Abstract
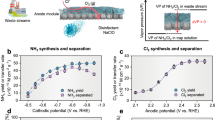
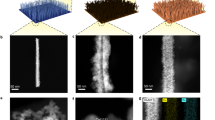
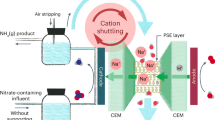
Electrocatalytic nitrate reduction has great potential for simultaneously achieving ammonia recovery and nitrate-rich wastewater treatment. However, the complexity of wastewater matrices has long hampered its implementation and commercialization in the wastewater treatment industry. Here we develop a membrane-free electrochemical system, called electrochemical NO3− conversion synchronized with NH3 recovery (ECSN), which synchronizes nitrate reduction with ammonia recovery for treating real nitrate-rich wastewater. Key components of this system include a 3D-printed metallic glass decorated Cu–Ni (MPCN) working electrode bearing good corrosion resistance and a UV-assisted stripping unit. When treating real electroplating wastewater, the ECSN system converts over 70% of nitrate into high-purity ammonia chloride. Long-term stability test demonstrates the robustness of the ECSN system in treating real wastewater. Further, the economic feasibility and environmental benefits of this system are evidenced by technoeconomic analysis and life-cycle analysis. Overall, this work brings the electrocatalytic nitrate reduction process one step closer to practical application, contributing to both environmental protection and the circularity of anthropogenic nitrogen flow.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated for this study are available in the paper and the Supplementary Information. Source experimental data are available via figshare (https://doi.org/10.6084/m9.figshare.26134027)50.
References
Xu, H., Ma, Y., Chen, J., Zhang, W.-X. & Yang, J. Electrocatalytic reduction of nitrate—a step towards a sustainable nitrogen cycle. Chem. Soc. Rev. 51, 2710–2758 (2022).
Chen, G.-F. et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 5, 605–613 (2020).
Chen, J. G. et al. Beyond fossil fuel-driven nitrogen transformations. Science 360, eaar6611 (2018).
Lim, J., Fernández, C. A., Lee, S. W. & Hatzell, M. C. Ammonia and nitric acid demands for fertilizer use in 2050. ACS Energy Lett. 6, 3676–3685 (2021).
Coates, J. D. et al. Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature 411, 1039–1043 (2001).
Li, R. et al. Large virtual transboundary hazardous waste flows: the case of China. Environ. Sci. Technol. 57, 8161–8173 (2023).
Gu, B., Ge, Y., Chang, S. X., Luo, W. & Chang, J. Nitrate in groundwater of China: sources and driving forces. Glob. Environ. Change 23, 1112–1121 (2013).
Guo, Y., Stroka, J. R., Kandemir, B., Dickerson, C. E. & Bren, K. L. A cobalt metallopeptide electrocatalyst for the selective reduction of nitrite to ammonium. J. Am. Chem. Soc. 140, 16888–16892 (2018).
Chen, P. et al. Interfacial engineering of cobalt sulfide/graphene hybrids for highly efficient ammonia electrosynthesis. Proc. Natl Acad. Sci. USA 116, 6635–6640 (2019).
Wang, Y., Wang, C., Li, M., Yu, Y. & Zhang, B. Nitrate electroreduction: mechanism insight, in situ characterization, performance evaluation, and challenges. Chem. Soc. Rev. 50, 6720–6733 (2021).
Sun, Y., Garg, S., Zhang, C., Xie, J. & Waite, T. D. Approaches to enhancing cathodic nickel recovery from Ni-EDTA containing synthetic wastewaters. ACS ES T Water 3, 2415–2426 (2023).
Park, D. et al. Metal recovery from electroplating wastewater using acidophilic iron oxidizing bacteria: pilot-scale feasibility test. Ind. Eng. Chem. Res. 44, 1854–1859 (2005).
Casademont, C., Pourcelly, G. & Bazinet, L. Bilayered self-oriented membrane fouling and impact of magnesium on CaCO3 formation during consecutive electrodialysis treatments. Langmuir 26, 854–859 (2010).
Yan, H., Xu, C., Li, W., Wang, Y. & Xu, T. Electrodialysis to concentrate waste ionic liquids: optimization of operating parameters. Ind. Eng. Chem. Res. 55, 2144–2152 (2016).
Liu, R.-T. et al. Recent advances in proton exchange membrane water electrolysis. Chem. Soc. Rev. 52, 5652–5683 (2023).
Wang, Y. et al. Enhanced nitrate-to-ammonia activity on copper–nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 142, 5702–5708 (2020).
Chen, F.-Y. et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst. Nat. Nanotechnol. 17, 759–767 (2022).
Wang, X. et al. Pivotal role of reversible NiO6 geometric conversion in oxygen evolution. Nature 611, 702–708 (2022).
Cioncoloni, G. et al. Proton-coupled electron transfer enhances the electrocatalytic reduction of nitrite to NO in a bioinspired copper complex. ACS Catal. 8, 5070–5084 (2018).
Sen, F. G., Alpas, A. T., van Duin, A. C. T. & Qi, Y. Oxidation-assisted ductility of aluminium nanowires. Nat. Commun. 5, 3959 (2014).
Wu, Z.-Z. et al. Identification of Cu(100)/Cu(111) interfaces as superior active sites for CO dimerization during CO2 electroreduction. J. Am. Chem. Soc. 144, 259–269 (2022).
Leow, W. R. et al. Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science 368, 1228–1233 (2020).
Daiyan, R. et al. Nitrate reduction to ammonium: from CuO defect engineering to waste NOx-to-NH3 economic feasibility. Energy Environ. Sci. 14, 3588–3598 (2021).
Liu, B. et al. Metal 3D nanoprinting with coupled fields. Nat. Commun. 14, 4920 (2023).
Chattot, R. et al. Surface distortion as a unifying concept and descriptor in oxygen reduction reaction electrocatalysis. Nat. Mater. 17, 827–833 (2018).
Panwisawas, C., Tang, Y. T. & Reed, R. C. Metal 3D printing as a disruptive technology for superalloys. Nat. Commun. 11, 2327 (2020).
Ge, J. et al. Joule-heated graphene-wrapped sponge enables fast clean-up of viscous crude-oil spill. Nat. Nanotechnol. 12, 434–440 (2017).
Okulov, I. V. et al. Flash joule heating for ductilization of metallic glasses. Nat. Commun. 6, 7932 (2015).
Qiang, G. et al. Synthesis of core/shell nanocrystals with ordered intermetallic single-atom alloy layers for nitrate electroreduction to ammonia. Nat. Synthesis 2, 624–634 (2023).
Xu, B., Chen, Z., Zhang, G. & Wang, Y. On-demand atomic hydrogen provision by exposing electron-rich cobalt sites in an open-framework structure toward superior electrocatalytic nitrate conversion to dinitrogen. Environ. Sci. Technol. 56, 614–623 (2022).
Fang, J.-Y. et al. Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature. Nat. Commun. 13, 7899 (2022).
Ataka, K.-i, Yotsuyanagi, T. & Osawa, M. Potential-dependent reorientation of water molecules at an electrode/electrolyte interface studied by surface-enhanced infrared absorption spectroscopy. J. Phys. Chem. 100, 10664–10672 (1996).
Bu, Y. et al. Electrical pulse-driven periodic self-repair of Cu–Ni tandem catalyst for efficient ammonia synthesis from nitrate. Angew. Chem. Int. Ed. 62, e202217337 (2023).
Deng, Y., Handoko, A. D., Du, Y., Xi, S. & Yeo, B. S. In situ Raman spectroscopy of copper and copper oxide surfaces during electrochemical oxygen evolution reaction: identification of CuIII oxides as catalytically active species. ACS Catal. 6, 2473–2481 (2016).
Faid, A. Y., Barnett, A. O., Seland, F. & Sunde, S. Ni/NiO nanosheets for alkaline hydrogen evolution reaction: in situ electrochemical–Raman study. Electrochim. Acta 361, 137040 (2020).
Kang, X. et al. A corrosion-resistant RuMoNi catalyst for efficient and long-lasting seawater oxidation and anion exchange membrane electrolyzer. Nat. Commun. 14, 3607 (2023).
Peng, J. et al. Surface coordination layer passivates oxidation of copper. Nature 586, 390–394 (2020).
Chuang, Y.-H. et al. Critical role of trichloramine interaction with dichloramine for N-nitrosamine formation during breakpoint chlorination. Environ. Sci. Technol. 57, 15232–15242 (2023).
Pang, R., Miseki, Y., Okunaka, S. & Sayama, K. Photocatalytic production of hypochlorous acid over Pt/WO3 under simulated solar light. ACS Sustain. Chem. Eng. 8, 8629–8637 (2020).
Cross, J. H. Reevaluation of the employment of Fick’s law for diffusion dosimeters. Environ. Sci. Technol. 37, 1633–1638 (2003).
Peng, W., Xiao, L., Huang, B., Zhuang, L. & Lu, J. Inhibition effect of surface oxygenated species on ammonia oxidation reaction. J. Phys. Chem. C 115, 23050–23056 (2011).
Kumar, A., Phillips, K. R., Thiel, G. P., Schröder, U. & Lienhard, J. H. Direct electrosynthesis of sodium hydroxide and hydrochloric acid from brine streams. Nat. Catal. 2, 106–113 (2019).
Zhang, C. et al. Capillary effect-enabled water electrolysis for enhanced electrochemical ozone production by using bulk porous electrode. J. Am. Chem. Soc. 139, 16620–16629 (2017).
Baker, D. V., Bao, C. & Kim, W. S. Highly conductive 3D printable materials for 3D structural electronics. ACS Appl. Electron. Mater. 3, 2423–2433 (2021).
Yu, Z. J., Carpenter, J. V. & Holman, Z. C. Techno-economic viability of silicon-based tandem photovoltaic modules in the United States. Nat. Energy 3, 747–753 (2018).
Wang, Y., Levis, J. W. & Barlaz, M. A. Life-cycle assessment of a regulatory compliant US municipal solid waste landfill. Environ. Sci. Technol. 55, 13583–13592 (2021).
Zhang, Y. et al. Designing magnesium phosphate cement for stabilization/solidification of Zn-rich electroplating sludge. Environ. Sci. Technol. 56, 9398–9407 (2022).
Yuan, W. et al. Understanding of adopting flat-top laser in laser powder bed fusion processed Inconel 718 alloy: simulation of single-track scanning and experiment. J. Mater. Res. Technol. 16, 1388–1401 (2022).
Yuan, W., Chen, H., Cheng, T. & Wei, Q. Effects of laser scanning speeds on different states of the molten pool during selective laser melting: simulation and experiment. Mater. Des. 189, 108542 (2020).
Zhang, G. et al. Source data for ‘Ammonia recovery from nitrate-rich wastewater using a membrane-free electrochemical system’. figshare https://doi.org/10.6084/m9.figshare.26134027 (2024).
Acknowledgements
We acknowledge the National Natural Science Foundation of China (grant no. 52221004 to H.L., 22022606 to G. Zhang). We thank B. Xu and Y. Wang from Tongji University for their helpful suggestions with in situ DEMS measurement, L. Liu from Nanjing University for assistance in DFT calculation, and B. Zhang from Beijing University of Science and Technology for help in the 3D printing process. We thank F. Xu from Suzhou Suwater Environmental Science and Technology Co., Ltd for the real electroplating wastewater treatment.
Author information
Authors and Affiliations
Contributions
H.L. directed the project. G. Zhang designed the experiments. G. Zhang, J.M. and B.L. performed materials synthesis, beach-scale degradation experiments and electrochemical experiments. B.L., Q.J. and Y.S. analysed the results of FTIR and DEMS experiments. S.Y. and W.Y. performed the physics-based modelling approach for the 3D printing process. S.M. and B.L. performed the resourceful treatment on a real electroplating wastewater. G. Zhou and W.-J.F. carried out and analysed the DFT calculations. Q.Z., G. Zhang, B.L. and J.M. performed LCA analysis. G. Zhang, B.L., Y.S., W.-J.F., G. Zhou, Q.J. and J.Q. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Huiyuan Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–47, Tables 1–13 and Discussion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, G., Li, B., Shi, Y. et al. Ammonia recovery from nitrate-rich wastewater using a membrane-free electrochemical system. Nat Sustain 7, 1251–1263 (2024). https://doi.org/10.1038/s41893-024-01406-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41893-024-01406-7
This article is cited by
-
Revealing and modulating catalyst reconstruction for highly efficient electrosynthesis of ammonia
Nature Communications (2025)
-
Controlling solvation in conducting redox polymers for selective electrochemical separation of nitrate from wastewater
Nature Communications (2025)
-
Catalytic resource recovery for transformation of the wastewater industry
Nature Water (2025)
-
Unconventional phase metal heteronanostructures with tunable exposed interface for efficient tandem nitrate electroreduction to ammonia
Nature Communications (2025)
-
Copper–palladium hydride interfaces promote electrochemical ammonia synthesis
Nature Synthesis (2025)