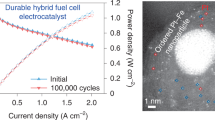
Abstract
Platinum (Pt) nanocatalysts are essential for facilitating the cathodic oxygen reduction reaction in proton exchange membrane fuel cells but suffer from a trade-off between activity and durability. Here we present the design of a fine nanocatalyst comprising Pt nanoparticles with sparsely embedded cobalt oxide clusters (CoOx@Pt). This design exploits the strong Pt/oxide interaction, which grants the catalyst its high structural and chemical durability without sacrificing activity. The CoOx@Pt nanocatalyst delivers a high initial mass activity of 1.10 A mgPt−1, a rated power density of 1.04 W cm−2 and a Pt utilization of 10.4 W mgPt−1 in a membrane electrode assembly. It exhibits a notably high durability that features a mass activity retention of 88.2%, a voltage loss of 13.3 mV at 0.8 A cm−2 and a small rated power loss of 7.5% after accelerated stress testing. This durability could offer a long projected lifetime of 15,000 hours and may greatly reduce the lifetime-adjusted cost.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the major findings of this study are available in the main text or the Supplementary Information. Data on the local minima structures described in the main text that support the findings of this study are available via Zenodo at https://doi.org/10.5281/zenodo.11174993 (ref. 74). Further data are available from the corresponding authors upon reasonable request.
Code availability
Proprietary codes are available from the corresponding authors upon reasonable request under a license agreement with the owner.
References
Debe, M. K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486, 43–51 (2012).
Cullen, D. A. et al. New roads and challenges for fuel cells in heavy-duty transportation. Nat. Energy 6, 462–474 (2021).
Kodama, K., Nagai, T., Kuwaki, A., Jinnouchi, R. & Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 16, 140–147 (2021).
Fan, J. et al. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 6, 475–486 (2021).
Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan (US Department of Energy, 2017).
Lohse-Busch, H. et al. Automotive fuel cell stack and system efficiency and fuel consumption based on vehicle testing on a chassis dynamometer at minus 18 °C to positive 35 °C temperatures. Int. J. Hydrogen Energy 45, 861–872 (2020).
Average Annual Vehicle Miles Traveled by Major Vehicle Category (EERE Alternative Fuels Data Center, 2020); https://afdc.energy.gov/data/
Li, M. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 354, 1414–1419 (2016).
Escudero-Escribano, M. et al. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 352, 73–76 (2016).
Chen, C. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343, 1339–1343 (2014).
Zhang, L. et al. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 349, 412–416 (2015).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Li, W., Chen, Z., Xu, L. & Yan, Y. A solution-phase synthesis method to highly active Pt-Co/C electrocatalysts for proton exchange membrane fuel cell. J. Power Sources 195, 2534–2540 (2010).
Wang, X. X. et al. Ordered Pt3Co intermetallic nanoparticles derived from metal–organic frameworks for oxygen reduction. Nano Lett. 18, 4163–4171 (2018).
Huang, L., Zheng, C. Y., Shen, B. & Mirkin, C. A. High-index-facet metal-alloy nanoparticles as fuel cell electrocatalysts. Adv. Mater. 32, 2002849 (2020).
Ge, Y. et al. Seeded synthesis of unconventional 2H-phase Pd alloy nanomaterials for highly efficient oxygen reduction. J. Am. Chem. Soc. 143, 17292–17299 (2021).
Liu, H. et al. Synthesis of ultrathin platinum nanoplates for enhanced oxygen reduction activity. Chem. Sci. 9, 398–404 (2018).
Liu, Z., Zhao, Z., Peng, B., Duan, X. & Huang, Y. Beyond extended surfaces: understanding the oxygen reduction reaction on nanocatalysts. J. Am. Chem. Soc. 142, 17812–17827 (2020).
Zhao, Z. et al. Pt-based nanocrystal for electrocatalytic oxygen reduction. Adv. Mater. 31, 1808115 (2019).
Borup, R. L. et al. Recent developments in catalyst-related PEM fuel cell durability. Curr. Opin. Electrochem. 21, 192–200 (2020).
Braaten, J. P., Xu, X., Cai, Y., Kongkanand, A. & Litster, S. Contaminant cation effect on oxygen transport through the ionomers of polymer electrolyte membrane fuel cells. J. Electrochem. Soc. 166, F1337–F1343 (2019).
Dumont, J. H. et al. Effect of cerium, cobalt and nickel contaminants on the oxygen reduction reaction at platinum electrodes. ECS Trans. 80, 861–867 (2017).
Cai, Y., Kongkanand, A., Gu, W. & Moylan, T. E. Effects of cobalt cation on low Pt-loaded PEM fuel cell performance. ECS Trans. 69, 1047–1061 (2015).
Han, B. et al. Record activity and stability of dealloyed bimetallic catalysts for proton exchange membrane fuel cells. Energy Environ. Sci. 8, 258–266 (2015).
Ko, M., Padgett, E., Yarlagadda, V., Kongkanand, A. & Muller, D. A. Revealing the nanostructure of mesoporous fuel cell catalyst supports for durable, high-power performance. J. Electrochem. Soc. 168, 024512 (2021).
Qiao, Z. et al. Atomically dispersed single iron sites for promoting Pt and Pt3Co fuel cell catalysts: performance and durability improvements. Energy Environ. Sci. 14, 4948–4960 (2021).
Duan, X. et al. Cobalt-doping stabilized active and durable sub-2 nm Pt nanoclusters for low-Pt-loading PEMFC cathode. Adv. Energy Mater. 12, 2103144 (2022).
Li, J. et al. Hard-magnet L10-CoPt nanoparticles advance fuel cell catalysis. Joule 3, 124–135 (2019).
Yang, C.-L. et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 374, 459–464 (2021).
Qiu, H. J. et al. Platinum cluster/nanoparticle on CoO nanosheets with coupled atomic structure and high electrocatalytic durability. ACS Appl. Energy Mater. 1, 1840–1845 (2018).
Xu, S. et al. Direct integration of strained-Pt catalysts into proton-exchange-membrane fuel cells with atomic layer deposition. Adv. Mater. 33, 2007885 (2021).
Sievers, G. W. et al. Self-supported Pt–CoO networks combining high specific activity with high surface area for oxygen reduction. Nat. Mater. 20, 208–213 (2021).
Zhu, E. et al. Stability of platinum-group-metal-based electrocatalysts in proton exchange membrane fuel cells. Adv. Funct. Mater. 32, 2203883 (2022).
Bertram, M. et al. Cobalt oxide-supported Pt electrocatalysts: intimate correlation between particle size, electronic metal–support interaction and stability. J. Phys. Chem. Lett. 11, 8365–8371 (2020).
Zeng, Z., Chang, K.-C., Kubal, J., Markovic, N. M. & Greeley, J. Stabilization of ultrathin (hydroxy)oxide films on transition metal substrates for electrochemical energy conversion. Nat. Energy 2, 17070 (2017).
Schmies, H. et al. Unravelling degradation pathways of oxide-supported Pt fuel cell nanocatalysts under in situ operating conditions. Adv. Energy Mater. 8, 1701663 (2018).
Xu, C. et al. Physical vapor deposition process for engineering Pt based oxygen reduction reaction catalysts on NbOx templated carbon support. J. Power Sources 451, 227709 (2020).
Luo, M. & Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2, 17059 (2017).
Jia, Q. et al. Activity descriptor identification for oxygen reduction on platinum-based bimetallic nanoparticles: in situ observation of the linear composition–strain–activity relationship. ACS Nsno 9, 387–400 (2015).
Stariha, S. et al. Recent advances in catalyst accelerated stress tests for polymer electrolyte membrane fuel Cells. J. Electrochem. Soc. 165, F492–F501 (2018).
Zhao, Z. et al. Tailoring a three-phase microenvironment for high-performance oxygen reduction reaction in proton exchange membrane fuel cells. Matter 3, 1774–1790 (2020).
Chong, L. et al. Ultralow-loading platinum–cobalt fuel cell catalysts derived from imidazolate frameworks. Science 362, 1276–1281 (2018).
Spendelow, J. S. Advanced Electro-Catalysts through Crystallographic Enhancement (US Department of Energy, 2019).
Yarlagadda, V. et al. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett. 3, 618–621 (2018).
Zhao, Z. et al. Graphene-nanopocket-encaged PtCo nanocatalysts for highly durable fuel cell operation under demanding ultralow-Pt-loading conditions. Nat. Nanotechnol. 17, 968–975 (2022).
Padgett, E. et al. Mitigation of PEM fuel cell catalyst degradation with porous carbon supports. J. Electrochem. Soc. 166, F198–F207 (2019).
Nilsson, A. et al. The electronic structure effect in heterogeneous catalysis. Catal. Lett. 100, 111–114 (2005).
Aprà, E., Ferrando, R. & Fortunelli, A. Density-functional global optimization of gold nanoclusters. Phys. Rev. B 73, 205414 (2006).
Sundararaman, R., Goddard, W. A. & Arias, T. A. Grand canonical electronic density-functional theory: algorithms and applications to electrochemistry. J. Chem. Phys. 146, 114104 (2017).
Lopes, P. P. et al. Relationships between atomic level surface structure and stability/activity of platinum surface atoms in aqueous environments. ACS Catal. 6, 2536–2544 (2016).
Huang, J. et al. Experimental Sabatier plot for predictive design of active and stable Pt-alloy oxygen reduction reaction catalysts. Nat. Catal. 5, 513–523 (2022).
Fairley, N. et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. 5, 100112 (2021).
Arruda, T. M., Shyam, B., Ziegelbauer, J. M., Mukerjee, S. & Ramaker, D. E. Investigation into the competitive and site-specific nature of anion adsorption on Pt using in situ X-ray absorption spectroscopy. J. Phys. Chem. C 112, 18087–18097 (2008).
Newville, M. IFEFFIT: interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 8, 322–324 (2001).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Newville, M., Līviņš, P., Yacoby, Y., Rehr, J. J. & Stern, E. A. Near-edge x-ray-absorption fine structure of Pb: A comparison of theory and experiment. Phys. Rev. B 47, 14126–14131 (1993).
Ankudinov, A. L., Ravel, B., Rehr, J. J. & Conradson, S. D. Real-space multiple-scattering calculation and interpretation of x-ray-absorption near-edge structure. Phys. Rev. B 58, 7565–7576 (1998).
Garrick, T. R., Moylan, T. E., Carpenter, M. K. & Kongkanand, A. Electrochemically active surface area measurement of aged Pt alloy catalysts in PEM fuel cells by CO stripping. J. Electrochem. Soc. 164, F55–F59 (2016).
Fuel cell fixture. Scribner https://www.scribner.com/products/fuel-cell-test-accessories/fuel-cell-fixture/ (2022).
Application note – minimum load and wiring resistance. Scribner https://www.scribner.com/wp-content/uploads/2019/02/Minimum-Load-Resistance.pdf (2022).
Marcinkoski, J. et al. Hydrogen Class 8 Long Haul Truck Targets (US Department of Energy, 2019).
Wales, D. J. & Doye, J. P. K. Global optimization by basin-hopping and the lowest energy structures of Lennard-Jones clusters containing up to 110 atoms. J. Phys. Chem. A 101, 5111–5116 (1997).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Ozaki, T. Variationally optimized atomic orbitals for large-scale electronic structures. Phys. Rev. B. 67, 155108 (2003).
Ozaki, T. and Kino, H. Efficient projector expansion for the ab initio LCAO method. Phys. Rev. B 72, 045121 (2005).
Duy, T. V. T. & Ozaki, T. A decomposition method with minimum communication amount for parallelization of multi-dimensional FFTs. Comput. Phys. Commun. 185, 153–164 (2014).
Ozaki, T. & Kino, H. Numerical atomic basis orbitals from H to Kr. Phys. Rev. B 69, 195113 (2004).
Barber, C. B., Dobkin, D. P. & Huhdanpaa, H. The Quickhull algorithm for convex hulls. ACM Trans. Math. Softw. 22, 469–483 (1996).
Giannozzi, P. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21, 395502 (2009).
Garrity, K. F., Bennett, J. W., Rabe, K. M. & Vanderbilt, D. Pseudopotentials for high-throughput DFT calculations. Comput. Mater. Sci. 81, 446–452 (2014).
Dabo, I., Kozinsky, B., Singh-Miller, N. E. & Marzari, N. Electrostatics in periodic boundary conditions and real-space corrections. Phys. Rev. B 77, 115139 (2008).
Andreussi, O. & Marzari, N. Electrostatics of solvated systems in periodic boundary conditions. Phys. Rev. B 90, 245101 (2014).
Koch, C. Determination of core structure periodicity and point defect density along dislocations. Dissertation, Arizona State University (2002). https://www.physik.hu-berlin.de/en/sem/images/koch02_phdthesis.pdf
Sementa, L. & Fortunelli, A. DFT global minimization of Pt-based nanoclusters. Zenodo https://doi.org/10.5281/zenodo.11174993 (2024).
Acknowledgements
B.P., Z.L., Q.J., Z.Z., J.H., X.D. and Y.H. gratefully acknowledge the Office of Naval Research (ONR) grant N00014-18-1-2155 for initial studies. Z.L. also acknowledges support from the UCLA Dissertation Year Fellowship. A.F. and L.S. gratefully acknowledge computational support from the Cineca Supercomputing Center (Italy). We acknowledge the use of facilities in the Irvine Materials Research Institute (IMRI) at the University of California Irvine. We also thank the Electron Imaging Center of Nanomachines at CNSI for TEM support. We acknowledge the training and help from I. Martini for the XPS data collection at the UCLA Molecular Instrumentation Center. The XAS data were collected at beamlines 6-BM and 8-ID (ISS) of the National Synchrotron Light Source II, a DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. Networking within the COST Action CA21101 ‘Confined molecular systems: from a new generation of materials to the stars’ (COSY) supported by COST (European Cooperation in Science and Technology) is also acknowledged.
Author information
Authors and Affiliations
Contributions
Research design and conceptualization was by Y.H. and X.D. Experimental design and execution was by B.P. and Z.L. Synthesis of the electrocatalysts and structural characterization was carried out by B.P., Z.L. and Y.-H.T. MEA fabrication and electrochemical testing was performed by B.P., Z.L. and Z.Z. S/TEM and EDS characterization was by M.X., Z.L., B.P. and J.H. STEM-EELS characterization was performed by X.Y. XAS data collection and analyses were conducted by Q.S., Q.J., C.U.S. and E.L. Modelling and data analyses were by L.S. and A.F. Supervision was by Y.H. (experimental design and MEA studies), X.P. (microscopic characterization) and A.F. (simulation studies) Writing (original draught) was by B.P., X.D. and Y.H. Writing (reviewing and editing) was by B.P., Z.L., A.F., L.S., X.D. and Y.H.
Corresponding authors
Ethics declarations
Competing interests
Y.H., X.D., B.P. and Z.L. are inventors on patents (US Provisional Application No. 63/580,271) relating to the developed catalysts in this study filed by the University of California, Los Angeles. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Jasna Jankovic and Tej S. Choksi for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–32 and Tables 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, B., Liu, Z., Sementa, L. et al. Embedded oxide clusters stabilize sub-2 nm Pt nanoparticles for highly durable fuel cells. Nat Catal 7, 818–828 (2024). https://doi.org/10.1038/s41929-024-01180-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-024-01180-x
This article is cited by
-
Pt catalyst protected by graphene nanopockets enables lifetimes of over 200,000 h for heavy-duty fuel cell applications
Nature Nanotechnology (2025)
-
Intrinsic metal-support interactions break the activity-stability dilemma in electrocatalysis
Nature Communications (2025)
-
Artificial-intelligence-guided design of ordered gas diffusion layers for high-performing fuel cells via Bayesian machine learning
Nature Communications (2025)
-
Ionomer distribution control via thiophene S-modification of carbon support for high-power proton exchange membrane fuel cells
Nature Communications (2025)
-
Regulating orbital interaction to construct quasi-covalent bond networks in Pt intermetallic alloys for high-performance fuel cells
Nature Communications (2025)