Abstract
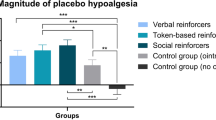
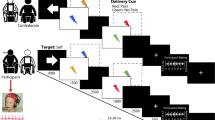
Digital environments are increasingly used to study social and pain-related behaviors. Empathy and contextual factors influence observationally induced placebo analgesia. We tested whether state empathy (i.e., immediate affective and cognitive responses to another’s experience) differs when observing a demonstrator in immersive VR versus 2D video, and whether this modulation affects placebo hypoalgesia. Forty-seven participants observed a human or avatar demonstrator receiving painful stimulation with or without placebo, then experienced the same stimulations. Observation induced significant placebo hypoalgesia for pain intensity and unpleasantness. Human demonstrators evoked greater cognitive empathy, while placebo treatments reduced empathy across contexts. Analgesic effects were stronger in 2D after observing humans, but in VR, avatars induced greater placebo effects. Placebo responsiveness was related to trait empathy in the VR-Human condition; however, state empathy did not mediate the effect. Our findings highlight that demonstrator characteristics and immersion critically shape the social transfer of placebo effects.
Similar content being viewed by others
Data availability
All behavioral data and the VR video footage we created for this study are available upon request to the corresponding author.
References
Shen, L. On a scale of state empathy during message processing. West. J. Commun. 74, 504–524 (2010).
Li, A., Montaño, Z., Chen, V. J. & Gold, J. I. Virtual reality and pain management: current trends and future directions. Pain. Manag. 1, 147–157 (2011).
Colloca, L. & Benedetti, F. Placebo analgesia induced by social observational learning. PAIN 144, 28–34 (2009).
Schenk, L. A., Krimmel, S. R. & Colloca, L. Observe to get pain relief: current evidence and potential mechanisms of socially learned pain modulation. Pain 158, 2077–2081 (2017).
Goubert, L., Vlaeyen, J. W., Crombez, G. & Craig, K. D. Learning about pain from others: an observational learning account. J. Pain. 12, 167–174 (2011).
Raghuraman, N. et al. Neural and behavioral changes driven by observationally-induced hypoalgesia. Sci. Rep. 9, 19760 (2019).
Fusaro, M., Tieri, G. & Aglioti, S. M. Seeing pain and pleasure on self and others: behavioral and psychophysiological reactivity in immersive virtual reality. J. Neurophysiol. 116, 2656–2662 (2016).
Waber, R. L., Shiv, B., Carmon, Z. & Ariely, D. Commercial features of placebo and therapeutic efficacy. JAMA 299, 1016–1017 (2008).
Kam-Hansen, S. et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci. Transl. Med. 6, 218ra215 (2014).
Faasse, K., Martin, L. R., Grey, A., Gamble, G. & Petrie, K. J. Impact of brand or generic labeling on medication effectiveness and side effects. Health Psychol. 35, 187–190 (2016).
Blackwell, B., Bloomfield, S. S. & Buncher, C. R. Demonstration to medical students of placebo responses and non-drug factors. Lancet 1, 1279–1282 (1972).
de Craen, A. J., Kaptchuk, T. J., Tijssen, J. G. & Kleijnen, J. Placebos and placebo effects in medicine: historical overview. J. R. Soc. Med. 92, 511–515 (1999).
Meissner, K. et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern. Med. 173, 1941–1951 (2013).
de Craen, A. J., Tijssen, J. G., de Gans, J. & Kleijnen, J. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J. Neurol. 247, 183–188 (2000).
Colloca, L., Lopiano, L., Lanotte, M. & Benedetti, F. Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol. 3, 679–684 (2004).
Koban, L., Jepma, M., Geuter, S. & Wager, T. D. What’s in a word? How instructions, suggestions, and social information change pain and emotion. Neurosci. Biobehav Rev. 81, 29–42 (2017).
Hunter, T., Siess, F. & Colloca, L. Socially induced placebo analgesia: a comparison of a pre-recorded versus live face-to-face observation. Eur. J. Pain. 18, 914–922 (2014).
Egorova, N. et al. Not seeing or feeling is still believing: conscious and non-conscious pain modulation after direct and observational learning. Sci. Rep. 5, 16809 (2015).
Vogtle, E., Barke, A. & Kroner-Herwig, B. Nocebo hyperalgesia induced by social observational learning. Pain 154, 1427–1433 (2013).
Swider, K. & Babel, P. The effect of the sex of a model on nocebo hyperalgesia induced by social observational learning. Pain 154, 1312–1317 (2013).
Swider, K. & Babel, P. The effect of the type and colour of placebo stimuli on placebo effects induced by observational learning. PLoS ONE 11, e0158363 (2016).
Meeuwis, S. H. et al. Learning pain from others: a systematic review and meta-analysis of studies on placebo hypoalgesia and nocebo hyperalgesia induced by observational learning. Pain 164, 2383–2396 (2023).
Betti, V., Zappasodi, F., Rossini, P. M., Aglioti, S. M. & Tecchio, F. Synchronous with your feelings: sensorimotor {gamma} band and empathy for pain. J. Neurosci. 29, 12384–12392 (2009).
Lee, J.-H., Lee, S. E. & Kwon, Y.-S. Exploring empathic engagement in immersive media: an EEG study on mu rhythm suppression in VR. PloS ONE 19, e0303553 (2024).
Van Loon, A., Bailenson, J., Zaki, J., Bostick, J. & Willer, R. Virtual reality perspective-taking increases cognitive empathy for specific others. PloS ONE 13, e0202442 (2018).
Cloninger, C. R. The dynamics of social learning. Science 213, 858–859 (1981).
Rotter, J. B. Some implications of a social learning theory for the prediction of goal directed behavior from testing procedures. Psychol. Rev. 67, 301–316 (1960).
Olsson, A., Nearing, K. I. & Phelps, E. A. Learning fears by observing others: the neural systems of social fear transmission. Soc. Cognit. Affect Neurosci. 2, 3–11 (2007).
Olsson, A. & Phelps, E. A. Social learning of fear. Nat. Neurosci. 10, 1095–1102 (2007).
Colloca, L. & Miller, F. G. How placebo responses are formed: a learning perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 1859–1869 (2011).
Gupta, A., Scott, K. & Dukewich, M. Innovative technology using virtual reality in the treatment of pain: does it reduce pain via distraction, or is there more to it? Pain. Med. 19, 151–159 (2018).
Hoffman, H. G., Doctor, J. N., Patterson, D. R., Carrougher, G. J. & Furness, T. A. 3rd. Virtual reality as an adjunctive pain control during burn wound care in adolescent patients. Pain 85, 305–309 (2000).
Hoffman, H. G., Patterson, D. R., Carrougher, G. J. & Sharar, S. R. Effectiveness of virtual reality-based pain control with multiple treatments. Clin. J. Pain. 17, 229–235 (2001).
Keefe, F. J. et al. Virtual reality for persistent pain: a new direction for behavioral pain management. Pain 153, 2163–2166 (2012).
Morris, L. D., Louw, Q. A. & Grimmer-Somers, K. The effectiveness of virtual reality on reducing pain and anxiety in burn injury patients: a systematic review. Clin. J. Pain. 25, 815–826 (2009).
Perry, B. N., Mercier, C., Pettifer, S. R., Cole, J. & Tsao, J. W. Virtual reality therapies for phantom limb pain. Eur. J. Pain. 18, 897–899 (2014).
Won, A. S. et al. Immersive Virtual Reality for Pediatric Pain. Children 4, https://doi.org/10.3390/children4070052 (2017).
Preis, M. A. & Kroener-Herwig, B. Empathy for pain: the effects of prior experience and sex. Eur. J. Pain. 16, 1311–1319 (2012).
Mohr, C., Rowe, A. C. & Blanke, O. The influence of sex and empathy on putting oneself in the shoes of others. Br. J. Psychol. 101, 277–291 (2010).
Oswald, P. A. Subtle sex bias in empathy and helping behavior. Psychol. Rep. 87, 545–551 (2000).
Hoffman, M. L. Sex differences in empathy and related behaviors. Psychol. Bull. 84, 712–722 (1977).
Hoffmann, F., Koehne, S., Steinbeis, N., Dziobek, I. & Singer, T. Preserved Self-other distinction during empathy in autism is linked to network integrity of right supramarginal gyrus. J. Autism Dev. Disord. 46, 637–648 (2016).
Gilpin, H. R., Bellan, V., Gallace, A. & Moseley, G. L. Exploring the roles of body ownership, vision and virtual reality on heat pain threshold. Eur. J. Pain. 18, 900–901 (2014).
Hoffman, H. G. et al. Manipulating presence influences the magnitude of virtual reality analgesia. Pain 111, 162–168 (2004).
Martingano, A. J., Hererra, F. & Konrath, S. Virtual reality improves emotional but not cognitive empathy: a meta-analysis. Technol. Mind Behav. 2, 7–21 (2021).
Hapuarachchi, H. et al. Empathic embarrassment towards non-human agents in virtual environments. Sci. Rep. 13, 1–12 (2023).
Kegel, L. C. et al. Dynamic human and avatar facial expressions elicit differential brain responses. Soc. Cognit. Affect Neurosci. 15, 303–317 (2020).
Hoffman, H. G. et al. Virtual reality as an adjunctive non-pharmacologic analgesic for acute burn pain during medical procedures. Ann. Behav. Med. 41, 183–191 (2011).
Leibovici, V., Magora, F., Cohen, S. & Ingber, A. Effects of virtual reality immersion and audiovisual distraction techniques for patients with pruritus. Pain. Res. Manag 14, 283–286 (2009).
Wiederhold, B. K., Gao, K., Sulea, C. & Wiederhold, M. D. Virtual reality as a distraction technique in chronic pain patients. Cyberpsychol. Behav. Soc. Netw. 17, 346 (2014).
Allen, J. G. Mentalizing. Bull. Menninger Clin. 67, 91–112 (2003).
Kampe, K. K., Frith, C. D. & Frith, U. Hey John”: signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. J. Neurosci. 23, 5258–5263 (2003).
Frith, C. D. & Frith, U. The neural basis of mentalizing. Neuron 50, 531–534 (2006).
Longmire, N. H. & Harrison, D. A. Seeing their side versus feeling their pain: Differential consequences of perspective-taking and empathy at work. J. Appl. Psychol. 103, 894 (2018).
Lockwood, P. L., Apps, M. A., Roiser, J. P. & Viding, E. Encoding of vicarious reward prediction in anterior cingulate cortex and relationship with trait empathy. J. Neurosci. 35, 13720–13727 (2015).
Lair, C. V. Empathy and its relation to stimulus meaning. J. Clin. Psychol. 14, 175–177 (1958).
Raghuraman, N. et al. Neuropsychological mechanisms of observational learning in human placebo effects. Psychopharmacology) 242, 889–900 (2025). .
Bajcar, E. A. & Babel, P. The role of observational learning in the formation of placebo and nocebo effects. Handb. Clin. Neurol. 213, 59–69 (2025).
Schenk, L. A. & Colloca, L. The neural processes of acquiring placebo effects through observation. Neuroimage 209, 116510 (2020).
Meeuwis, S. H. et al. I do not feel your pain: exploring the impact of state empathy on placebo and nocebo effects evoked by observational learning. J. Pain 35, 105526 (2025).
Bieniek, H. & Bąbel, P. The effect of the model’s social status on placebo analgesia induced by social observational learning. Pain. Med. 23, 81–88 (2022).
Braczyk, J. & Babel, P. The role of the observers’ perception of a model’s self-confidence in observationally induced placebo analgesia. J. Pain. 22, 1672–1680 (2021).
Rutgen, M. et al. Beyond sharing unpleasant affect-evidence for pain-specific opioidergic modulation of empathy for pain. Cereb. Cortex 31, 2773–2786 (2021).
Okusogu, C. et al. Placebo hypoalgesia: racial differences. Pain 161, 1872–1883 (2020).
Adhanom, I. B., MacNeilage, P. & Folmer, E. Eye tracking in virtual reality: a broad review of applications and challenges. Virtual Real. 27, 1481–1505 (2023).
Clay, V., König, P. & König, S. Eye tracking in virtual reality. J. Eye Mov. Res. 12, 1–18 (2019).
Lee, Y., Shin, H. & Gil, Y.-H. Measurement of empathy in virtual reality with head-mounted displays: a systematic review. IEEE Trans. Vis. Comput. Graph. 30, 2485–2495 (2024).
Roberts, K. et al. Contact heat evoked potentials using simultaneous EEG and fMRI and their correlation with evoked pain. BMC Anesthesiol. 8, 1–12 (2008).
Persky, S. & Colloca, L. Medical extended reality trials: building robust comparators, controls, and sham. J. Med. Internet Res. 25, e45821 (2023).
Davis, M. A. A multidimensional approach to individual differences in empathy. JSAS Cat Sel. Docs Psychol. 10,85 (1980).
Beck, A. T., Epstein, N., Brown, G. & Steer, R. A. An inventory for measuring clinical anxiety: psychometric properties. J. Consulting Clin. Psychol. 56, 893 (1988).
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J. & Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571 (1961).
Harris, P. A. et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inf. 95, 103208 (2019).
Harris, P. A. et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 42, 377–381 (2009).
Hayes, A. F. & Rockwood, N. J. Conditional process analysis: concepts, computation, and advances in the modeling of the contingencies of mechanisms. Am. Behav. Sci. 64, 19–54 (2020).
Hu, X. et al. Using multilevel mediation model to measure the contribution of beliefs to judgments of learning. Front. Psychol. 11, 637 (2020).
Acknowledgements
The authors acknowledge the support of the National Institute of Dental and Craniofacial Research Helping to End Addiction Long-term (NIDCR HEAL) Initiative (1R21 DE032532-01, Dorsey/Colloca; J. White), the University of Maryland Baltimore’s Institute for Translational and Clinical Research (5TL1TR003100-05, L. Watson) and the National Center for Complementary and Integrative Health (R01AT010333 and R01AT011347, L. Colloca). The funding source was not involved in this work. The authors would like to thank Nandini Raghuraman for helping with the procedure setup and Yavin Shaham for feedback on the manuscript.
Author information
Authors and Affiliations
Contributions
J.N.W. and L.C. conceived and designed the study. J.N.W., L.W., R.S., J.M.H., S.L., and B.B. contributed to data collection and preprocessing. J.N.W. and L.W. performed initial data analysis. A.V. provided expertise and oversight on virtual reality design and implementation. L.C. supervised the study and secured funding. G.C., Y.W., and C.-É.B.-R conducted intendent analyses of the data collected and helped interpret the findings. J.N.W., L.W., and C.-É.B.-R drafted the manuscript. L.C. and C.-É.B.-R. reviewed and approved the final version of the manuscript. All authors contributed to manuscript revisions and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
White, J.N., Watson, L., Wang, Y. et al. Context-dependent placebo hypoalgesia through observational learning: the role of empathy in immersive and non-immersive environments. npj Digit. Med. (2026). https://doi.org/10.1038/s41746-026-02373-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-026-02373-3