Abstract
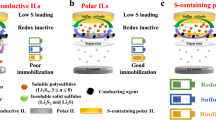
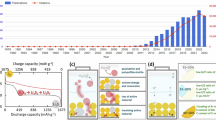
Among the emerging ‘beyond lithium-ion’ technologies for maximized sustainability, lithium–sulfur (Li–S) is a favoured chemistry because of its exceptional energy density from the conversion of sulfur, an element in abundant supply. However, the dissolution of several intermediate polysulfides formed during conversion leads to rapid performance degradation over cycling. Here we address this issue by sulfurizing a hybrid polymer network with polyphosphazene and carbon as a cathode for Li–S batteries. With rich sites to re-bond and adsorb dissociative sulfur species, this hybrid polymer network circumvents the formation of soluble polysulfides and enables a unique, reversible inserting conversion reaction. Thus, our cathode delivers both high capacity (~900 mAh g−1cathode) and excellent cycling stability in Li–S coin cells, with a pouch cell demonstration of projected energy density of ~300 Wh kg−1 and 84.9% capacity retention after 150 cycles. The strategy can be extended to other cost-effective, recyclable polymers, advancing sulfur-based batteries towards practical energy storage application.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information.
References
Bauer, C. et al. Charging sustainable batteries. Nat. Sustain. 5, 176–178 (2022).
Zhou, G., Chen, H. & Cui, Y. Formulating energy density for designing practical lithium–sulfur batteries. Nat. Energy 7, 312–319 (2022).
Manthiram, A., Fu, Y., Chung, S.-H., Zu, C. & Su, Y.-S. Rechargeable lithium-sulfur batteries. Chem. Rev. 114, 11751–11787 (2014).
Zhang, S. S. Sulfurized carbon: a class of cathode materials for high performance lithium/sulfur batteries. Front. Energy Res. 1, 1–9 (2013).
Chung, W. J. et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 5, 518–524 (2013).
Chen, Y. et al. Advances in lithium-sulfur batteries: from academic research to commercial viability. Adv. Mater. 33, 2003666 (2021).
Ko, S. et al. Electrolyte design for lithium-ion batteries with a cobalt-free cathode and silicon oxide anode. Nat. Sustain. 6, 1705–1714 (2023).
Wang, J. et al. Sustainable upcycling of spent LiCoO2 to an ultra-stable battery cathode at high voltage. Nat. Sustain. 6, 797–805 (2023).
Zhou, S. et al. Visualizing interfacial collective reaction behaviour of Li-S batteries. Nature 621, 75–81 (2023).
Liu, R. et al. Establishing reaction networks in the 16-electron sulfur reduction reaction. Nature 626, 98–104 (2024).
Zhao, C. et al. A high-energy and long-cycling lithium-sulfur pouch cell via a macroporous catalytic cathode with double-end binding sites. Nat. Nanotechnol. 16, 166–173 (2021).
Pang, Q., Liang, X., Kwok, C. Y. & Nazar, L. F. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 1, 16132 (2016).
Ji, X., Lee, K. T. & Nazar, L. F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 8, 500–506 (2009).
Zhou, L., Danilov, D. L., Eichel, R. A. & Notten, P. H. L. Host materials anchoring polysulfides in Li–S batteries reviewed. Adv. Energy Mater. 11, 2001304 (2021).
Pan, H. et al. Non-encapsulation approach for high-performance Li–S batteries through controlled nucleation and growth. Nat. Energy 2, 813–820 (2017).
Yin, L., Wang, J., Lin, F., Yang, J. & Nuli, Y. Polyacrylonitrile/graphene composite as a precursor to a sulfur-based cathode material for high-rate rechargeable Li-S batteries. Energy Environ. Sci. 5, 6966–6972 (2012).
Li, Z., Zhang, J., Lu, Y. & Lou, X. W. D. A pyrolyzed polyacrylonitrile/selenium disulfide composite cathode with remarkable lithium and sodium storage performances. Sci. Adv. 4, eaat1687 (2018).
Tan, S. et al. Structural and interphasial stabilities of sulfurized polyacrylonitrile (SPAN) cathode. ACS Energy Lett. 8, 2496–2504 (2023).
Wang, S. et al. Structural transformation in a sulfurized polymer cathode to enable long-life rechargeable lithium-sulfur batteries. J. Am. Chem. Soc. 145, 9624–9633 (2023).
Yang, H., Chen, J., Yang, J. & Wang, J. Prospect of sulfurized pyrolyzed poly(acrylonitrile) (S@pPAN) cathode materials for rechargeable lithium batteries. Angew. Chem. Int. Ed. Engl. 59, 7306–7318 (2020).
Song, J. et al. Strong lithium polysulfide chemisorption on electroactive sites of nitrogen-doped carbon composites for high-performance lithium-sulfur battery cathodes. Angew. Chem. Int. Ed. Engl. 54, 4325–4329 (2015).
Song, J. et al. Nitrogen-doped mesoporous carbon promoted chemical adsorption of sulfur and fabrication of high-areal-capacity sulfur cathode with exceptional cycling stability for lithium-sulfur batteries. Adv. Funct. Mater. 24, 1243–1250 (2014).
Yan, J. et al. Enhanced Na+ pseudocapacitance in a P, S co-doped carbon anode arising from the surface modification by sulfur and phosphorus with C–S–P coupling. J. Mater. Chem. A 8, 422–432 (2020).
Wu, Z. et al. Understanding the roles of the electrode/electrolyte interface for enabling stable Li∥sulfurized polyacrylonitrile batteries. ACS Appl. Mater. Interfaces 13, 31733–31740 (2021).
Li, X. et al. A high-energy sulfur cathode in carbonate electrolyte by eliminating polysulfides via solid-phase lithium-sulfur transformation. Nat. Commun. 9, 4509 (2018).
Chen, X. et al. Ether-compatible sulfurized polyacrylonitrile cathode with excellent performance enabled by fast kinetics via selenium doping. Nat. Commun. 10, 1021 (2019).
Li, Z. et al. Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium–sulfur batteries. Nat. Energy 8, 84–93 (2023).
Persson, I., Klysubun, W. & Lundberg, D. A K-edge P XANES study of phosphorus compounds in solution. J. Mol. Struct. 1179, 608–611 (2019).
Luo, C. et al. A chemically stabilized sulfur cathode for lean electrolyte lithium sulfur batteries. Proc. Natl Acad. Sci. USA 117, 14712–14720 (2020).
Li, Z., Chen, C., McCaffrey, M., Yang, H. & Allcock, H. R. Polyphosphazene elastomers with alkoxy and trifluoroethoxy side groups. ACS Appl. Polym. Mater. 2, 475–480 (2020).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy (DOE), through an Advanced Battery Materials Research (BMR) Program award no. DE-EE0009650. C.W. thanks the support of Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US DOE under the Advanced BMR Program and the United States-Germany Cooperation on Energy Storage under contract no. DE-LC-000L072. The in situ TEM was conducted in the William R. Wiley Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the US DOE’s Office of Biological and Environmental Research and located at the Pacific Northwest National Laboratory. The Pacific Northwest National Laboratory is operated by Battelle for the US DOE under contract no. DE-AC05-76RL01830. The work at Brookhaven National Laboratory is supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Vehicle Technology Office of the US DOE through the Advanced BMR Program under contract no. DE-SC0012704. This research used the 8-BM and 28-ID-2 beamlines of the National Synchrotron Light Source II, US DOE Office of Science User Facilities, operated for the DOE’s Office of Science by the Brookhaven National Laboratory under contract no. DE-SC0012704. We thank the computing resources provided by the Laboratory Computing Resource Center at Argonne National Laboratory for performing the density functional theory calculations.
Author information
Authors and Affiliations
Contributions
Donghai Wang conceived and designed the research project. M.L. performed the experiments on sulfur cathode materials and Li–S batteries. Y.X. and C.W. performed the in situ TEM measurements and analysed the data. M.M.R., S.T. and E.H. conducted the synchrotron-based sXAS and PDF characterizations and analysed the data. Daiwei Wang, L.L., R.K. and L.Y. performed the electrochemical measurements of Li–S batteries. K.W. collected the HR-TEM and electron energy loss spectroscopy characterization data. N.K.D. and A.T.N. conducted the molecular simulations. Q.L. and A.N. collected the XPS characterization data. G.L., H.J. and P.S. performed the experiments on the pouch cells. All authors discussed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Hao Chen, Gui-Liang Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16, Table 1, Captions for Videos 1–8 and References.
Supplementary Video 1
Beam control experiments of S-HYB materials (high-magnification, shown at a speed 20 times the real-time process; scale bar, 10 nm).
Supplementary Video 2
Beam control experiments of S-HOM materials (high-magnification, shown at a speed 20 times the real-time process; scale bar, 10 nm).
Supplementary Video 3
First in situ lithiation of S-HYB particles (high-magnification, shown at a speed 20 times the real-time process; scale bar, 10 nm).
Supplementary Video 4
First in situ delithiation of S-HYB particles (high-magnification, shown at a speed 20 times the real-time process; scale bar, 10 nm).
Supplementary Video 5
Second in situ lithiation of S-HYB particles (high-magnification, shown at a speed 20 times the real-time process; scale bar, 10 nm).
Supplementary Video 6
First in situ lithiation of S-HYB particles (low-magnification, shown at a speed 64 times the real-time process; scale bar, 200 nm).
Supplementary Video 7
First in situ delithiation of S-HYB particles (low-magnification, shown at a speed 32 times the real-time process; scale bar, 200 nm).
Supplementary Video 8
Second in situ lithiation of S-HYB particles (low-magnification, shown at a speed 32 times the real-time process; scale bar, 200 nm).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, M., Xu, Y., Rahman, M.M. et al. Hybrid polymer network cathode-enabled soluble-polysulfide-free lithium–sulfur batteries. Nat Sustain 7, 1709–1718 (2024). https://doi.org/10.1038/s41893-024-01453-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41893-024-01453-0