Abstract
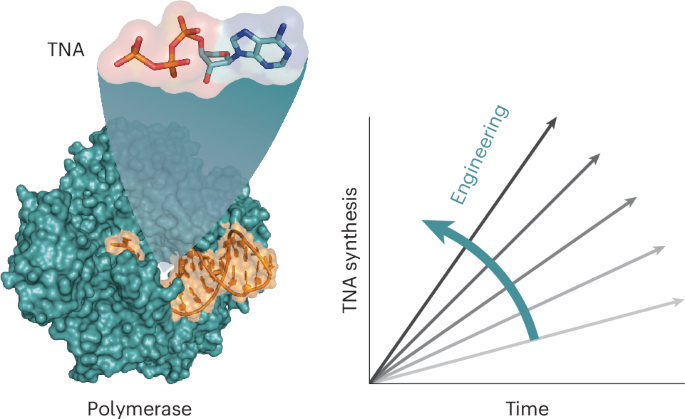
Reprogramming DNA polymerases to synthesize xeno-nucleic acids (XNAs) is an important challenge that tests current enzyme engineering tools. Here we describe an evolutionary campaign aimed at generating an XNA polymerase that can efficiently make α-l-threofuranosyl nucleic acid (TNA)—an artificial genetic polymer that is recalcitrant to nucleases and resistant to acid-mediated degradation. Starting from a homologous recombination library, iterative cycles of selection were performed to traverse the fitness landscape in search of neutral mutations with increased evolutionary potential. Subsequent directed evolution of focused mutagenic libraries yielded 10–92, a newly engineered TNA polymerase that functions with a catalytic rate of ∼1 nt s−1 and >99% fidelity. A crystal structure of the closed ternary complex reveals the degree of structural change required to remodel the active site pocket for improved TNA synthesis activity. Together, these data demonstrate the importance of recombination as a strategy for evolving XNA polymerases with considerable practical value for biotechnology and medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The crystal structure of 10–92 was deposited in the Protein Data Bank [PDB:8T3X]. The repository furthermore contains PDB structures of all ensemble refinements presented in this work. Other data are available in the main text, Supplementary Information or from the authors upon reasonable request. Source data are provided with this paper.
References
Nikoomanzar, A., Chim, N., Yik, E. J. & Chaput, J. C. Engineering polymerases for applications in synthetic biology. Q. Rev. Biophys. 53, e8 (2020).
Houlihan, G., Arangundy-Franklin, S. & Holliger, P. Engineering and application of polymerases for synthetic genetics. Curr. Opin. Biotechnol. 48, 168–179 (2017).
Aschenbrenner, J. & Marx, A. DNA polymerases and biotechnological applications. Curr. Opin. Biotechnol. 48, 187–195 (2017).
Tunyasuvunakool, K. et al. Highly accurate protein structure prediction for the human proteome. Nature 596, 590–596 (2021).
Wang, J. et al. Scaffolding protein functional sites using deep learning. Science 377, 387–394 (2022).
Chim, N., Meza, R. A., Trinh, A. M., Yang, K. & Chaput, J. C. Following replicative DNA synthesis by time-resolved X-ray crystallography. Nat. Commun. 12, 2641 (2021).
Doublie, S., Tabor, S., Long, A. M., Richardson, C. C. & Ellenberger, T. Crystal structure of a bacteriophage T7 DNA replication complex at 2.2 Å resolution. Nature 391, 251–258 (1998).
Larsen, A. C. et al. A general strategy for expanding polymerase function by droplet microfluidics. Nat. Commun. 7, 11235 (2016).
Nikoomanzar, A., Vallejo, D. & Chaput, J. C. Elucidating the determinants of polymerase specificity by microfluidic-based deep mutational scanning. ACS Synth. Biol. 8, 1421–1429 (2019).
Nikoomanzar, A., Vallejo, D., Yik, E. J. & Chaput, J. C. Programmed allelic mutagenesis of a DNA polymerase with single amino acid resolution. ACS Synth. Biol. 9, 1873–1881 (2020).
Pinheiro, V. B. et al. Synthetic genetic polymers capable of heredity and evolution. Science 336, 341–344 (2012).
Hoshino, H., Kasahara, Y., Kuwahara, M. & Obika, S. DNA polymerase variants with high processivity and accuracy for encoding and decoding locked nucleic acid sequences. J. Am. Chem. Soc. 142, 21530–21537 (2020).
Liu, C. et al. Phosphonomethyl oligonucleotides as backbone-modified artificial genetic polymers. J. Am. Chem. Soc. 140, 6690–6699 (2018).
Kuwahara, M. et al. Systematic analysis of enzymatic DNA polymerization using oligo-DNA templates and triphosphate analogs involving 2′,4′-bridged nucleosides. Nucleic Acids Res. 36, 4257–4265 (2008).
Loakes, D., Gallego, J., Pinheiro, V. B., Kool, E. T. & Holliger, P. Evolving a polymerase for hydrophobic base analogues. J. Am. Chem. Soc. 131, 14827–14837 (2009).
Ramsay, N. et al. CyDNA: synthesis and replication of highly Cy-dye substituted DNA by an evolved polymerase. J. Am. Chem. Soc. 132, 5096–5104 (2010).
Chen, T. et al. Evolution of thermophilic DNA polymreases for the recognition and amplification of C2′-modified DNA. Nat. Chem. 8, 556–562 (2016).
Freund, N. et al. A two-residue nascent-strand steric gate controls synthesis of 2′-O-methyl- and 2′-O-(2-methoxyethyl)-RNA. Nat. Chem. 15, 91–100 (2023).
Lelyveld, V. S., Fang, Z. & Szostak, J. W. Trivalent rare earth metal cofactors confer rapid NP-DNA polymerase activity. Science 382, 423–429 (2023).
Arangundy-Franklin, S. et al. A synthetic genetic polymer with an uncharged backbone chemistry based on alkyl phosphonate nucleic acids. Nat. Chem. 11, 533–542 (2019).
Chim, N., Shi, C., Sau, S. P., Nikoomanzar, A. & Chaput, J. C. Structural basis for TNA synthesis by an engineered TNA polymerase. Nat. Commun. 8, 1810 (2017).
Crameri, A., Raillard, S.-A., Bermudez, E. & Stemmer, W. P. C. DNA shuffling of a family of genes from diverse species accelerates directed evolution. Nature 391, 288–291 (1998).
Ness, J. E. et al. Synthetic shuffling expands functional protein diversity by allowing amino acids to recombine independently. Nat. Biotechnol. 20, 1251–1255 (2002).
Drummond, D. A., Silberg, J. J., Meyer, M. M., Wilke, C. O. & Arnold, F. H. On the conservative nature of intragenic recombination. Proc. Natl Acad. Sci. USA 102, 5380–5385 (2005).
d’Abbadie, M. et al. Molecular breeding of polymerases for amplification of ancient DNA. Nat. Biotechnol. 25, 939–943 (2007).
Baar, C. et al. Molecular breeding of polymerases for resistance to environmental inhibitors. Nucleic Acids Res. 39, e51 (2011).
Leconte, A. M. et al. Directed evolution of DNA polymerases for next-generation sequencing. Angew. Chem. Int. Ed. Engl. 49, 5921–5924 (2010).
Schöning, K. U. et al. Chemical etiology of nucleic acid structure: the α-threofuranosyl-(3′ → 2′) oligonucleotide system. Science 290, 1347–1351 (2000).
Eschenmoser, A. Chemical etiology of nucleic acid structure. Science 284, 2118–2124 (1999).
Culbertson, M. C. et al. Evaluating TNA stability under simulated physiological conditions. Bioorg. Med. Chem. Lett. 26, 2418–2421 (2016).
Lee, E. M., Setterholm, N. A., Hajjar, M., Barpuzary, B. & Chaput, J. C. Stability and mechanism of threose nucleic acid toward acid-mediated degradation. Nucleic Acids Res. 51, 9542–9551 (2023).
Dunn, M. R., Jimenez, R. M. & Chaput, J. C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 1, 0076 (2017).
Yu, H., Zhang, S. & Chaput, J. C. Darwinian evolution of an alternative genetic system provides support for TNA as an RNA progenitor. Nat. Chem. 4, 183–187 (2012).
Dunn, M. R., McCloskey, C. M., Buckley, P., Rhea, K. & Chaput, J. C. Generating biologically stable TNA aptamers that function with high affinity and thermal stability. J. Am. Chem. Soc. 142, 7721–7724 (2020).
McCloskey, C. M. et al. Evolution of functionally enhanced α-l-threofuranosyl nucleic acid aptamers. ACS Synth. Biol. 10, 3190–3199 (2021).
Li, X., Li, Z. & Yu, H. Selection of threose nucleic acid aptamers to block PD-1/PD-L1 interaction for cancer immunotherapy. Chem. Commun. 56, 14653–14656 (2020).
Wang, Y. et al. An RNA-cleaving threose nucleic acid enzyme capable of single point mutation discrimination. Nat. Chem. 14, 350–359 (2022).
Wang, Y. et al. A threose nucleic acid enzyme with RNA ligase activity. J. Am. Chem. Soc. 143, 8154–8163 (2021).
Yang, K., McCloskey, C. M. & Chaput, J. C. Reading and writing digital information in TNA. ACS Synth. Biol. 9, 2936–2942 (2020).
Wang, Y., Nguyen, K., Spitale, R. C. & Chaput, J. C. A biologically stable DNAzyme that efficiently silences gene expression in cells. Nat. Chem. 13, 319–326 (2021).
Wang, F. et al. Synthetic α-l-threose nucleic acids targeting BcL-2 show gene silencing and in vivo antitumor activity for cancer therapy. ACS Appl. Mater. Interfaces 11, 38510–38518 (2019).
Shigeo, M. et al. Shorter is better: the α-(l)-threofuranosyl nucleic acid modification improves stability, potency, safety, and Ago2 binding and mitigates off-target effects of small interfering RNAs. J. Am. Chem. Soc. 145, 19691–19706 (2023).
Gould, S. J. Wonderful Life: The Burgess Shale and the Nature of History, 1st edn (W.W. Norton, 1989).
Hottin, A. & Marx, A. Structural insights into the processing of nucleobase-modified nucleotides by DNA polymerases. Acc. Chem. Res. 49, 418–427 (2016).
Dunn, M. R., Otto, C., Fenton, K. E. & Chaput, J. C. Improving polymerase activity with unnatural substrates by sampling mutations in homologous protein architectures. ACS Chem. Biol. 11, 1210–1219 (2016).
Yik, E. J., Maola, V. A. & Chaput, J. C. Engineering TNA polymerases through iterative cycles of directed evolution. Methods Enzymol. 691, 29–59 (2023).
Minshull, J. & Stemmer, W. P. Protein evolution by molecular breeding. Curr. Opin. Chem. Biol. 3, 284–290 (1999).
Vallejo, D., Nikoomanzar, A., Paegel, B. M. & Chaput, J. C. Fluorescence-activated droplet sorting for single-cell directed evolution. ACS Synth. Biol. 8, 1430–1440 (2019).
Liao, J.-Y., Bala, S., Ngor, A. K., Yik, E. J. & Chaput, J. C. P(V) reagents for the scalable synthesis of natural and modified nucleoside triphosphates. J. Am. Chem. Soc. 141, 13286–13289 (2019).
Mei, H. et al. Synthesis and evolution of a threose nucleic acid aptamer bearing 7-deaza-7-substituted guanosine residues. J. Am. Chem. Soc. 140, 5706–5713 (2018).
Nikoomanzar, A., Dunn, M. R. & Chaput, J. C. Evaluating the rate and substrate specificity of laboratory evolved XNA polymerases. Anal. Chem. 89, 12622–12625 (2017).
Goodman, M. F., Creighton, S., Bloom, L. B. & Petruska, J. Biochemical basis of DNA replication fidelity. Crit. Rev. Biochem. Mol. Biol. 28, 83–126 (1993).
Kundu, N., McCloskey, C. M., Hajjar, M. & Chaput, J. C. Parameterizing the binding properties of XNA aptamers isolated from a low stringency selection. Biochemistry 62, 3245–3254 (2023).
Lozoya-Colinas, A., Yu, Y. & Chaput, J. C. Functionally enhanced XNA aptamers discovered by parallelized library screening. J. Am. Chem. Soc. 145, 25789–25796 (2023).
Bala, S. et al. Synthesis of 2′-deoxy-α-l-threofuranosyl nucleoside triphosphates. J. Org. Chem. 83, 8840–8850 (2018).
Rodriguez, A. C., Park, H.-W., Mao, C. & Beese, L. S. Crystal structure of a Pol A family DNA polymerase from the hyperthermophilic Archaeon Thermococcus sp. 9°N-7. J. Mol. Biol. 299, 447–462 (2000).
Kropp, H. M., Betz, K., Wirth, J., Diederichs, K. & Marx, A. Crystal structures of ternary complexes of archaeal B-family DNA polymerases. PLoS One 12, e0188005 (2017).
Wang, Y., Ngor, A. K., Nikoomanzar, A. & Chaput, J. C. Evolution of a general RNA-cleaving FANA enzyme. Nat. Commun. 9, 5067 (2018).
Chaput, J. C. & Szostak, J. W. TNA synthesis by DNA polymerases. J. Am. Chem. Soc. 125, 9274–9275 (2003).
Vastmans, K. et al. Enzymatic incorporation in DNA of 1,5-anhydrohexitol nucleotides. Biochemistry 39, 12757–12765 (2000).
Kool, E. T. Active site tightness and substrate fit in DNA replication. Annu. Rev. Biochem. 71, 191–219 (2002).
Steitz, T. A., Smerdon, S. J., Jager, J. & Joyce, C. M. A unified polymerase mechanism for nonhomologous DNA and RNA polymerases. Science 266, 2022–2025 (1994).
Hogrefe, H. H., Cline, J., Lovejoy, A. E. & Nielson, K. B. DNA polymerases from hyperthermophiles. Methods Enzymol. 334, 91–116 (2001).
Sau, S. P., Fahmi, N. E., Liao, J.-Y., Bala, S. & Chaput, J. C. A scalable synthesis of α-l-threose nucleic acid monomers. J. Org. Chem. 81, 2302–2307 (2016).
Vallejo, D., Nikoomanzar, A. & Chaput, J. C. Directed evolution of custom polymerases using droplet microfluidics. Methods Enzymol. 644, 227–253 (2020).
Nikoomanzar, A., Dunn, M. R. & Chaput, J. C. Engineered polymerases with altered substrate specificity: expression and purification. Curr. Protoc. Nucleic Acid Chem. 69, 4.75 (2017).
Cadwell, R. C. & Joyce, G. F. Randomization of genes by PCR mutagenesis. PCR Methods Appl. 2, 28–33 (1992).
Chaput, J. C. & Szostak, J. W. Evolutionary optimization of a nonbiological ATP binding protein for improved folding stability. Chem. Biol. 11, 865–874 (2004).
Li, Q. et al. Synthesis and polymerase recognition of threose nucleic acid triphosphates equipped with diverse chemical functionalities. J. Am. Chem. Soc. 143, 17761–17768 (2021).
Medina, E., Yik, E. J., Herdewijn, P. & Chaput, J. C. Functional comparison of laboratory-evolved XNA polymerases for synthetic biology. ACS Synth. Biol. 10, 1429–1437 (2021).
Kabsch, W. Xds. Acta Crystallogr. D 66, 125–132 (2010).
Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D 66, 133–144 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D 68, 352–367 (2012).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
PyMOL version 2.5.2 (DeLano Scientific, 2002).
Acknowledgements
This work was supported by a grant to J.C.C. from the NSF (MCB 1946312).
Author information
Authors and Affiliations
Contributions
J.C.C., V.A.M. and E.J.Y. conceived the project and designed the experiments. E.J.Y. and V.A.M. evolved the enzyme. E.J.Y., V.A.M. and M.H. performed the biochemical characterization. V.A.M., J.J.L., M.H., M.J.H., R.N.Q., K.K.N., K.L.H., J.V.M. and N.C. contributed to protein structure determination. J.C.C. wrote the paper. All authors reviewed and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
J.C.C., V.A.M., E.J.Y. and the University of California, Irvine have filed a patent application (PCT/US24/11595) on the composition and activity of the 10–92 TNA polymerase. The other authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Masayasu Kuwahara, Vitor Pinheiro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–5, Figs. 1–19, and uncropped gels for Supplementary Figs. 2, 5, 8, 9, 10, 13–15.
Source data
Source Data Fig. 3
Unprocessed Urea-PAGE gel.
Source Data Fig. 4
Kinetics raw and processed data.
Source Data Fig. 5
Unprocessed Urea-PAGE gel.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maola, V.A., Yik, E.J., Hajjar, M. et al. Directed evolution of a highly efficient TNA polymerase achieved by homologous recombination. Nat Catal 7, 1173–1185 (2024). https://doi.org/10.1038/s41929-024-01233-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-024-01233-1
This article is cited by
-
Directed evolution of a TNA polymerase identifies independent paths to fidelity and catalysis
Nature Communications (2025)