et al. Clinical outcome of biomarker-guided therapies in adult patients with tumors of the nervous system. Neurooncol. Adv. 5, vdad012 (2023).
Blobner, J. et al. Significance of molecular diagnostics for therapeutic decision-making in recurrent glioma. Neurooncol. Adv. 5, vdad060 (2023).
Acknowledgements
This research was funded by the Center for Personalized Medicine, Eberhard-Karls University, Tuebingen, Germany. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We acknowledge support for the Open Access Publication Fund of the University of Tuebingen.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
B.P.G.: conceptualization of the study, study design, data collection, data analysis, interpretation of data, wrote the first draft. M.B.: conceptualization of the study, study design, data collection, data analysis, interpretation of data, wrote the first draft. J.B.: study design, data collection, interpretation of data. A.H.: data collection, data analysis, interpretation of data. P.M.: study design, data analysis and interpretation of data. N.M.: conceptualization and study design, interpretation of data. All the authors approved the final version of the manuscript for submission. All authors accept to be accountable for the accuracy and integrity of the data investigated.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gladstone, B.P., Beha, J., Hakariya, A. et al. Systematic review and meta-analysis of molecular tumor board data on clinical effectiveness and evaluation gaps. npj Precis. Onc. 9, 96 (2025). https://doi.org/10.1038/s41698-025-00865-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-00865-1
This article is cited by
-
Convergence of machine learning and genomics for precision oncology
Nature Reviews Cancer (2026)
-
PROs als Kompass der intersektoralen onkologischen Versorgung – von der Praxis in das MTB und zurück
Die Onkologie (2026)
-
Benchmarking progression-free survival ratio as primary endpoint in precision oncology clinical trials
npj Precision Oncology (2025)
-
Zertifizierte Zentren für Personalisierte Medizin in Deutschland: Struktur, Qualität und Translation
Die Gynäkologie (2025)