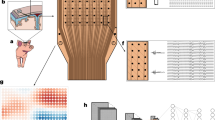
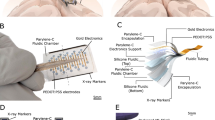
Abstract
High-density, large-area electronic interfaces are a key component of brain–computer interface technologies. However, current designs typically require patients to undergo invasive procedures, which can lead to various complications. Here, we report a biodegradable and self-deployable tent electrode for brain cortex interfacing. The system can be integrated with multiplexing arrays and a wireless module for near-field communication and data transfer. It can be programmably packaged and self-deployed using a syringe for minimally invasive delivery through a small hole. Following delivery, it can expand to cover an area around 200 times its initial size. The electrode also naturally decomposes within the body after use, minimizing the impact of subsequent removal surgery. Through in vivo demonstrations, we show that our cortical-interfacing platform can be used to stimulate large populations of cortical activities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the paper. Source data are provided with this paper.
References
Sporns, O. Structure and function of complex brain networks. Dialogues Clin. Neurosci. 15, 247–262 (2013).
Vaidya, A. R., Pujara, M. S., Petrides, M., Murray, E. A. & Fellows, L. K. Lesion studies in contemporary neuroscience. Trends Cogn. Sci. 23, 653–671 (2019).
Liu, J. et al. Complex brain network analysis and its applications to brain disorders: a survey. Complexity 2017, 8362741 (2017).
Mullin, J. P., Sexton, D., Al-Omar, S., Bingaman, W. & Gonzalez-Martinez, J. Outcomes of subdural grid electrode monitoring in the stereoelectroencephalography era. World Neurosurg. 89, 255–258 (2016).
Pittau, F. et al. Mapping epileptic activity: sources or networks for the clinicians? Front. Neurol. 5, 218 (2014).
Hartings, J. A. et al. Spreading depolarization and late secondary insults after traumatic brain injury. J. Neurotrauma 26, 1857–1866 (2009).
Nicolelis, M. A. L. et al. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc. Natl Acad. Sci. USA 100, 11041–11046 (2003).
Hatsopoulos, N. G. & Donoghue, J. P. The science of neural interface systems. Annu. Rev. Neurosci. 32, 249–266 (2009).
Nicolelis, M. A. L. Actions from thoughts. Nature 409, 403–407 (2001).
Lebedev, M. A. & Nicolelis, M. A. L. Brain–machine interfaces: from basic science to neuroprostheses and neurorehabilitation. Physiol. Rev. 97, 767–837 (2017).
Schalk, G. & Leuthardt, E. C. Brain–computer interfaces using electrocorticographic signals. IEEE Rev. Biomed. Eng. 4, 140–154 (2011).
Talke, P. O. & Gelb, A. W. Postcraniotomy pain remains a real headache! Eur. J. Anaesthesiol. 22, 325–327 (2005).
Basali, A., Mascha, E. J., Kalfas, I. & Schubert, A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology 93, 48–54 (2000).
Chiang, H. -Y. et al. Risk factors and outcomes associated with surgical site infections after craniotomy or craniectomy. J. Neurosurg. 120, 509–521 (2014).
Hall-Stoodley, L., Costerton, J. W. & Stoodley, P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2, 95–108 (2004).
VanEpps, J. S. & Younger, J. G. Implantable device related infection. Shock 46, 597–608 (2016).
Stice, P. & Muthuswamy, J. Assessment of gliosis around moveable implants in the brain. J. Neural Eng. 6, 046004 (2009).
Xie, C. et al. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 14, 1286–1292 (2015).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015).
Hettick, M. et al. The layer 7 cortical interface: a scalable and minimally invasive brain–computer interface platform. Preprint at www.biorxiv.org/content/10.1101/2022.01.02.474656v2 (2022).
Oxley, T. J. et al. Minimally invasive endovascular stent-electrode array for high-fidelity, chronic recordings of cortical neural activity. Nat. Biotechnol. 34, 320–327 (2016).
Wei, S. et al. Shape-changing electrode array for minimally invasive large-scale intracranial brain activity mapping. Nat. Commun. 15, 715 (2024).
Song, S., Fallegger, F., Trouillet, A., Kim, K. & Lacour, S. P. Deployment of an electrocorticography system with a soft robotic actuator. Sci. Robot. 8, eadd1002 (2023).
Liu, Y. et al. Ferromagnetic flexible electronics for brain-wide selective neural recording. Adv. Mater. 35, 2208251 (2022).
Jeong, U.-J. et al. A minimally invasive flexible electrode array for simultaneous recording of ECoG signals from multiple brain regions. Lab Chip 21, 2383–2397 (2021).
Yu, K. J. et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 15, 782–791 (2016).
Huang, Y. et al. Bioresorbable thin-film silicon diodes for the optoelectronic excitation and inhibition of neural activities. Nat. Biomed. Eng. 7, 486–498 (2023).
Gross, R. A. & Kalra, B. Biodegradable polymers for the environment. Science 297, 803–807 (2002).
Hwang, S.-W. et al. A physically transient form of silicon electronics. Science 337, 1640–1644 (2012).
Kang, S.-K. et al. Dissolution behaviors and applications of silicon oxides and nitrides in transient electronics. Adv. Funct. Mater. 24, 4427–4434 (2014).
Yin, L. et al. Dissolvable metals for transient electronics. Adv. Funct. Mater. 24, 645–658 (2014).
Woodington, B. J. et al. X-ray markers for thin film implants. Adv. Healthc. Mater. 11, 2200739 (2022).
Ding, Y. et al. 3D-printed radiopaque bioresorbable stents to improve device visualization. Adv. Healthc. Mater. 11, 2201955 (2022).
Nave, M. D. & Barnett, M. R. Microstructures and textures of pure magnesium deformed in plane-strain compression. Scr. Mater. 51, 881–885 (2004).
Salvatore, G. A. et al. Biodegradable and highly deformable temperature sensors for the internet of things. Adv. Funct. Mater. 27, 1702390 (2017).
Bae, J.-Y. et al. Biodegradable metallic glass for stretchable transient electronics. Adv. Sci. 8, 2004029 (2021).
Kang, S.-K. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016).
Shim, J.-S., Rogers, J. A. & Kang, S.-K. Physically transient electronic materials and devices. Mater. Sci. Eng.: R: Rep. 145, 100624 (2021).
Zhang, H. & Grinstaff, M. W. Recent advances in glycerol polymers: chemistry and biomedical applications. Macromol. Rapid Commun. 35, 1906–1924 (2014).
Cruccu, G. et al. Recommendations for the clinical use of somatosensory-evoked potentials. Clin. Neurophysiol. 119, 1705–1719 (2008).
Daube, J. R. & Rubin, D. I. Clinical Neurophysiology (ed. Daube, J. R.) Ch. 15 (Oxford Univ. Press, 2009).
Park, D.-W. et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 5, 5258 (2014).
Williams, J. C., Hippensteel, J. A., Dilgen, J., Shain, W. & Kipke, D. R. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J. Neural Eng. 4, 410–423 (2007).
Roh, D. & Park, S. Brain multimodality monitoring: updated perspectives. Curr. Neurol. Neurosci. Rep. 16, 56 (2016).
Konrad, B. et al. A simple and reliable technique to monitor intracranial pressure in the rat. Neurosurgery 30, 138–140 (1992).
Giuseppe, C. et al. Induced abdominal compartment syndrome increases intracranial pressure in neurotrauma patients: a prospective study. Crit. Care Med. 29, 1466–1471 (2001).
Viventi, J. et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 14, 1599–1605 (2011).
Li, J. et al. Conductively coupled flexible silicon electronic systems for chronic neural electrophysiology. Proc. Natl Acad. Sci. USA 115, 9542–9549 (2018).
Kim, J. et al. Miniaturized flexible electronic systems with wireless power and near-field communication capabilities. Adv. Funct. Mater. 25, 4761–4767 (2015).
Kim, S. et al. Enhancement of gene editing and base editing with therapeutic ribonucleoproteins through in vivo delivery based on absorptive silica nanoconstruct. Adv. Healthc. Mater. 12, 2201825 (2022).
Acknowledgements
More than half of this work is supported by the National R&D Programme through the National Research Foundation of Korea funded by the Ministry of Science and ICT (Grant No. 2022M3H4A1A04096393). Further support is provided by National R&D Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (Grant Nos. 2022R1C1C1008513, RS-2023-00302145, RS-2023-00217968, 2023R1A2C2007705 and RS-2024-00419269).
Author information
Authors and Affiliations
Contributions
J.-Y.B., G.-S.H., Y.-S.K., J.-Y.K. and S.-K.K. designed the research. J.-Y.B., Y.-S.K., S.-G.C., J.-Y.L., J.-H.L., K.-S.K., J.-H.P, W.-J.L. and S.-K.K. designed, fabricated and analysed the devices and interfaces. J.-Y.B., Y.-S.K., J.-W.K., J.K. and S.-K.K. designed and fabricated the NFC-based wireless system with devices. G.-S.H. and J.-Y.K. designed and performed the mechanical modelling. J.-Y.B., Y.-S.K. J.J., M.C., K.-S.L. and J.K.H. designed, performed and analysed the in vivo experiments. M.C., S.K., S.-H.L, S.L., Y.-C.K., K.-S.L. and H.L. analysed the biocompatibility and immunohistochemistry. J.-Y.B., G.-S.H., Y.-S.K., J.K.H., J.-Y.K. and S.-K.K. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Electronics thanks Meining Zhang, Hongbian Li and and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 In vivo multimodal monitoring of brain activities.
(a) A photograph of deployed multimodal platform for physiological signal monitoring. (b) Device locations (left) and corresponding ECoG recordings (right). (c) Temperature monitoring with infrared lamp irradiation. (d) Strain monitoring with flank squeezing. (e) pH monitoring with saline injection through drilled hole.
Supplementary information
Supplementary Information
Supplementary Notes 1–25, references, Figs. 1–69 and Table 1.
Supplementary Video 1
Programmable packaging and self-deployment (in vitro).
Supplementary Video 2
Wireless LED operation after deployment (in vitro).
Supplementary Video 3
FEM analysis during programmable packaging.
Supplementary Video 4
Real-time deployment in canine model.
Supplementary Video 5
Wireless monitoring (temperature).
Supplementary Video 6
Animal’s quick recovery (after 1 d movement).
Supplementary Data
Source data for supplementary figures.
Source data
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Fig. 5
Source data for Fig. 5.
Source Data Fig. 6
Source data for Fig. 6.
Source Data Extended Data Fig. 1
Source data for Extended Fig. 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bae, JY., Hwang, GS., Kim, YS. et al. A biodegradable and self-deployable electronic tent electrode for brain cortex interfacing. Nat Electron 7, 815–828 (2024). https://doi.org/10.1038/s41928-024-01216-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41928-024-01216-x
This article is cited by
-
Soft sonocapacitor with topologically integrated piezodielectric nanospheres enables wireless epidural closed-loop neuromodulation
Nature Communications (2026)
-
Fully biodegradable and mass-producible conductive fiber based on tungsten–poly(butylene adipate-co-terephthalate) composite
npj Flexible Electronics (2025)
-
Solution-processable and photo-curable system for low-cost and scalable transient electronics
Nature Communications (2025)
-
Materials strategy and device fabrication for stable closed-loop bioelectronics
npj Biosensing (2025)
-
Bio-inspired electronics: Soft, biohybrid, and “living” neural interfaces
Nature Communications (2025)