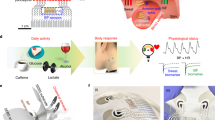
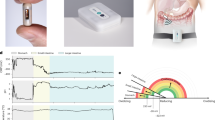
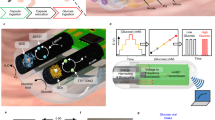
Abstract
The gastrointestinal tract contains a wealth of chemical information that can be used to decipher the health of the digestive and nervous systems. Traditional methods of analysis, such as faecal analysis and biopsies, are invasive, costly and incapable of providing real-time metabolic and hormone profiling across the gastrointestinal tract. Commercial ingestible capsule sensors have been developed, but only monitor basic markers, such as pH and pressure, neglecting detailed chemical analysis. Here we report an integrated smart capsule that can simultaneously detect a spectrum of biochemical markers, including electrolytes, metabolites and hormones. The capsule, which is termed PillTrek, is 7 mm in diameter and 25 mm in length, and houses a miniaturized wireless electrochemical workstation capable of executing a range of electrochemical measurement techniques (potentiometry, amperometry, voltammetry and impedimetry), allowing it to interface with a variety of electrochemical sensors and detect various parameters in the gut. Using an array of sensors (serotonin, glucose, pH, ionic strength and temperature), we illustrate the capabilities of the system in vitro and in vivo in animal studies involving rat and rabbit models, monitoring the dynamic profile of these crucial biomarkers and their responsiveness to different dietary intakes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that supports the findings of this study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Steiger, C. et al. Ingestible electronics for diagnostics and therapy. Nat. Rev. Mater. 4, 83–98 (2019).
Said, H. M. & Ghishan, F. K. (eds) Physiology of the Gastrointestinal Tract (Academic Press, 2018).
Dieterich, W., Schink, M. & Zopf, Y. Microbiota in the gastrointestinal tract. Med. Sci. 6, 116 (2018).
McCallum, G. & Tropini, C. The gut microbiota and its biogeography. Nat. Rev. Microbiol. 22, 105–118 (2024).
Smith, P. A. The tantalizing links between gut microbes and the brain. Nature 526, 312–314 (2015).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Rea, K., Dinan, T. G. & Cryan, J. F. The microbiome: a key regulator of stress and neuroinflammation. Neurobiol. Stress 4, 23–33 (2016).
Bhattacharyya, A., Chattopadhyay, R., Mitra, S. & Crowe, S. E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 94, 329–354 (2014).
Vernia, P. et al. Fecal lactate and ulcerative colitis. Gastroenterology 95, 1564–1568 (1988).
Mawe, G. M. & Hoffman, J. M. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 10, 473–486 (2013).
Gershon, M. D. & Tack, J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007).
Martin-Gallausiaux, C., Marinelli, L., Blottière, H. M., Larraufie, P. & Lapaque, N. SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 80, 37–49 (2021).
Dalile, B., Van Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019).
Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 46, 183–196 (1999).
Press et al. Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment Pharmacol. Ther. 12, 673–678 (1998).
Chang, E. B. & Leung, P. S. in The Gastrointestinal System (ed. Leung, P. S.) 107–134 (Springer, 2014).
Maggs, D., MacDonald, I. & Nauck, M. A. Glucose homeostasis and the gastrointestinal tract: insights into the treatment of diabetes. Diabetes Obes. Metab. 10, 18–33 (2008).
Fournel, A. et al. Glucosensing in the gastrointestinal tract: impact on glucose metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G645–G658 (2016).
Antunes, L. C. M., Davies, J. E. & Finlay, B. B. Chemical signaling in the gastrointestinal tract. F1000 Biol. Rep. 3, 4 (2011).
Shalon, D. et al. Profiling the human intestinal environment under physiological conditions. Nature 617, 581–591 (2023).
Kalantar-Zadeh, K., Ha, N., Ou, J. Z. & Berean, K. J. Ingestible sensors. ACS Sens. 2, 468–483 (2017).
Cao, Q. et al. Robotic wireless capsule endoscopy: recent advances and upcoming technologies. Nat. Commun. 15, 4597 (2024).
Nan, K. et al. Mucosa-interfacing electronics. Nat. Rev. Mater. 7, 908–925 (2022).
Min, J., Yang, Y., Wu, Z. & Gao, W. Robotics in the gut. Adv. Ther. 3, 1900125 (2020).
Abdigazy, A. et al. End-to-end design of ingestible electronics. Nat. Electron. 7, 102–118 (2024).
Traverso, G. et al. First-in-human trial of an ingestible vitals-monitoring pill. Device 1, 100125 (2023).
You, S. S. et al. An ingestible device for gastric electrophysiology. Nat. Electron. 7, 497–508 (2024).
Kalantar-Zadeh, K. et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat. Electron. 1, 79–87 (2018).
Hou, B. et al. A swallowable X-ray dosimeter for the real-time monitoring of radiotherapy. Nat. Biomed. Eng. 7, 1242–1251 (2023).
Inda-Webb, M. E. et al. Sub-1.4 cm3 capsule for detecting labile inflammatory biomarkers in situ. Nature 620, 386–392 (2023).
Mimee, M. et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science 360, 915–918 (2018).
De La Paz, E. et al. A self-powered ingestible wireless biosensing system for real-time in situ monitoring of gastrointestinal tract metabolites. Nat. Commun. 13, 7405 (2022).
Xu, C. et al. A physicochemical-sensing electronic skin for stress response monitoring. Nat. Electron. 7, 168–179 (2024).
Maines, A., Ashworth, D. & Vadgama, P. Diffusion restricting outer membranes for greatly extended linearity measurements with glucose oxidase enzyme electrodes. Anal. Chim. Acta 333, 223–231 (1996).
Venton, B. J. & Cao, Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 145, 1158–1168 (2020).
Li, J. et al. A tissue-like neurotransmitter sensor for the brain and gut. Nature 606, 94–101 (2022).
Li, S. et al. A wrinkled structure of gold film greatly improves the signaling of electrochemical aptamer-based biosensors. RSC Adv. 11, 671–677 (2020).
Li, H., Dauphin-Ducharme, P., Ortega, G. & Plaxco, K. W. Calibration-free electrochemical biosensors supporting accurate molecular measurements directly in undiluted whole blood. J. Am. Chem. Soc. 139, 11207–11213 (2017).
Jang, H.-S. et al. Nafion coated Au nanoparticle-graphene quantum dot nanocomposite modified working electrode for voltammetric determination of dopamine. Inorg. Chem. Commun. 105, 174–181 (2019).
Ren, J., Shi, W., Li, K. & Ma, Z. Ultrasensitive platinum nanocubes enhanced amperometric glucose biosensor based on chitosan and Nafion film. Sens. Actuators B 163, 115–120 (2012).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Margolis, K. G. et al. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 63, 928–937 (2014).
Zelkas, L. et al. Serotonin-secreting enteroendocrine cells respond via diverse mechanisms to acute and chronic changes in glucose availability. Nutr. Metab. 12, 55 (2015).
O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G. & Cryan, J. F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277, 32–48 (2015).
Nath, S. et al. Topographical and biometrical anatomy of the digestive tract of white New Zealand rabbit (Oryctolagus cuniculus). J. Adv. Vet. Anim. Res. 3, 145–151 (2016).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Brodkorb, A. et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 14, 991–1014 (2019).
Del-Rio-Ruiz, R. et al. Soft autonomous ingestible device for sampling the small-intestinal microbiome. Device 2, 100406 (2024).
Levy, J. A., Straker, M. A., Stine, J. M., Beardslee, L. A. & Ghodssi, R. Magnetically triggered ingestible capsule for localized microneedle drug delivery. Device 2, 100438 (2024).
Acknowledgements
This project was supported by the National Science Foundation grant no. 2145802, National Institutes of Health grant nos. R01HL155815 and R21DK13266, American Cancer Society Research Scholar grant no. RSG-21-181-01-CTPS, Army Research Office grant no. W911NF-23-1-0041, US Army Medical Research Acquisition Activity grant no. HT9425-24-1-0249, National Research Foundation of Korea grant no. RS-2024-00411904 and Heritage Medical Research Institute (all to W.G.). We gratefully acknowledge critical support and infrastructure provided for this work by the Kavli Nanoscience Institute at Caltech. J.M. acknowledges fellowship support from the National Institutes of Health Training grant (no. T32EB023858). H.A. and H.-T.J. acknowledge the support from the KAIST-UC Berkeley-VNU Climate Change Research Center grant no. 2021K1A4A8A01079356. Lemberg (Austria) helped with porting code.
Author information
Authors and Affiliations
Contributions
W.G. and J.M. initiated the concept of and designed the studies. W.G. supervised the work. J.M. and H.A. led the experiments and collected the overall data. H.L., R.B. and G.K. contributed to sensor characterization and validation. X.M., S.-H.S., C.W., Y.X. and D.R.Y contributed to the animal studies. Z.L., T.K.H., A.E. and H.-T.J. contributed to the study design. All authors contributed to the data analysis and provided the feedback on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Electronics thanks Rabia Yazicigil and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1 and 2, Figs. 1–41, Tables 1–5, Videos 1 and 2 and Refs. 1–12.
Supplementary Video 1
PillTrek performing multiparametric measurements while moving through a phantom intestine model.
Supplementary Video 2
PillTrek with protective cap repeatedly contacting rabbit intestinal tissue.
Source data
Source Data Figs. 2–5
Source data for sensor characterization studies in Fig. 2. Source data for electronic system characterization studies in Fig. 3. Source data for ex-vivo rat studies in Fig. 4. Source data for in vivo rabbit studies in Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Min, J., Ahn, H., Lukas, H. et al. Continuous biochemical profiling of the gastrointestinal tract using an integrated smart capsule. Nat Electron 8, 844–855 (2025). https://doi.org/10.1038/s41928-025-01407-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41928-025-01407-0
This article is cited by
-
Magnetically controllable battery-free multifunctional ingestible and versatile smart e-pill
npj Flexible Electronics (2026)
-
The potential of miniaturized ingestible electronics
Nature Electronics (2026)
-
Wearable biomolecular sensing nanotechnologies in chronic disease management
Nature Nanotechnology (2025)
-
A novel insight into the femtosecond induced nonlinear response of monoamine neurotransmitters through experimental and in silico approaches
Scientific Reports (2025)