Abstract
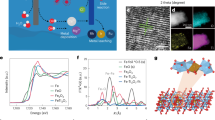
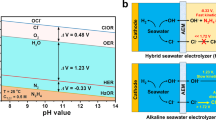
Active chlorine, including HClO and ClO−, is one of the most extensively used disinfectants. However, it is mainly produced through energy-consuming three-step chlor-alkali electrolysis of saturated brine using Cl2 gases as intermediates. Here we report a photoelectrochemical synthetic pathway from natural seawater using a chloride-mediated NbClOx/BiVO4 photoanode. The photoanode presents an onset potential of 0.6 V versus a reversible hydrogen electrode (VRHE) and over 500 h of stability in seawater under one sun illumination. The faradaic efficiency and selectivity of hypochlorite are close to 100% at 1.2–1.8 VRHE with a yield of 119.9 ± 9 μmol cm−2 h−1 at 1.72 VRHE. Meanwhile, value-added products of Mg(OH)2 and CaCO3 are obtained on the cathode, accompanied by hydrogen production. Further analyses show that the present process reduces electricity consumption by 77.16% and CO2 emissions by 75.31%. Our findings suggest a strategy with combined safety, efficiency and economic feasibility for direct synthesis of active chlorine from seawater.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Chung, M. et al. Direct propylene epoxidation via water activation over Pd–Pt electrocatalysts. Science 383, 49–55 (2024).
Fan, R. et al. Ultrastable electrocatalytic seawater splitting at ampere-level current density. Nat. Sustain. 7, 158–167 (2024).
Choi, S. et al. A reflection on sustainable anode materials for electrochemical chloride oxidation. Adv. Mater. 35, 2300429 (2023).
Zhao, S. et al. Selective electrosynthesis of chlorine disinfectants from seawater. Nat. Sustain. 7, 148–157 (2024).
Yang, J. et al. CO2-mediated organocatalytic chlorine evolution under industrial conditions. Nature 617, 519–523 (2023).
Zeradjanin, A. R. The era of stable electrocatalysis. Nat. Catal. 6, 458–459 (2023).
Janssen, L. J. J., Starmans, L. M. C., Visser, J. G. & Barendrecht, E. Mechanism of the chlorine evolution on a ruthenium oxide/titanium oxide electrode and on a ruthenium electrode. Electrochim. Acta 22, 1093–1100 (1977).
Faita, G. & Fiori, G. Anodic discharge of chloride ions on oxide electrodes. J. Appl. Electrochem. 2, 31–35 (1972).
Ha, H. et al. Highly selective active chlorine generation electrocatalyzed by Co3O4 nanoparticles: mechanistic investigation through in situ electrokinetic and spectroscopic analyses. J. Phys. Chem. Lett. 10, 1226–1233 (2019).
Karlsson, R. K. B. & Cornel, A. Selectivity between oxygen and chlorine evolution in the chlor-alkali and chlorate processes. Chem. Rev. 116, 2982–3028 (2016).
Exner, K. S. Design criteria for the competing chlorine and oxygen evolution reactions: avoid the OCl adsorbate to enhance chlorine selectivity. Phys. Chem. Chem. Phys. 22, 22451–22458 (2020).
Vos, J. G., Venugopal, A., Smith, W. A. & Koper, M. T. M. Competition and selectivity during parallel evolution of bromine, chlorine and oxygen on IrOx electrodes. J. Catal. 389, 99–110 (2020).
Lim, T. et al. Atomically dispersed Pt–N4 sites as efficient and selective electrocatalysts for the chlorine evolution reaction. Nat. Commun. 11, 412 (2020).
Liu, H. et al. High-performance alkaline seawater electrolysis with anomalous chloride promoted oxygen evolution reaction. Angew. Chem. Int. Ed. 62, e202311674 (2023).
Yao, Y. et al. Single atom Ru monolithic electrode for efficient chlorine evolution and nitrate reduction. Angew. Chem. Int. Ed. 61, e202208215 (2022).
Moreno-Hernandez, I. A., Brunschwig, B. S. & Lewis, N. S. Crystalline nickel, cobalt, and manganese antimonates as electrocatalysts for the chlorine evolution reaction. Energy Environ. Sci. 12, 1241–1248 (2019).
Liu, J. et al. Rationally designing efficient electrocatalysts for direct seawater splitting: challenges, achievements, and promises. Angew. Chem. Int. Ed. 61, e202210753 (2022).
Tong, W. et al. Electrolysis of low-grade and saline surface water. Nat. Energy 5, 367–377 (2020).
Miao, J. et al. Supramolecular catalyst with [FeCl4] unit boosting photoelectrochemical seawater splitting via water nucleophilic attack pathway. Nat. Commun. 15, 2023 (2024).
Zhao, Y. et al. A hydrogen farm strategy for scalable solar hydrogen production with particulate photocatalysts. Angew. Chem. Int. Ed. 59, 9653–9658 (2020).
Qi, Y. et al. Efficient overall water splitting of a suspended photocatalyst boosted by metal-support interaction. Joule 8, 193–203 (2024).
Kuang, Y. et al. Ultrastable low-bias water splitting photoanodes via photocorrosion inhibition and in situ catalyst regeneration. Nat. Energy 2, 16191 (2016).
Ye, S. et al. Mimicking the key functions of photosystem II in artificial photosynthesis for photoelectrocatalytic water splitting. J. Am. Chem. Soc. 140, 3250–3256 (2018).
Rahaman, M. et al. Solar-driven liquid multi-carbon fuel production using a standalone perovskite–BiVO4 artificial leaf. Nat. Energy 8, 629–638 (2023).
Qi, Y. et al. Unraveling of cocatalysts photodeposited selectively on facets of BiVO4 to boost solar water splitting. Nat. Commun. 13, 484 (2022).
Kim, T. W. & Choi, K.-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 343, 990–994 (2014).
Lee, D. K. & Choi, K.-S. Enhancing long-term photostability of BiVO4 photoanodes for solar water splitting by tuning electrolyte composition. Nat. Energy 3, 53–60 (2018).
Gao, R.-T. et al. Dynamic semiconductor–electrolyte interface for sustainable solar water splitting over 600 hours under neutral conditions. Sci. Adv. 9, eade4589 (2023).
Haverkamp, R. G., Kappen, P., Sizeland, K. H. & Wallwork, K. S. Niobium K-edge X-ray absorption spectroscopy of doped TiO2 produced from ilmenite digested in hydrochloric acid. ACS Omega 7, 28258–28264 (2022).
Kumar, A. et al. Probing the electronic and local structure of Sr2−xLaxCoNbO6 using near-edge and extended x-ray absorption fine structures. Phys. Rev. B 105, 245155 (2022).
Markina, D. I. et al. Perovskite nanowire laser for hydrogen chloride gas sensing. ACS Nano 17, 1570–1582 (2023).
Liang, J. et al. Electroreduction of alkaline/natural seawater: self-cleaning Pt/carbon cathode and on-site co-synthesis of H2 and Mg hydroxide nanoflakes. Chem 10, 3067–3087 (2024).
Pardo-Jaramillo, S., Munoz-Villamizar, A. & Gomez-Gonzalez, J. E. Unveiling the influence of COVID-19 on the online retail market: a comprehensive exploration. J. Retail. Consum. Serv. 75, 103538 (2023).
Zhu, W. et al. Energy-efficient electrosynthesis of high value-added active chlorine coupled with H2 generation from direct seawater electrolysis through decoupling electrolytes. Angew. Chem. Int. Ed. 63, e202319798 (2024).
Mukherjee, I., Ghosh, A., Bhadury, P. & De, P. Matrix assisted antibacterial activity of polymer conjugates with pendant antibiotics, and bioactive and biopassive moieties. J. Mater. Chem. B 7, 3007–3018 (2019).
Behler, J. & Parrinello, M. Generalized neural-network representation of high-dimensional potential-energy surfaces. Phys. Rev. Lett. 98, 146401 (2007).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Yu, J. & Kudo, A. Effects of structural variation on the photocatalytic performance of hydrothermally synthesized BiVO4. Adv. Funct. Mater. 16, 2163–2169 (2006).
Guo, J. et al. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 8, 264–272 (2023).
Hao, Y. et al. Switching the oxygen evolution mechanism on atomically dispersed Ru for enhanced acidic reaction kinetics. J. Am. Chem. Soc. 145, 23659–23669 (2023).
Acknowledgements
The work was supported by the National Science and Technology Major Project (grant no. 2022YFA1205200), the National Natural Science Foundation of China (grant nos 22269016, 22479083 and 22405138) and the Group Project of Developing Inner Mongolia through Talents from the Talents Work Leading Group under the CPC Inner Mongolia Autonomous Regional Committee (grant no. 2025TYL03). N.T.N. acknowledges NSERC, FRQNT and QCAM for financial support. We thank Y. Li at the University of Electronic Science and Technology of China for the discussion of this work.
Author information
Authors and Affiliations
Contributions
L. Wu, R.-T.G. and L. Wang conceived the idea, designed the experiments and supervised the research project. R.-T.G. and Z.G. conducted the experiments and characterizations under discussion with N.T.N., X.L. and L. Wang. R.-T.G. analysed the data and wrote the paper. J.C. performed the modelling and simulation. L. Wu and L. Wang revised the paper. All the authors contributed to the experiments and discussion of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Hao Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–67, Notes 1–13, Tables 1–21 and Methods.
Source data
Source Data Figs. 1–5
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, RT., Gao, Z., Nguyen, N.T. et al. Photoelectrochemical production of disinfectants from seawater. Nat Sustain 8, 672–681 (2025). https://doi.org/10.1038/s41893-025-01530-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41893-025-01530-y
This article is cited by
-
Characterization of CQDs/ZnIn2S4 composite photocatalyst and photocatalytic performance for hydrogen production
Journal of Materials Science (2026)
-
Hybrid island-and-sea approach for corrosion protection of Si photocathode in neutral-pH water splitting
Scientific Reports (2025)
-
Turning sun and seawater into disinfectant
Nature Sustainability (2025)
-
Sustainable chlorine cycle enabled by single atom catalysis
Nature Communications (2025)
-
Direct ammonia and dihydroxyacetone production in an unbiased photoelectrochemical cell
Nature Communications (2025)