Abstract
A key hallmark of cancer tumorigenesis is the maintenance of telomere length, which occurs canonically through the reactivation of telomerase. Alternative lengthening of telomeres (ALT) is an atypical, non-canonical telomere maintenance mechanism that uses homologous recombination (HR) to maintain telomere length and is associated with replication stress and defects in genome maintenance. In preclinical models, ALT positivity (ALT+) sensitizes tumor cells to ataxia telangiectasia and Rad3-related (ATR) inhibitors. Camonsertib is a novel potent, and highly selective ATR inhibitor that is synthetic lethal with genomic alterations affecting HR and DNA damage response (DDR). Here we describe a case of confirmed clinical and molecular response to pharmacological ATR inhibition through camonsertib, in a patient with ALT+ metastatic melanoma. To our knowledge, this is the first clinical report of synthetic lethal targeting of a confirmed ALT+ tumor with an ATR inhibitor.
Similar content being viewed by others
Introduction
Cumulative telomeric attrition with each cellular division leads to replicative senescence1. To bypass replicative senescence and achieve replicative immortality, cancer cells preferentially exploit one of two known telomere maintenance mechanisms to reconstitute telomere length—either (i) the de-silencing of hTERT telomerase gene expression, occurring in the majority of human cancers, or (ii) through ALT2. While most prevalent in mesenchymal tumors, ALT has been described in up to 18% of melanomas3.
ALT+ cells synthesize telomeric DNA through a homology-directed, recombination-dependent replication pathway similar to break-induced replication, generating chromosomal instability and replication stress2. Furthermore, ALT is highly associated with the loss-of-function of related chromatin remodelers (such as ATRX and DAXX), which leads to replication stress through the promotion of aberrant HR at repetitive elements, including telomeres4. Though clinical experience is limited, preclinical studies have suggested that the high replication stress state arising through ALT is therapeutically exploitable by targeting ATR, a key mediator of cellular response to replication stress, in a synthetic lethal approach5,6. We now present our experience treating a patient with ALT+ metastatic melanoma with the novel, potent, and selective ATR inhibitor, camonsertib7.
Results
Patient treatment history
The patient, a 63-year-old Caucasian male, was diagnosed in July 2019 with metastatic cutaneous melanoma and treated with nivolumab and ipilimumab with initial radiological partial response (PR) after 3 months, which was confirmed on subsequent nivolumab maintenance. Ipilimumab was resumed following suspected pseudoprogression in March 2020 and continued until confirmed disease progression in June 2020 (Fig. 1A). The patient then received additional off-label targeted treatments with the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib, initially in combination with nivolumab, before switching to olaparib plus trametinib. However, no anti-tumor benefit was observed with both combination regimens and disease progression was noted in April 2021, prior to enrollment on the current study (Fig. 1A).
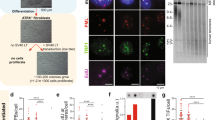
A Treatment history from diagnosis through TRESR trial. B TVR counts derived from Telomere Hunter analysis of WGS data in the tumor compared to blood normal sample collected from this patient. C A representative confocal microscopy image of the ssTeloC foci (green) detected in the retroperitoneal metastatic biopsy (scale bar = 20 µm). The pie chart shows the proportion of cells with various numbers of the foci from the total number of cells analyzed (N = 44,616). Scans of the peritoneal mass at (D) baseline (3.3 cm × 3.2 cm), and 11 months on treatment (0.0 cm × 0.0 cm). E RECIST v1.1 measurements at baseline and on-treatment. Green box indicates −30% change from baseline. F Longitudinal ctDNA monitoring was performed using tumor uninformed variant-based approach using Tempus xF and methylation-based circulating tumor fraction estimation using Guardant Infinity™, and a tumor-informed dPCR based approach from Tracer Biotechnologies. Approximate LOQs for each assay type are marked in the dotted lines.
Tumor genomic findings
Contemporaneous genomic analyses of liver biopsy and paired blood specimens collected at the time of disease progression after front-line nivolumab plus ipilimumab revealed pathogenic somatic alterations in TP53 (p.S127F, p.R65fs), NF1 (p.K1263*), BRCA2 (p.R2482C), ATRX (p.R418*), high tumor mutational burden, microsatellite stable status, and a germline alteration in CHEK2 (p.T367fs) (Table 1). Furthermore, retrospective genomic analysis of a retroperitoneal metastatic biopsy taken after disease progression on the combinations of olaparib plus nivolumab and olaparib plus trametinib revealed the previously detected alterations, as well as an additional pathogenic germline NF1 alteration, and provided further molecular information on allele zygosity and mutational signatures (Table 1, Fig. S1). Importantly, these analyses demonstrated that the BRCA2 and gCHEK2 mutations were monoallelic, which was consistent with an HR proficient (HRP) genomic signature, as well as the lack of anti-tumor clinical benefit from olaparib. Compound germline and somatic NF1 pathogenic mutations point to a potentially early initiating event for this patient’s tumor.
Assessment of telomere maintenance mechanisms
Whole genome sequencing (WGS) confirmed biallelic loss of ATRX, which was consistent with the presence of an ALT+ genomic signature (Table 1), driven by markedly higher telomere variant repeats (TVR) counts compared with normal tissue (Table 1, Fig. 1B). To confirm the ALT+ status in tumor cells, Native Fluorescence In Situ Hybridization (FISH) was performed on a tissue section from the retroperitoneum metastasis (same specimen as WGS). Single-strand telomeric C-rich (ssTeloC) foci were observed in 28.3% (12,648/44,616) of tumor cells (Fig. 1C), providing functional confirmation for ALT activity in the patient’s tumor. In total, we provide 3 independent lines of evidence supporting ALT+ status from the same tumor tissue specimen.
Safety, dosing, and adverse events
The patient was enrolled on the TRESR trial based on his previously identified BRCA2 alteration and received camonsertib monotherapy at a dose of 120 mg given on Days 1–3 of each week of a 21-day cycle. The patient tolerated treatment well and continued at the same dose for 13 cycles (9 months), after which an intermittent weekly schedule (2 weeks on/1-week off) was adopted due to the development of grade 3 anemia requiring blood transfusion. Anemia was the only reported toxicity considered related to study drug treatment. Following the change in dosing schedule, the patient’s hemoglobin levels remained stable for the rest of the duration of therapy (a further 13 months).
Radiological and molecular response
The patient achieved a PR (RECIST v1.1; sum of target lesions -36% from baseline) after 3 months of treatment which was confirmed and sustained with further deepening of the target lesion response (sum of target lesions -74% from baseline) after 11 months of camonsertib (Fig. 1D, E). Circulating tumor DNA (ctDNA) clearance was observed by three weeks after camonsertib initiation and remained low throughout treatment monitored using variant-, methylation- and personalized digital polymerase chain reaction (dPCR)-based assays (Fig. 1F). The cancer began to progress at 19 months (+105% from nadir, -47% from baseline), but due to ongoing clinical benefit, treatment was continued for a total duration of 22 months.
Discussion
We describe the first clinical report of synthetic lethal targeting of ALT+ melanoma with an ATR inhibitor. This patient’s tumor was characterized by the biallelic loss of ATRX, markedly elevated TVR counts and ssTeloC foci, consistent with an ALT+ phenotype. Upon treatment with camonsertib, dosed at 120 mg weekly on Days 1–3 of each week, the patient experienced marked and sustained RECIST PR and ctDNA molecular response, and had a total of 22 months of treatment with a safe and well-tolerated oral targeted therapy.
Camonsertib was well-tolerated with anemia being the only treatment-related toxicity observed, which is a known class effect related to orally available ATR inhibitor. ALT is an emerging predictive biomarker of response for ATR inhibitors, but to the best of our knowledge, has never been formally assessed or demonstrated in the clinic5,6. Indeed, in ALT+ cells, telomeres are prone to replication stress caused by a variety of physical impediments to replication such as RNA-DNA hybrids, G-quadruplexes, or repetitive DNA sequence8. ATRX loss, which is highly associated with the ALT phenotype, further exacerbates replication stress at telomeres through defective nucleosome assembly9. In ALT+ cells, DNA double-stranded breaks at telomeres arising from cleaved replication forks encourage inter/intra-telomeric HR and break-induced telomere synthesis leading to marked cell-to-cell telomere length heterogeneity and chromosomal instability5. ATRX loss has also been described to lead to persistent association of replication protein A (RPA) with telomeres after DNA replication, creating a recombinogenic RPA-single-stranded DNA structure, which serves not only as an HR intermediate but a recruiter of ATR, which itself regulates HR5. Treatment of ALT+ cell lines with an ATR inhibitor was demonstrated to trigger chromosomal fragmentation and apoptosis5, providing preclinical rationale for the targeting of ATR in ALT+ tumors.
While this patient’s tumor harbored other genomic alterations of interest (namely, somatic BRCA2 and germline CHEK2 mutations), which have also been described as predictive biomarkers for ATR inhibitor sensitivity, both alterations were monoallelic and associated with an HRP genomic signature, consistent with the patient’s lack of clinical benefit from PARP inhibitor-based therapy given as a prior line of treatment. The HRP genomic signature detected in the patient’s tumor is furthermore consistent with its ALT dependence, as ALT+ cells actively rely on intact HR to sustain replicative immortality. Furthermore, the identification of compound heterozygous NF1 loss of function is notable, but not predicted to predispose to ATR inhibitor sensitivity, and more likely represents an early event in tumorigenesis.
Several molecular markers may be used to provide evidence of ALT activity in cancers, each with their own inherent advantages and disadvantages. These markers include Native FISH10,11, Telo-FISH4, colocalization of telomeres with promyelocytic leukemia protein (PML) bodies9, and extrachromosomal telomeric repeats including circles of partially double-stranded C-rich telomeric DNA (C-circles) assay12. A C-circle assay is a highly specific and sensitive method to detect ALT activity and may be amenable to testing in plasma samples, although this has not yet been clinically validated13. However, C-circle detection requires multiple DNA extraction, enzymatic, and detection steps that may not be convenient for a routine histopathological laboratory14. Detection of ‘ultrabright’ telomeric foci by Telo-FISH and telomere localization in PML bodies have been used to detect ALT in archival FFPE samples15, although, especially in the case of ALT-associated PML bodies, routine use is challenged by technical limitations and potential for false positives or negatives10. In this study, whole-genome sequencing analysis using a published bioinformatic classifier3,16 was combined with a Native FISH assay, providing orthogonal lines of evidence for ALT activity. The Native FISH assay is a convenient readout for ALT detection that is compatible with routine FISH-based assays in clinical histopathological laboratories10. This assay was shown to reliably detect ALT+ tumor models when a threshold was applied of as few as 10% of cells showing ≥1 nuclear focus, highlighting its specificity and sensitivity. We believe that further diagnostic development of Native FISH, including a robust validation of the relevant cut point, could enable the prospective selection of patients harboring ALT+ tumors. Computational sequence analysis approaches are also showing promise for the stratification of telomere maintenance mechanisms3,16,17. The classifier3 adapted in this study has been shown to robustly detect ALT+ tumors with a C-circle assay, based on the quantification of relative telomere content and the presence of specific TVRs (e.g., TTTGGG, TTCGGG, TTGGGG, and GTAGGG). However, the requirement of whole-genome sequencing data may be yet limiting for routine prospective diagnostic use. We envision that a future diagnostic assay for ALT status may combine multiple orthogonal readouts, allowing for the highest specificity and sensitivity.
Another important area of future investigation will be to understand the dynamics of ALT activity over the course of cancer development and in response to therapy. In this study, genomic analysis of ctDNA at progression did not identify any emergent resistance mechanisms, but was limited by low circulating tumor fraction. While little is currently known about acquired resistance mechanisms to ALT+ targeted therapies, one could speculate that reactivation of telomerase-mediated telomere elongation through hTERT could render ALT+ tumor cells resistant to ATR inhibitors.
In conclusion, treatment with the ATR inhibitor, camonsertib, led to impressive and durable radiological and ctDNA molecular responses in a patient with ATRX mutant/ALT+ heavily pre-treated metastatic melanoma. While the clinical experience of ATR inhibitor therapy in patients with ALT+ tumors remains limited and broad conclusions about the efficacy of ATR inhibitor therapy in ALT+ melanoma cannot be made from this single-patient case report, our report provides the first clinical example of the potential utility of ALT+ as a predictive biomarker of response. Further investigation into ALT+ as a patient selection biomarker is warranted in future ATR inhibitor clinical trials.
Methods
TRESR study of camonsertib in advanced solid tumors
Camonsertib is a highly potent (50% inhibitory concentration, 0.33 nM in cell-based assays), orally bioavailable, and highly selective ATR inhibitor, which is undergoing clinical investigation in several biomarker-selected early phase clinical trials as monotherapy or in combination with other targeted- or chemo-therapies18. Clinical responses to camonsertib have been reported in patients harboring biallelic loss-of-function mutations in genes including ATM, BRCA1/2, RAD51C, SETD2, and CDK127. While key toxicities of clinical grade ATR inhibitors have included a substantial risk of hematological side effects, namely anemia and neutropenia19, the high selectivity of camonsertib for ATR18 and the adoption of intermittent drug dosing schedules have led to camonsertib being associated with acceptable tolerability on recent trials7,20. Doses of camonsertib greater than 100 mg/day were projected as biologically active and determined to be clinically efficacious7. A dose of 160 mg on Days 1–3 for the first 2 weeks of a 3-week cycle was selected as the optimized monotherapy dosing regimen based on a combination of safety, pharmacokinetics, pharmacodynamics, and efficacy data20.
The patient reported herein was enrolled in a monotherapy arm of TRESR (NCT04497116), a first-in-human, Phase 1/2a, nonrandomized study of camonsertib in patients with advanced solid tumors harboring genomic biomarkers predicted to sensitize tumors to ATR inhibitors, including somatic or germline pathogenic alterations in selected genes (ATM, ATRIP, BRCA1/2, CDK12, CHTF8, FZR1, MRE11, NBN, PALB2, RAD51B/C/D, RNASEH2A/B, RAD17, REV3L, RAD50, SETD2). The patient provided written informed consent which allowed for in-depth genomic investigation of tumor and blood specimens, and consented to the publication and presentation of study results. The protocol was approved by the Institutional Review Board at The University of Texas, MD Anderson Cancer Center (Federalwide Assurance Documentation number FWA00000363, trial number 2020-0284) and complied with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Tumor genomic analyses
Available next-generation sequencing (NGS) reports were reviewed at the time of patient enrollment to the TRESR trial for evidence of a genomic alteration to fulfill study eligibility criteria (Tempus xT, Tempus) (Table 1). Subsequently, longitudinally collected, archival, formalin-fixed, paraffin-embedded (FFPE) tumor samples from the patient were retrospectively subjected to detailed genomic analyses (Table 1), including central NGS testing using Synthetic Lethal Interactions for Precision Diagnostics (SNiPDx™), and WGS. SNiPDx™ is a novel, targeted sequencing panel consisting of 26 DNA damage response (DDR) genes, capable of distinguishing monoallelic and biallelic loss-of-function alterations21. WGS was performed as previously described22. Genes were considered to have biallelic loss if one of the following criteria was met: (1) homozygous deletion; (2) compound heterozygous mutation; (3) mutation and loss of heterozygosity; or (4) mutation and non-overlapping heterozygous loss. Genes were considered to have monoallelic loss if the mutation was detected without loss of heterozygosity. VAF, inferred tumor purity and ploidy were also considered in characterizing gene allelic status. Mutational signatures were decomposed using SigProfileExtractor23. HRD classification was determined using CHORD24. Telomere variant repeats and other telomeric features were quantified using TelomereHunter16, and ALT status was classified according to a model trained on known ALT+ samples from Pan-cancer Analysis of Whole Genomes3.
Efficacy and safety outcomes
Radiological tumor response was assessed by the investigator according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). Camonsertib adverse events (AEs), assessed using National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0, were recorded and graded from the start of study treatment until 30 days after the last administration of trial therapy. A comprehensive list of secondary outcomes is provided in the protocol.
Molecular response analysis
Molecular response by ctDNA level was assessed, defined as ≥50% decline in circulating tumor fraction compared with study baseline for at least one timepoint. For ctDNA monitoring, plasma samples were collected from the patient at baseline and at each cycle of treatment. ctDNA analysis was performed using Tempus xF (Tempus), a genomic-variant detection and methylation-based tumor fraction assessment using Guardant Infinity™ (Guardant Health), and TracerDx (Tracer Biotechnologies), a tumor-informed dPCR-based approach using 48 variants selected from the patient WGS profile based on sequence context, sequencing quality metrics, and multiplex compatibility.
Native fluorescence in situ hybridization analysis
The protocol was adapted from Claude et al.,10. The FFPE tissue section was deparaffinized in HistoClear II (Electron Microscopy Sciences, 64111) three times for 3 minutes each and rehydrated in 100%, 95%, 70%, and 30% ethanol for 3 min each. The tissue section was then treated with permeabilization buffer (20 mM Tris-HCl pH 8.0, 50 mM sodium chloride (NaCl), 3 mM magnesium chloride, 300 mM sucrose, 0.5% Triton X-100) for 1 hour at 37 °C, followed by treatment with 500 µg/mL RNase A in RNase A blocking buffer (1 mg/ml bovine serum albumin, 3% normal goat serum, 1 mM EDTA, 0.1% Triton X-100 in phosphate-buffer saline) for 1 hour at 37 °C. Subsequently, the section was serially dehydrated in 70%, 85%, and 100% ethanol for 2 min each and air-dried.
Next, the tissue section was incubated with hybridization solution (100 nM TelG-Alexa488 (PNA Bio, F1008), 50% deionized formamide (Millipore, S4117), 2x Saline-Sodium Citrate (SSC), 1x Blocking Reagent (Roche, 11096176001) at room temperature for 2 h. It was then washed twice for 15 min in 50% formamide, 2x SSC, 20 mM Tris-HCl pH 7.4, and three times for 5 min in 150 mM NaCl, 0.05% Tween-20, 50 mM Tris-HCl pH 7.4. The section was serially dehydrated again, air-dried, and mounted with ProLong Gold Antifade Mountant with DNA Stain DAPI (Invitrogen, P36931).
The confocal microscopy images were acquired at 40X water immersion using the PreciScan function in Opera Phenix Plus (Revvity), and the ssTeloC foci were automatically quantified using Spot Count in Harmony (Revvity, version 5.2). Reporting of ssTeloC foci was restricted to tumor cells as annotated by a board-certified pathologist.
Data availability
Data will only be shared upon reasonable request. For eligible studies, qualified researchers may request access to individual patient-level clinical data by contacting Repare Therapeutics, Inc. Once approved, the data will be shared through a secure data sharing link.
Abbreviations
- ATR:
-
ataxia telangiectasia and Rad3-related
- DDR:
-
DNA damage response
- FFPE:
-
formalin-fixed, paraffin-embedded
- FISH:
-
flourescence in situ hybridization
- HRP:
-
homologous recombination proficient
- PML:
-
promyelocytic leukemia protein
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- RPA:
-
replication protein A
- SNiPDx:
-
synthetic lethal interactions for precision diagnostics
- 2/1w:
-
2 weeks on/1 week off
- 3/4:
-
3 days on/4 days off
- ALT:
-
alternative lengthening of telomeres
- Cam:
-
camonsertib
- ctDNA:
-
circulating tumor DNA
- cTF:
-
circulating tumor fraction
- dPCR:
-
digital polymerase chain reaction
- HRD:
-
homologous recombination deficiency
- LOQ:
-
limit of quantification
- MSI:
-
microsatellite instability
- mVAF:
-
mean variant allele frequency
- PR:
-
partial response
- QD:
-
once daily
- SBS:
-
single base substitution
- ssTeloC:
-
single-stranded C-rich telomeric DNA
- TMB:
-
tumor mutational burden
- TVR:
-
telomere variant repeats
- UV:
-
ultraviolet
- W:
-
Week
- WGS:
-
whole genome sequencing.
References
Feldser, D. M. & Greider, C. W. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell 11, 461–469 (2007).
Sobinoff, A. P. & Pickett, H. A. Alternative lengthening of telomeres: DNA repair pathways converge. Trends Genet. 33, 921–932 (2017).
de Nonneville, A. & Reddel, R. R. Alternative lengthening of telomeres is not synonymous with mutations in ATRX/DAXX. Nat. Commun. 12, 1552 (2021).
Heaphy, C. M. et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science 333, 425 (2011).
Flynn, R. L. et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science 347, 273–277 (2015).
Zimmermann, M. et al. Guiding ATR and PARP inhibitor combinations with chemogenomic screens. Cell Rep. 40, 111081 (2022).
Yap, T. A. et al. Camonsertib in DNA damage response-deficient advanced solid tumors: phase 1 trial results. Nat. Med. 29, 1400–1411 (2023).
Tarsounas, M. & Tijsterman, M. Genomes and G-quadruplexes: for better or for worse. J. Mol. Biol. 425, 4782–4789 (2013).
Lovejoy, C. A. et al. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet 8, e1002772 (2012).
Claude, E. et al. Detection of alternative lengthening of telomeres mechanism on tumor sections. Mol. Biomed. 2, 32 (2021).
Loe, T. K. et al. Telomere length heterogeneity in ALT cells is maintained by PML-dependent localization of the BTR complex to telomeres. Genes Dev. 34, 650–662 (2020).
Henson, J. D. et al. DNA C-circles are specific and quantifiable markers of alternative-lengthening-of-telomeres activity. Nat. Biotechnol. 27, 1181–1185 (2009).
Chen, Y. Y., et al. The C-circle biomarker is secreted by alternative-lengthening-of-telomeres positive cancer cells inside exosomes and provides a blood-based diagnostic for ALT activity. Cancers 13, 5369 (2021).
Henson, J. D. et al. The C-Circle Assay for alternative-lengthening-of-telomeres activity. Methods 114, 74–84 (2017).
Heaphy, C. M. et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am. J. Pathol. 179, 1608–1615 (2011).
Feuerbach, L. et al. TelomereHunter—n silico estimation of telomere content and composition from cancer genomes. BMC Bioinforma. 20, 272 (2019).
Sieverling, L. et al. Genomic footprints of activated telomere maintenance mechanisms in cancer. Nat. Commun. 11, 733 (2020).
Roulston, A. et al. RP-3500: a novel, potent, and selective ATR inhibitor that is effective in preclinical models as a monotherapy and in combination with PARP inhibitors. Mol. Cancer Ther. 21, 245–256 (2022).
Ngoi, N. Y. L. et al. Targeting ATR in patients with cancer. Nat. Rev. Clin. Oncol. 21, 278–293 (2024).
Fontana, E. et al. Ataxia telangiectasia and Rad3-related (ATR) inhibitor camonsertib dose optimization in patients with biomarker-selected advanced solid tumors (TRESR study). J. Natl. Cancer Inst. 116, 1439–1449 (2024).
Glodzik, D. et al. Detection of biallelic loss of DNA repair genes in formalin-fixed, paraffin-embedded tumor samples using a novel tumor-only sequencing panel. J. Mol. Diagn. 25, 295–310 (2023).
Pelster, M. S. et al. Post-therapy emergence of an NBN reversion mutation in a patient with pancreatic acinar cell carcinoma. NPJ Precis Oncol. 8, 82 (2024).
Islam, S. M. A., et al. Uncovering novel mutational signatures by de novo extraction with SigProfilerExtractor. Cell Genom. 2, (2022).
Nguyen, L., J, W. M. M., Van Hoeck, A. & Cuppen, E. Pan-cancer landscape of homologous recombination deficiency. Nat. Commun. 11, 5584 (2020).
Acknowledgements
We thank all the patients participating in TRESR for their selflessness and willingness to participate in clinical research. We thank the entire camonsertib study team for their contributions. We also thank additional treating physicians, Ecaterina E. Dumbrava, M.D., David S. Hong, MD, Shubham Pant, M.D., MBBS, and Sarina A. Piha-Paul, M.D. of MD Anderson for their excellent patient care. We also thank our partner labs, Tempus, Tracer Biotechnologies, and Guardant Health, for their technical assistance and data generation for this study. Medical writing support was provided by Justin L. Eddy, Ph.D. and Swetha Sungomla of Repare Therapeutics, and editorial support was provided by Peter Gray, Ph.D., Bella Cavanna, B.A., and Travis Taylor, B.A., and Rosie Henderson, M.Sc., all of Onyx (a division of Prime, London, UK). This study was funded by Repare Therapeutics. We also thank the Precision Oncology Decision Support Group at The University of Texas MD Anderson Cancer Center for providing genomic decision support through prospective annotation of all genomic alterations on next-generation sequencing testing at study enrollment. Additionally, we acknowledge that the authors from University of Texas MD Anderson Cancer Center were supported by NCI Cancer Center Support Grant CA016672 to The University of Texas MD Anderson Cancer Center. T.A.Y. is supported by Department of Defense grants W81XWH2210504_BC211174, W81XWH-21–1-0282_OC200482, and NIH R01 grant 1R01CA255074, and is supported in part by the NCI Cancer Center Support Grant P30CA016672 and Clinical and Translational Science Award Grant 1UM1 TR0045906 to The University of Texas MD Anderson Cancer Center. This study was funded by Repare Therapeutics.
Author information
Authors and Affiliations
Contributions
I.M.S., J.D.S., M.Z., and D.U. designed the study, which was supervised by V.R., M.K. and T.A.Y., N.Y.L.N., I.M.S., H.K., A.J., C.M., J.D.S., M.Z., D.U., C.T., C.S., and T.A.Y. analyzed the data. N.Y.L.N., C.V.B., C.S., C.T., J.R., and T.A.Y. provided patient care. All authors contributed to writing the report and approved the final version.
Corresponding author
Ethics declarations
Competing interests
Natalie Ngoi has received advisory board honoraria from AstraZeneca, MSD, and Pfizer; and research funding (to Institution) from iOnctura SA. Ian M. Silverman, Adrienne Johnson, Chenfeng Meng, Joseph D. Schonhoft, Michal Zimmermann, Danielle Ulanet, Victoria Rimkunas, Hyeyeon Kim, and Maria Koehler are current or former employees of Repare Therapeutics and may hold stock and/or stock options. Carlos Torrado, Carolina Salguero, and Christian Valladolid Brown have nothing to disclose. Jordi Rodon reports non-financial support and reasonable reimbursement for travel from European Society for Medical Oncology, American Society of Medical Oncology, National Taiwan University Cancer Center, 280-Biotech, Dava Oncology, STOP Cancer; receiving consulting and travel fees from Ellipses Pharma, IONCTURA, Sardona, Mekanistic, Amgen, Merus, MonteRosa, Aadi, and Bridgebio (including serving on the scientific advisory board); Consulting fees from Vall d’Hebron Institute of Oncology, Chinese University of Hong Kong, Boxer Capital, LLC, Tang Advisors LLC, Guidepoint, and Axiom; research funding from Blueprint Medicines, Merck Sharp & Dohme, Hummingbird, AstraZeneca, 280 Bio, Vall d’Hebron Institute of Oncology/Cancer Core Europe; and serving as investigator in clinical trials with Cancer Core Europe, Symphogen, BioAlta, Pfizer, Kelun-Biotech, GlaxoSmithKline, Taiho, Roche Pharmaceuticals, Hummingbird, Yingli, Bicycle Therapeutics, Merus, AadiBioscience, ForeBio, Loxo Oncology, Hutchinson MediPharma, Ideaya, Amgen, Tango Therapeutics, Mirati, Linnaeus Therapeutics, MonteRosa, Kinnate, Debio, BioTheryX, Storm Therapeutics, Beigene, MapKure, Relay, Novartis, FusionPharma, C4 Therapeutics, Scorpion Therapeutics, Incyte, Fog Pharmaceuticals, Tyra, Nuvectis Pharma, Hotspot Pharma, Adcentrix, Vividion, AstraZeneca, Alnylam, Immuneering Corp, Alterome, and Exelixis. Timothy A. Yap is an employee of The University of Texas MD Anderson Cancer Center, where he is Vice President, Head of Clinical Development in the Therapeutics Discovery Division, which has a commercial interest in DDR and other inhibitors (IACS30380/ART0380 was licensed to Artios); has received funding paid to their institution from Acrivon, Artios, AstraZeneca, Bayer, BeiGene, BioNTech, Blueprint, Bristol Myers Squibb, Boundless Bio, Clovis, Constellation, Cyteir, Eli Lilly, EMD Serono, Forbius, F-Star, GlaxoSmithKline, Genentech, Haihe, Ideaya ImmuneSensor, Insilico Medicine, Ionis, Ipsen, Jounce, Karyopharm, KSQ, Kyowa, Merck, Mirati, Novartis, Pfizer, Ribon Therapeutics, Regeneron, Repare, Rubius, Sanofi, Scholar Rock, Seattle Genetics, Tango, Tesaro, Vivace, and Zenith; has received consultancy funding from AbbVie, Acrivon, Adagene, Almac, Aduro, Amphista, Artios, Astex, AstraZeneca, Athena, Atrin, Avenzo, Avoro, Axiom, Baptist Health Systems, Bayer, BeiGene, BioCity Pharma, Blueprint, Boxer, Bristol Myers Squibb, C4 Therapeutics, Calithera, Cancer Research UK, Carrick Therapeutics, Circle Pharma, Clovis, Cybrexa, Daiichi Sankyo, Dark Blue Therapeutics, Diffusion, Duke Street Bio, 858 Therapeutics, EcoR1 Capital, Ellipses Pharma, EMD Serono, Entos, F-Star, Genesis Therapeutics, Genmab, Glenmark, GLG, Globe Life Sciences, GSK, Guidepoint, Ideaya Biosciences, Idience, Ignyta, I-Mab, ImmuneSensor, Impact Therapeutics, Institut Gustave Roussy, Intellisphere, Jansen, Kyn, MEI pharma, Mereo, Merck, Merit, Monte Rosa Therapeutics, Natera, Nested Therapeutics, Nexys, Nimbus, Novocure, Odyssey, OHSU, OncoSec, Ono Pharma, Onxeo, PanAngium Therapeutics, Pegascy, PER, Pfizer, Piper-Sandler, Pliant Therapeutics, Prolynx, Radiopharma Theranostics, Repare, resTORbio, Roche, Ryvu Therapeutics, SAKK, Sanofi, Schrodinger, Servier, Synnovation, Synthis Therapeutics, Tango, TCG Crossover, TD2, Terremoto Biosciences, Tessellate Bio, Theragnostics, Terns Pharmaceuticals, Tolremo, Tome, Thryv Therapeutics, Trevarx Biomedical, Varian, Veeva, Versant, Vibliome, Voronoi Inc, Xinthera, Zai Labs, and ZielBio; and is a stockholder in Seagen. He was supported by the NCI Cancer Center Support Grant CA016672 to The University of Texas MD Anderson Cancer Center, Department of Defense grants W81XWH2210504_BC211174 and W81XWH-21-1-0282_OC200482, V Foundation Scholar Grant VC2020-001, and NIH R01 grant 1R01CA255074.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ngoi, N.Y.L., Silverman, I.M., Johnson, A. et al. Exceptional response to the ATR inhibitor, camonsertib, in a patient with ALT+ metastatic melanoma. npj Precis. Onc. 9, 227 (2025). https://doi.org/10.1038/s41698-025-01025-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-01025-1
This article is cited by
-
Functional analysis of telomere maintenance mechanisms is more informative than immunohistochemistry for ATRX mutation interpretation in Gliomas
Acta Neuropathologica Communications (2025)