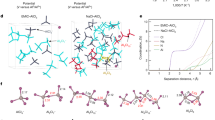
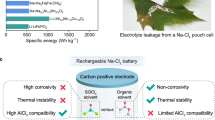
Abstract
The transition to clean energy necessitates the development of alternative or complementary battery chemistries to lithium-ion batteries. Aluminium (Al) metal batteries (AMBs) are a promising option owing to their high energy density and advantages in terms of abundance, recyclability, manufacturability and sustainability. However, state-of-the-art AMBs rely primarily on electrolytes featuring chloroaluminate anions such as Al2Cl7−. Unfortunately, such electrolytes create a disparity between achievable energy density and potential, in addition to having high corrosivity, high cost, high viscosity and poor transport kinetics. Here we break this paradigm by formulating an organochloro electrolyte with AlCl3 salt in dipropyl ether (DPE) solvent. Notably, this AlCl3/DPE electrolyte shows no corrosion behaviour towards stainless steel current collectors during 90-day soaking. It enables stable cycling of the Al anode for over 2,000 h at 0.2 mA cm−2 and a high Coulombic efficiency of 99.85%, and prototype Al//Mo6S8 full cells survive 300 cycles. Underlying these unprecedented performances is the unique organochloro solvation structure, AlCl2(DPE)2+, which is desolvatable and immobilizes free Cl−. By extending the horizon of electrolyte design into the organic domain, this work addresses the notorious issues facing AMBs and paves the way for the implementation of multivalent rechargeable batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the Article and Supplementary Information. Source data are provided with this paper.
References
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Tu, J. et al. Nonaqueous rechargeable aluminum batteries: progresses, challenges, and perspectives. Chem. Rev. 121, 4903–4961 (2021).
Ng, K. L., Amrithraj, B. & Azimi, G. Nonaqueous rechargeable aluminum batteries. Joule 6, 134–170 (2022).
Liu, W. et al. Pursuing high voltage and long lifespan for low-cost Al-based rechargeable batteries: dual-ion design and prospects. Energy Storage Mater. 62, 102922 (2023).
Long, Y. et al. Suppressing Al dendrite growth towards a long-life Al-metal battery. Energy Storage Mater. 34, 194–202 (2021).
Jiang, M. et al. Challenges and strategies of low-cost aluminum anodes for high-performance Al-based batteries. Adv. Mater. 34, e2102026 (2022).
Yang, H. et al. The rechargeable aluminum battery: opportunities and challenges. Angew. Chem. Int. Ed. 58, 11978–11996 (2019).
Meng, Y. S., Srinivasan, V. & Xu, K. Designing better electrolytes. Science 378, eabq3750 (2022).
Liang, Y., Dong, H., Aurbach, D. & Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 5, 646–656 (2020).
Del Duca, B. S. Electrochemical behavior of the aluminum electrode in molten salt electrolytes. J. Electrochem. Soc. 118, 405 (1971).
Holleck, G. L. & Giner, J. The aluminum electrode in AlCl3–alkali–halide melts. J. Electrochem. Soc. 119, 1161 (1972).
Pastel, G. R. et al. A sobering examination of the feasibility of aqueous aluminum batteries. Energy Environ. Sci. 15, 2460–2469 (2022).
Zhang, Y., Bian, Y., Lv, Z., Han, Y. & Lin, M.-C. Aqueous aluminum cells: mechanisms of aluminum anode reactions and role of the artificial solid electrolyte interphase. ACS Appl. Mater. Interfaces 13, 37091–37101 (2021).
Jayaprakash, N., Das, S. K. & Archer, L. A. The rechargeable aluminum-ion battery. Chem. Commun. 47, 12610–12612 (2011).
Lin, M.-C. et al. An ultrafast rechargeable aluminium-ion battery. Nature 520, 324–328 (2015).
Sun, H. et al. A new aluminium-ion battery with high voltage, high safety and low cost. Chem. Commun. 51, 11892–11895 (2015).
Elia, G. A. et al. An overview and prospective on Al and Al-ion battery technologies. J. Power Sources 481, 228870 (2021).
Faegh, E., Ng, B., Hayman, D. & Mustain, W. E. Practical assessment of the performance of aluminium battery technologies. Nat. Energy 6, 21–29 (2021).
Angell, M. et al. High coulombic efficiency aluminum-ion battery using an AlCl3-urea ionic liquid analog electrolyte. Proc. Natl Acad. Sci. USA 114, 834–839 (2017).
Chu, W. et al. A low-cost deep eutectic solvent electrolyte for rechargeable aluminum–sulfur battery. Energy Storage Mater. 22, 418–423 (2019).
Abood, H. M. A., Abbott, A. P., Ballantyne, A. D. & Ryder, K. S. Do all ionic liquids need organic cations? Characterisation of [AlCl2·nAmide]+AlCl4− and comparison with imidazolium based systems. Chem. Commun. 47, 3523–3525 (2011).
Winter, M., Barnett, B. & Xu, K. Before Li ion batteries. Chem. Rev. 118, 11433–11456 (2018).
Gálová, M. Electrodeposition of aluminium from organic aprotic solvents. Surf. Technol. 11, 357–369 (1980).
Legrand, L., Tranchant, A. & Messina, R. Aluminium behaviour and stability in AlCl3DMSO2 electrolyte. Electrochim. Acta 41, 2715–2720 (1996).
Jiang, T., Chollier Brym, M. J., Dubé, G., Lasia, A. & Brisard, G. M. Studies on the AlCl3/dimethylsulfone (DMSO2) electrolytes for the aluminum deposition processes. Surf. Coat. Technol. 201, 6309–6317 (2007).
Kitada, A., Nakamura, K., Fukami, K. & Murase, K. Electrochemically active species in aluminum electrodeposition baths of AlCl3/glyme solutions. Electrochim. Acta 211, 561–567 (2016).
Chiku, M., Matsumura, S., Takeda, H., Higuchi, E. & Inoue, H. Aluminum bis(trifluoromethanesulfonyl)imide as a chloride-free electrolyte for rechargeable aluminum batteries. J. Electrochem. Soc. 164, A1841–A1844 (2017).
Kumar, S. et al. Additive-driven interfacial engineering of aluminum metal anode for ultralong cycling life. Nanomicro Lett. 15, 21 (2022).
Yoo, H. D. et al. Fast kinetics of magnesium monochloride cations in interlayer-expanded titanium disulfide for magnesium rechargeable batteries. Nat. Commun. 8, 339 (2017).
Xu, Z.-L. et al. A new high-voltage calcium intercalation host for ultra-stable and high-power calcium rechargeable batteries. Nat. Commun. 12, 3369 (2021).
Chen, K. et al. Correlating the solvating power of solvents with the strength of ion-dipole interaction in electrolytes of lithium-ion batteries. Angew. Chem. Int. Ed. 62, e202312373 (2023).
Wu, Y. et al. Electrostatic potential as solvent descriptor to enable rational electrolyte design for lithium batteries. Adv. Energy Mater. 13, 2300259 (2023).
Yang, H. et al. An aluminum–sulfur battery with a fast kinetic response. Angew. Chem. Int. Ed. 57, 1898–1902 (2018).
Pang, Q. et al. Fast-charging aluminium–chalcogen batteries resistant to dendritic shorting. Nature 608, 704–711 (2022).
Akdeniz, Z., Pastore, G. & Tosi, M. P. An ionic model for molecular units in molten aluminium trichloride and alkali chloroaluminates. Phys. Chem. Liq. 32, 191–209 (1996).
Haouas, M., Taulelle, F. & Martineau, C. Recent advances in application of 27Al NMR spectroscopy to materials science. Prog. Nucl. Magn. Reson. Spectrosc. 94–95, 11–36 (2016).
von Cresce, A. & Xu, K. Preferential solvation of Li+ directs formation of interphase on graphitic anode. Electrochem. Solid-State Lett. 14, A154 (2011).
Abdul-Sada, A.aK., Greenway, A. M., Seddon, K. R. & Welton, T. A fast atom bombardment mass spectrometric study of room-temperature 1-ethyl-3-methylimidazolium chloroaluminate(III) ionic liquids. Evidence for the existence of the decachlorotrialuminate(III) anion. Org. Mass Spectrom. 28, 759–765 (1993).
Zhang, Y. et al. d–p hybridization-induced “trapping–coupling–conversion” enables high-efficiency Nb single-atom catalysis for Li–S batteries. J. Am. Chem. Soc. 145, 1728–1739 (2023).
Wang, L. et al. Design rules of a sulfur redox electrocatalyst for lithium–sulfur batteries. Adv. Mater. 34, e2110279 (2022).
Barthel, E. R., Martini, I. B. & Schwartz, B. J. How does the solvent control electron transfer? Experimental and theoretical studies of the simplest charge transfer reaction. J. Phys. Chem. B 105, 12230–12241 (2001).
Hou, S. et al. Solvation sheath reorganization enables divalent metal batteries with fast interfacial charge transfer kinetics. Science 374, 172–178 (2021).
Wang, H. et al. High-voltage and noncorrosive ionic liquid electrolyte used in rechargeable aluminum battery. ACS Appl. Mater. Interfaces 8, 27444–27448 (2016).
Tseng, C.-H. et al. Corrosion behaviors of materials in aluminum chloride–1-ethyl-3-methylimidazolium chloride ionic liquid. Electrochem. Commun. 12, 1091–1094 (2010).
Geng, L., Lv, G., Xing, X. & Guo, J. Reversible electrochemical intercalation of aluminum in Mo6S8. Chem. Mater. 27, 4926–4929 (2015).
Levi, E. & Aurbach, D. Chevrel phases, MxMo6T8 (M = metals, T = S, Se, Te) as a structural chameleon: changes in the rhombohedral framework and triclinic distortion. Chem. Mater. 22, 3678–3692 (2010).
Jadhav, A. L., Xu, J. H. & Messinger, R. J. Quantitative molecular-level understanding of electrochemical aluminum-ion intercalation into a crystalline battery electrode. ACS Energy Lett. 5, 2842–2848 (2020).
Lin, Z. et al. Electroactive-catalytic conductive framework for aluminum–sulfur batteries. Energy Storage Mater. 51, 266–272 (2022).
Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 92, 508–517 (1990).
Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 113, 7756–7764 (2000).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Frisch, M. J. et al. Gaussian 09, Revision A.01 (Gaussian, Inc., 2016).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Dronskowski, R. & Bloechl, P. E. Crystal orbital Hamilton populations (COHP): energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. A 97, 8617–8624 (1993).
Maintz, S., Deringer, V. L., Tchougréeff, A. L. & Dronskowski, R. LOBSTER: a tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem. 37, 1030–1035 (2016).
Deringer, V. L., Tchougréeff, A. L. & Dronskowski, R. Crystal orbital Hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 115, 5461–5466 (2011).
Vandevondele, J. & Hutter, J. An efficient orbital transformation method for electronic structure calculations. J. Chem. Phys. 118, 4365–4369 (2003).
Vandevondele, J. & Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 127, 114105 (2007).
Laun, J. & Bredow, T. BSSE-corrected consistent Gaussian basis sets of triple-zetavalence with polarization quality of the fifth period for solid-state calculations. J. Comput. Chem. 43, 839–846 (2022).
Acknowledgements
We gratefully acknowledge the technical support from the Advanced Instrumental Analysis Center, School of Chemical Engineering and Technology, Tianjin University, for their provision of high-performance characterization services. We thank BRUKER OPTICS for granting access to the Raman characterization instruments. We acknowledge the financial support from the National Natural Science Foundation of China (numbers 22479110, 22109116 and 22121004), the National Key Research and Development Program of China (number 2022YFB2404500), the Energy Revolution S&T Program of Yulin Innovation Institute of Clean Energy (number E411050316), the ‘Pandeng Plan’ Project in Tianjin University (number 2024XPD-0002), the Natural Science Foundation of Tianjin (number 23JCQNJC01750), the National Industry-Education Platform for Energy Storage (Tianjin University), the Fundamental Research Funds for the Central Universities and the Haihe Laboratory of Sustainable Chemical Transformations.
Author information
Authors and Affiliations
Contributions
Q.-H.Y. and Z.W. supervised the project. Z.W. and D.H. conceived and designed the experiments. B.Z., D.H. and Z.W. performed electrochemical, NMR, Raman, ESI–MS and SEM characterizations, and results analysis. Z.L., L.W. and Z.M. carried out molecular dynamics simulations and DFT calculations. Q.L. contributed to the synthesis of cathode materials. X.M. conducted the XRD measurement. B.Z. and D.H. wrote the first draft of the paper. D.H., Z.W. and Q.-H.Y. reviewed and edited the final version of the article with assistance from C.C. and Z.L. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Jan Bitenc, Shuqiang Jiao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–35, Tables 1–4, Notes 1–5 and references.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Li, Z., Han, D. et al. Non-corrosive organodichloro electrolyte for reversible aluminium metal batteries. Nat Sustain (2025). https://doi.org/10.1038/s41893-025-01706-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41893-025-01706-6