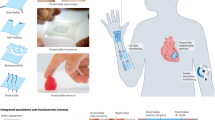
Abstract
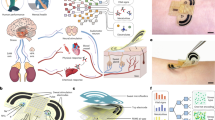
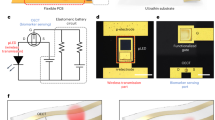
Biosensors based on organic field-effect transistors can offer mechanical flexibility, stretchability and operational stability for conformal on-skin monitoring. However, bending, stretching, moisture and temperature changes can lead to signal artefacts and drifts. Here we report skin-like drift-free biosensors based on stretchable diode-connected organic field-effect transistors. Our approach relies on capacitive coupling and the subtraction of interference signals using two extended gates functionalized separately with target and reference bioreceptors. It reduces signal distortion by up to two orders of magnitude compared with an unconnected organic field-effect transistor, despite changes in the sampling environment, including bias stress instability, uniaxial strain (up to 100%), compression (up to 50 mN) and temperature variations (25–40 °C). We apply the approach to aptamer-based sensing for cortisol, enzyme-based sensing for glucose and ion-selective membrane-based potentiometric sensing for sodium ions. We also develop a hybrid wearable system, including soft sensors and a flexible printed circuit board, which wirelessly communicates with a smartphone app. We show that the system can perform cortisol sensing from human sweat under acute stress events.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed in this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Zhao, C., Park, J., Root, S. E. & Bao, Z. Skin-inspired soft bioelectronic materials, devices and systems. Nat. Rev. Bioeng. 2, 671–690 (2024).
Wang, Y. et al. Skin bioelectronics towards long-term, continuous health monitoring. Chem. Soc. Rev. 51, 3759–3793 (2022).
Cho, K. W. et al. Soft bioelectronics based on nanomaterials. Chem. Rev. 122, 5068–5143 (2022).
Lin, M., Hu, H., Zhou, S. & Xu, S. Soft wearable devices for deep-tissue sensing. Nat. Rev. Mater. 7, 850–869 (2022).
Khodagholy, D. et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 4, 1575 (2013).
Jiang, Y. et al. Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 375, 1411–1417 (2022).
Wu, J. et al. Adhesive anti-fibrotic interfaces on diverse organs. Nature 630, 360–367 (2024).
Li, Y., Li, N., De Oliveira, N. & Wang, S. Implantable bioelectronics toward long-term stability and sustainability. Matter 4, 1125–1141 (2021).
Le Floch, P. et al. 3D spatiotemporally scalable in vivo neural probes based on fluorinated elastomers. Nat. Nanotechnol. 19, 319–329 (2024).
Zhu, C. et al. Stretchable temperature-sensing circuits with strain suppression based on carbon nanotube transistors. Nat. Electron. 1, 183–190 (2018).
Li, J. et al. Thin, soft, wearable system for continuous wireless monitoring of artery blood pressure. Nat. Commun. 14, 5009 (2023).
Schwartz, G. et al. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 4, 1859 (2013).
Sim, K. et al. An epicardial bioelectronic patch made from soft rubbery materials and capable of spatiotemporal mapping of electrophysiological activity. Nat. Electron. 3, 775–784 (2020).
Li, N. et al. Bioadhesive polymer semiconductors and transistors for intimate biointerfaces. Science 381, 686–693 (2023).
Wang, S. et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 555, 83–88 (2018).
Wang, W. et al. Neuromorphic sensorimotor loop embodied by monolithically integrated, low-voltage, soft e-skin. Science 380, 735–742 (2023).
Yuvaraja, S. et al. Organic field-effect transistor-based flexible sensors. Chem. Soc. Rev. 49, 3423–3460 (2020).
Dai, Y., Hu, H., Wang, M., Xu, J. & Wang, S. Stretchable transistors and functional circuits for human-integrated electronics. Nat. Electron. 4, 17–29 (2021).
Sugiyama, M. et al. An ultraflexible organic differential amplifier for recording electrocardiograms. Nat. Electron. 2, 351–360 (2019).
Kondo, M. et al. Imperceptible magnetic sensor matrix system integrated with organic driver and amplifier circuits. Sci. Adv. 6, eaay6094 (2020).
Mirshojaeian Hosseini, M. J. et al. 270 nm ultra-thin self-adhesive conformable and long-term air-stable complimentary organic transistors and amplifiers. npj Flex. Electron. 7, 38 (2023).
Kim, J., Campbell, A. S., de Ávila, B. E.-F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Li, J. et al. A tissue-like neurotransmitter sensor for the brain and gut. Nature 606, 94–101 (2022).
Zhao, C. et al. Implantable aptamer–field-effect transistor neuroprobes for in vivo neurotransmitter monitoring. Sci. Adv. 7, eabj7422 (2021).
Ye, C. et al. A wearable aptamer nanobiosensor for non-invasive female hormone monitoring. Nat. Nanotechnol. 19, 330–337 (2024).
Wang, B. et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 8, eabk0967 (2022).
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022).
Sempionatto, J. R. et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 5, 737–748 (2021).
Macchia, E. et al. Single-molecule detection with a millimetre-sized transistor. Nat. Commun. 9, 3223 (2018).
Park, S., Kim, S. H., Choi, H. H., Kang, B. & Cho, K. Recent advances in the bias stress stability of organic transistors. Adv. Funct. Mater. 30, 1904590 (2019).
Iqbal, H. F. et al. Suppressing bias stress degradation in high performance solution processed organic transistors operating in air. Nat. Commun. 12, 2352 (2021).
Kaisti, M. Detection principles of biological and chemical FET sensors. Biosens. Bioelectron. 98, 437–448 (2017).
Torsi, L., Magliulo, M., Manoli, K. & Palazzo, G. Organic field-effect transistor sensors: a tutorial review. Chem. Soc. Rev. 42, 8612–8628 (2013).
Picca, R. A. et al. Ultimately sensitive organic bioelectronic transistor sensors by materials and device structure design. Adv. Funct. Mater. 30, 1904513 (2020).
White, S. P., Dorfman, K. D. & Frisbie, C. D. Operating and sensing mechanism of electrolyte-gated transistors with floating gates: building a platform for amplified biodetection. J. Phys. Chem. C 120, 108–117 (2016).
Torricelli, F. et al. Electrolyte-gated transistors for enhanced performance bioelectronics. Nat. Rev. Methods Prim. 1, 66 (2021).
Kwon, J. et al. Nanoscale FET-based transduction toward sensitive extended-gate biosensors. ACS Sens. 4, 1724–1729 (2019).
Wang, W. et al. Strain-insensitive intrinsically stretchable transistors and circuits. Nat. Electron. 4, 143–150 (2021).
Thomas, M. S., Adrahtas, D. Z., Frisbie, C. D. & Dorfman, K. D. Modeling of quasi-static floating-gate transistor biosensors. ACS Sens. 6, 1910–1917 (2021).
Arcus, V. L., van der Kamp, M. W., Pudney, C. R. & Mulholland, A. J. Enzyme evolution and the temperature dependence of enzyme catalysis. Curr. Opin. Struct. Biol. 65, 96–101 (2020).
Zheng, Y. et al. A molecular design approach towards elastic and multifunctional polymer electronics. Nat. Commun. 12, 5701 (2021).
Li, S. G. et al. Implantable hydrogel-protective DNA aptamer-based sensor supports accurate, continuous electrochemical analysis of drugs at multiple sites in living rats. ACS Nano 17, 18525–18538 (2023).
Chan, D. et al. Combinatorial polyacrylamide hydrogels for preventing biofouling on implantable biosensors. Adv. Mater. 34, e2109764 (2022).
Zhong, D. et al. High-speed and large-scale intrinsically stretchable integrated circuits. Nature 627, 313–320 (2024).
Wu, C. et al. Strain-induced performance variation in stretchable carbon-nanotube thin-film transistors and the solution through a circular channel design. IEEE Trans. Electron Dev. 71, 3411–3416 (2024).
Yoo, H., Jo, H. & Oh, S. S. Detection and beyond: challenges and advances in aptamer-based biosensors. Mater. Adv. 1, 2663–2687 (2020).
Nakatsuka, N. et al. Aptamer–field-effect transistors overcome debye length limitations for small-molecule sensing. Science 362, 319–324 (2018).
Poudineh, M. et al. A fluorescence sandwich immunoassay for the real-time continuous detection of glucose and insulin in live animals. Nat. Biomed. Eng. 5, 53–63 (2021).
Gerson, J. et al. High-precision monitoring of and feedback control over drug concentrations in the brains of freely moving rats. Sci. Adv. 9, eadg3254 (2023).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Xu, C. et al. A physicochemical-sensing electronic skin for stress response monitoring. Nat. Electron. 7, 168–179 (2024).
Zdrachek, E. & Bakker, E. Potentiometric sensing. Anal. Chem. 91, 2–26 (2019).
Chung, H. U. et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363, eaau0780 (2019).
Matsuhisa, N., Chen, X., Bao, Z. & Someya, T. Materials and structural designs of stretchable conductors. Chem. Soc. Rev. 48, 2946–2966 (2019).
Widlund, T., Yang, S., Hsu, Y.-Y. & Lu, N. Stretchability and compliance of freestanding serpentine-shaped ribbons. Int. J. Solids Struct. 51, 4026–4037 (2014).
Jiang, Y. et al. A universal interface for plug-and-play assembly of stretchable devices. Nature 614, 456–462 (2023).
Schwabe, L., Haddad, L. & Schachinger, H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology 33, 890–895 (2008).
Torrente-Rodríguez, R. M. et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mhealth system. Matter 2, 921–937 (2020).
Tang, W. et al. Touch-based stressless cortisol sensing. Adv. Mater. 33, 2008465 (2021).
Wang, G.-J. N. et al. Nonhalogenated solvent processable and printable high-performance polymer semiconductor enabled by isomeric nonconjugated flexible linkers. Macromolecules 51, 4976–4985 (2018).
Acknowledgements
We acknowledge financial support from the National Science Foundation (SENSE-2037304). C. Zhao acknowledges funding from an F32 fellowship from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (F32EB034156, C. Zhao). R.K.M. was supported by the Department of Defense (DoD) through the National Defense Science and Engineering Graduate (NDSEG) Fellowship Program. A.B. acknowledges the National Science Foundation Graduate Research Fellowship (grant number DGE-1656518, A.B.) and the Stanford Graduate Fellowship. Z.B. is a Chan Zuckerberg Biohub San Francisco investigator and an Arc Institute innovation investigator. Z.B. acknowledges support from the Tianqiao and Chrissy Chen Ideation and Prototyping Lab and Stanford Wearable Electronics Initiative (eWEAR) seed funding. We thank the Bin Lin and Daisy Liu Family Fund for the generous support of the Bao Group’s research at Stanford University. Part of this work was performed at the Stanford Nano Shared Facilities (SNSF), supported by the NSF award ECCS-2026822. We thank the Asahi Kasei Corporation for providing SEBS. We thank E. Luis for feedback on the paper and J.-Y. Chang for technical assistance on data collection.
Author information
Authors and Affiliations
Contributions
C. Zhao, J.P. and Z.B. designed the project and experiments. C. Zhao, J.P. and D.M. carried out the experiments and collected the related data. C. Zhao, D.M., Y.Y., W.W. and Q.L. fabricated the OFET biosensors. C. Zhao, J.P., D.M., D.Z., C.W. and C. Zhu carried out the circuit model design and analysis of diode-connected OFETs for biosensing. C. Zhao, J.P., D.M. and Changhao Xu carried out the biosensing tests. Y.Z., X.J. and Z.Y. designed and synthesized the azide compounds (azide, BA and BH). R.K.M. and X.J. designed and synthesized the conjugated polymer (DPPTT). H.L. carried out the scanning electron microscopy imaging. Ying Jiang fabricated the BIND interconnects for the soft-flexible interface. C. Zhao and J.P. conducted and analysed the on-body data. Chengyi Xu and B.S. carried out the laser cutting for the microfluidics. A.B. printed out the 3D resins for encapsulation. L.M., Yuanwen Jiang, S.W., J.S., A.A., E.K. and M.K. helped with the experimental design and device fabrication. C. Zhao, J.P. and Z.B. wrote the paper. All authors reviewed and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Electronics thanks Magnus Berggren and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–4, Figs. 1–31 and Table 1.
Supplementary Data 1
Statistical source data.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, C., Park, J., Maulà, D. et al. Skin-like drift-free biosensors with stretchable diode-connected organic field-effect transistors. Nat Electron 8, 981–993 (2025). https://doi.org/10.1038/s41928-025-01465-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41928-025-01465-4
This article is cited by
-
Wearables that learn to read gestures on the move
Nature Sensors (2026)
-
Sweat sensors that won’t catch your drift
Nature Electronics (2025)