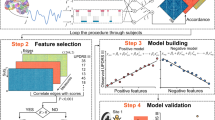
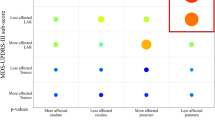
Abstract
Parkinson’s disease (PD) is increasingly recognized as a brain network-disconnection syndrome. However, there is little consistent evidence on multimodal global topological alterations and their diagnostic value. We systematically searched PubMed, Embase and Web of Science up to March 2025 for articles reporting brain network topology in PD, to which we applied a multilevel random-effects meta-analyses with robust variance estimation to account for statistical dependencies. Our case-control meta-analysis included 80 studies (42 fMRI, 25 dMRI, 10 EEG, 4 sMRI, 3 others) involving 3736 PD patients and 2384 healthy controls. Compared to controls, PD patients showed lower structural and functional network segregation, especially when cognitively impaired. Structural network integration was also lower in PD, such deficits appearing to correlate with disease progression. Drug and network construction strategies were identified as potential moderating factors. Our diagnostic meta-analysis of 10 studies yielded a pooled diagnostic odds ratio of 16.4 and a pooled area under the curve of 0.86, with better diagnostic performance observed in studies using combined network metrics. These results support the clinical relevance of topological metrics in PD as potential biomarkers for disease characterization, prognosis and patient stratification, and underscore the importance of methodological harmonization and prospective validation in future research.
Similar content being viewed by others
Data availability
All the data included in this study are available within the paper and its supplementary information files. The codes used in this paper are available on GitHub: https://github.com/chao9791/PD-brain-network-topological-properties-alterations.
References
Tanner, C. M. & Ostrem, J. L. Parkinson’s disease. N. Engl. J. Med. 391, 442–452 (2024).
Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet 397, 2284–2303 (2021).
Zarkali, A., Thomas, G. E. C., Zetterberg, H. & Weil, R. S. Neuroimaging and fluid biomarkers in Parkinson’s disease in an era of targeted interventions. Nat. Commun. 15, 5661 (2024).
Lui, S., Zhou, X. J., Sweeney, J. A. & Gong, Q. Psychoradiology: the frontier of neuroimaging in psychiatry. Radiology 281, 357–372 (2016).
Pan, G. Q. et al. Identification of Parkinson's disease subtypes with distinct brain atrophy progression and its association with clinical progression. Psychoradiology. 4, kkae002 (2024).
Bullmore, E. & Sporns, O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198 (2009).
Shamir, I. & Assaf, Y. Tutorial: a guide to diffusion MRI and structural connectomics. Nat. Protoc. 20, 317–335 (2025).
Sebenius, I. et al. Structural MRI of brain similarity networks. Nat. Rev. Neurosci. 26, 42–59 (2025).
van den Heuvel, M. P. & Sporns, O. A cross-disorder connectome landscape of brain dysconnectivity. Nat. Rev. Neurosci. 20, 435–446 (2019).
Suo, X. S. et al. Psychoradiological patterns of small-world properties and a systematic review of connectome studies of patients with 6 major psychiatric disorders. J. Psychiatry Neurosci. 43, 427 (2018).
Farahani, F. V., Karwowski, W. & Lighthall, N. R. Application of graph theory for identifying connectivity patterns in human brain networks: a systematic review. Front Neurosci. 13, 585 (2019).
Cronin-Golomb, A. Parkinson’s disease as a disconnection syndrome. Neuropsychol. Rev. 20, 191–208 (2010).
Filippi, M. et al. Longitudinal brain connectivity changes and clinical evolution in Parkinson’s disease. Mol. Psychiatry 26, 5429–5440 (2021).
Chung, S. J. et al. Association between white matter connectivity and early dementia in patients with Parkinson disease. Neurology 98, e1846–e1856 (2022).
Dan, X. J. et al. Reorganization of intrinsic functional connectivity in early-stage Parkinson’s disease patients with probable REM sleep behavior disorder. npj Parkinsons Dis. 10, 5 (2024).
Zuo, C. et al. Global alterations of whole brain structural connectome in Parkinson’s disease: a meta-analysis. Neuropsychol. Rev. 33, 783–802 (2023).
Cheung, M. W. A guide to conducting a meta-analysis with non-independent effect sizes. Neuropsychol. Rev. 29, 387–396 (2019).
Fotiadis, P. et al. Structure-function coupling in macroscale human brain networks. Nat. Rev. Neurosci. 25, 688–704 (2024).
Xiao, P. et al. Combined brain topological metrics with machine learning to distinguish essential tremor and tremor-dominant Parkinson’s disease. Neurol. Sci. 45, 4323–4334 (2024).
Devignes, Q. et al. Resting-state functional connectivity in frontostriatal and posterior cortical subtypes in Parkinson’s disease-mild cognitive impairment. Mov. Disord. 37, 502–512 (2022).
Suo, X. et al. Brain functional network abnormalities in parkinson’s disease with mild cognitive impairment. Cereb. Cortex 32, 4857–4868 (2022).
Abbasi, N. et al. Predicting severity and prognosis in Parkinson’s disease from brain microstructure and connectivity. Neuroimage Clin. 25, 102111 (2020).
De Micco, R. et al. Functional connectomics and disease progression in drug-naïve Parkinson’s disease patients. Mov. Disord. 36, 1603–1616 (2021).
Du, J. et al. Levodopa responsiveness and white matter alterations in Parkinson’s disease: a DTI-based study and brain network analysis: a cross-sectional study. Brain Behav. 12, e2825 (2022).
Wang, L. et al. Altered brain structural topological properties in Parkinson’s disease with levodopa-induced dyskinesias. Parkinsonism Relat. Disord. 67, 36–41 (2019).
Albano, L. et al. Functional connectivity in Parkinson’s disease candidates for deep brain stimulation. npj Parkinsons Dis. 8, 4 (2022).
Liu, W. et al. The whole-brain structural and functional connectome in Alzheimer’s disease spectrum: a multimodal Bayesian meta-analysis of graph theoretical characteristics. Neurosci. Biobehav Rev. 174, 106174 (2025).
Slinger, G., Otte, W. M., Braun, K. P. J. & van Diessen, E. An updated systematic review and meta-analysis of brain network organization in focal epilepsy: looking back and forth. Neurosci. Biobehav Rev. 132, 211–223 (2022).
Gao, Z. et al. The whole-brain connectome landscape in patients with schizophrenia: a systematic review and meta-analysis of graph theoretical characteristics. Neurosci. Biobehav Rev. 148, 105144 (2023).
Biswal, B. B. & Uddin, L. Q. The history and future of resting-state functional magnetic resonance imaging. Nature 641, 1121–1131 (2025).
Park, H. J. & Friston, K. Structural and functional brain networks: from connections to cognition. Science 342, 1238411 (2013).
Suárez, L. E., Markello, R. D., Betzel, R. F. & Misic, B. Linking structure and function in macroscale brain networks. Trends Cogn. Sci. 24, 302–315 (2020).
Yuan, J. et al. The structural basis for interhemispheric functional connectivity: evidence from individuals with agenesis of the corpus callosum. Neuroimage Clin. 28, 102425 (2020).
Uddin, L. Q. et al. Residual functional connectivity in the split-brain revealed with resting-state functional MRI. Neuroreport 19, 703–709 (2008).
Shine, J. M. et al. Dopamine depletion alters macroscopic network dynamics in Parkinson’s disease. Brain 142, 1024–1034 (2019).
Simuni, T. et al. A biological definition of neuronal α-synuclein disease: towards an integrated staging system for research. Lancet Neurol. 23, 178–190 (2024).
Huang, L. C. et al. Effects of levodopa on motor and cerebellar network connectivity in Parkinson’s disease. Neurol Sci. 46, 6563-6573 (2025).
Ben-Shlomo, Y. et al. The epidemiology of Parkinson’s disease. Lancet 403, 283–292 (2024).
Caminiti, S. P. et al. Male sex accelerates cognitive decline in GBA1 Parkinson’s disease. npj Parkinsons Dis. 11, 41 (2025).
Iwaki, H. et al. Differences in the presentation and progression of Parkinson’s disease by sex. Mov. Disord. 36, 106–117 (2021).
Sarasso, E. et al. MRI biomarkers of freezing of gait development in Parkinson’s disease. npj Parkinsons Dis. 8, 158 (2022).
Vachha, B. & Huang, S. Y. MRI with ultrahigh field strength and high-performance gradients: challenges and opportunities for clinical neuroimaging at 7 T and beyond. Eur. Radio. Exp. 5, 35 (2021).
Barisano, G. et al. Clinical 7 T MRI: are we there yet? A review about magnetic resonance imaging at ultra-high field. Br. J. Radio. 92, 20180492 (2019).
Arslan, S. et al. Human brain mapping: a systematic comparison of parcellation methods for the human cerebral cortex. Neuroimage 170, 5–30 (2018).
Eickhoff, S. B., Yeo, B. T. T. & Genon, S. Imaging-based parcellations of the human brain. Nat. Rev. Neurosci. 19, 672–686 (2018).
Vijiaratnam, N., Simuni, T., Bandmann, O., Morris, H. R. & Foltynie, T. Progress towards therapies for disease modification in Parkinson’s disease. Lancet Neurol. 20, 559–572 (2021).
Stocchi, F., Bravi, D., Emmi, A. & Antonini, A. Parkinson disease therapy: current strategies and future research priorities. Nat. Rev. Neurol. 20, 695–707 (2024).
Bočková, M. et al. Cortical network organization reflects clinical response to subthalamic nucleus deep brain stimulation in Parkinson’s disease. Hum. Brain Mapp. 42, 5626–5635 (2021).
Berman, B. D. et al. Levodopa modulates small-world architecture of functional brain networks in Parkinson’s disease. Mov. Disord. 31, 1676–1684 (2016).
Borchert, R. J. et al. Atomoxetine and citalopram alter brain network organization in Parkinson’s disease. Brain Commun. 1, fcz013 (2019).
van Balkom, T. D., van den Heuvel, O. A., Berendse, H. W., van der Werf, Y. D. & Vriend, C. Eight-week multi-domain cognitive training does not impact large-scale resting-state brain networks in Parkinson’s disease. Neuroimage Clin. 33, 102952 (2022).
Rajpurkar, P. & Lungren, M. P. The current and future state of AI interpretation of medical images. N. Engl. J. Med. 388, 1981–1990 (2023).
Wang, J. et al. Diagnostic performance of artificial intelligence-assisted PET imaging for Parkinson’s disease: a systematic review and meta-analysis. NPJ Digit Med 7, 17 (2024).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71 (2021).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069 (2010).
Goetz, C. G., Stebbins, G. T. & Tilley, B. C. Calibration of unified Parkinson’s disease rating scale scores to movement disorder society-unified Parkinson’s disease rating scale scores. Mov. Disord. 27, 1239–1242 (2012).
Hentz, J. G. et al. Simplified conversion method for unified Parkinson’s disease rating scale motor examinations. Mov. Disord. 30, 1967–1970 (2015).
Drevon, D., Fursa, S. R. & Malcolm, A. L. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav. Modif. 41, 323–339 (2017).
Wan, X., Wang, W., Liu, J. & Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135 (2014).
Higgins, J. et al. Cochrane Handbook for Systematic Reviews of Interventions. (Cochrane, 2024).
Engels, G. et al. Clinical pain and functional network topology in Parkinson’s disease: a resting-state fMRI study. J. Neural Transm. 125, 1449–1459 (2018).
Guan, X. et al. Iron-related nigral degeneration influences functional topology mediated by striatal dysfunction in Parkinson’s disease. Neurobiol. Aging 75, 83–97 (2019).
Olde Dubbelink, K. T. et al. Disrupted brain network topology in Parkinson’s disease: a longitudinal magnetoencephalography study. Brain 137, 197–207 (2014).
Van den Noortgate, W., López-López, J. A., Marín-Martínez, F. & Sánchez-Meca, J. Three-level meta-analysis of dependent effect sizes. Behav. Res. Methods 45, 576–594 (2013).
Cheung, M. W. Modeling dependent effect sizes with three-level meta-analyses: a structural equation modeling approach. Psychol. Methods 19, 211–229 (2014).
Griffin, J. W. et al. Investigating the face inversion effect in autism across behavioral and neural measures of face processing: a systematic review and bayesian meta-analysis. JAMA Psychiatry 80, 1026–1036 (2023).
Borenstein, M., Hedges, L. V., Higgins, J. P. & Rothstein, H. R. Introduction to meta-analysis. (Wiley, 2021).
Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48 (2010).
Fernandez-Castilla, B. et al. Detecting selection bias in meta-analyses with multiple outcomes: a simulation study. J. Exp. Educ. 89, 125–144 (2021).
Viechtbauer, W. & Cheung, M. W. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 1, 112–125 (2010).
Xu, H. L. et al. Artificial intelligence performance in image-based ovarian cancer identification: a systematic review and meta-analysis. EClinicalMedicine 53, 101662 (2022).
Sounderajah, V. et al. A quality assessment tool for artificial intelligence-centered diagnostic test accuracy studies: QUADAS-AI. Nat. Med. 27, 1663–1665 (2021).
Deeks, J. J., Bossuyt, P. M., Leeflang, M. M. & Takwoingi, Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. (Cochrane, 2023).
Acknowledgements
This research was supported by the National Key R&D Program of China (No. 2022YFC2009904), National Natural Science Foundation of China (Grant Nos. 82001800), Young Elite Scientists Sponsorship Program by China Association for Science and Technology (CAST) (No. 2022QNRC001), and Sichuan Science and Technology Program (No. 2025ZNSFSC0661). The authors would like to express their sincere gratitude to Dr Angeliki Zarkali, Dr Kathy Dujardin, Dr Chuanxi Tang, Dr Muthuraman Muthuraman, and Dr Madhura Ingalhalikar for generously providing data and/or additional information essential to the completion of this study.
Author information
Authors and Affiliations
Contributions
X.L.S. and Q.Y.G. designed the study. C.Z., W.X.L., X.L.S., and H.L. contributed to the literature search, data collection and interpretation. C.Z. contributed to statistical analysis of case-control meta-analysis. W.X.L. contributed to statistical analysis of diagnostic meta-analysis. C.Z. and W.X.L. drafted the manuscript. L.C., N.L., Y.Y., L.L., C.L., G.J.K., S.L., X.L.S., and Q.Y.G. critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zuo, C., Liu, W., Lan, H. et al. Multimodal brain network topology and enhanced computer-aided diagnosis in Parkinson’s Disease: a systematic review and meta-analysis. npj Digit. Med. (2026). https://doi.org/10.1038/s41746-025-02301-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-025-02301-x