Abstract
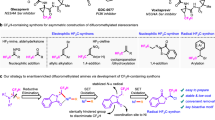
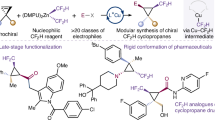
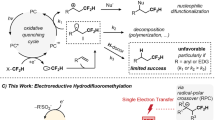
Stereochemically controlled hydrogen bond donors play essential roles in the pharmaceutical industry. Consequently, organic molecules that bear difluoromethyl (CF2H) groups at chiral centres are emerging as pivotal components in pharmaceuticals owing to their distinct hydrogen-bonding property. However, a general approach for introducing CF2H groups in an enantioselective manner has remained elusive. Here we show that enantioconvergent difluoromethylation of racemic alkyl electrophiles, through alkyl radical intermediates, represents a strategy for constructing CF2H-containing stereocentres. This strategy is enabled by using copper catalysts bound with a chiral diamine ligand bearing electron-deficient phenyl groups, and a nucleophilic CF2H-zinc reagent. This method allows the high-yield conversion of a diverse range of alkyl halides into their alkyl-CF2H analogues with excellent enantioselectivity. Mechanistic studies reveal a route involving asymmetric difluoromethylation of alkyl radicals and crucial non-covalent interactions in the enantiodetermining steps. This copper-catalysed difluoromethylation process opens an avenue for the efficient preparation of CF2H-containing pharmaceuticals.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data generated in this study are provided in Supplementary Information and related files provided with this paper. Data are also available from the corresponding author upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers 2300997 and 2303333. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Kenny, P. W. Hydrogen-bond donors in drug design. J. Med. Chem. 65, 14261–14275 (2022).
Zafrani, Y. et al. Difluoromethyl bioisostere: examining the ‘lipophilic hydrogen bond donor’ concept. J. Med. Chem. 60, 797–804 (2017).
Zafrani, Y. et al. CF2H, a functional group-dependent hydrogen-bond donor: is it a more or less lipophilic bioisostere of OH, SH, and CH3? J. Med. Chem. 62, 5628–5637 (2019).
Muller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Hanan, E. J. et al. Discovery of GDC-0077 (Inavolisib), a highly selective inhibitor and degrader of mutant PI3Kα. J. Med. Chem. 65, 16589–16621 (2022).
Zhao, J. et al. Preclinical safety and efficacy characterization of an LpxC inhibitor against Gram-negative pathogens. Science Transl. Med. 15, eadf5668 (2023).
Sap, J. B. I. et al. Late-stage difluoromethylation: concepts, developments and perspective. Chem. Soc. Rev. 50, 8214–8247 (2021).
Briand, M., Anselmi, E., Dagousset, G. & Magnier, E. The revival of enantioselective perfluoroalkylation—update of new synthetic approaches from 2015–2022. Chem. Rec. 23, e202300114 (2023).
Yang, X. Y., Wu, T., Phipps, R. J. & Toste, F. D. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 115, 826–870 (2015).
Aikawa, K., Yoshida, S., Kondo, D., Asai, Y. & Mikami, K. Catalytic asymmetric synthesis of tertiary alcohols and oxetenes bearing a difluoromethyl group. Org. Lett. 17, 5108–5111 (2015).
Liu, Y.-L. et al. Organocatalytic asymmetric strecker reaction of di- and trifluoromethyl ketoimines. Remarkable fluorine effect. Org. Lett. 13, 3826–3829 (2011).
Middleton, W. J. New fluorinating reagents. Dialkylaminosulfur fluorides. J. Org. Chem. 40, 574–578 (1975).
Xu, Y. & Prestwich, G. D. Concise synthesis of acyl migration-blocked 1,1-difluorinated analogues of lysophosphatidic acid. J. Org. Chem. 67, 7158–7161 (2002).
Ni, C., Wang, F. & Hu, J. Enantioselective nucleophilic difluoromethylation of aromatic aldehydes with Me3SiCF2SO2Ph and PhSO2CF2H reagents catalyzed by chiral quaternary ammonium salts. Beilstein J. Org. Chem. 4, 21 (2008).
Zhao, Y., Huang, W., Zheng, J. & Hu, J. Efficient and direct nucleophilic difluoromethylation of carbonyl compounds and Imines with Me3SiCF2H at ambient or low temperature. Org. Lett. 13, 5342–5345 (2011).
Peng, L., Wang, H. & Guo, C. Copper-catalyzed enantioselective difluoromethylation of amino acids via difluorocarbene. J. Am. Chem. Soc. 143, 6376–6381 (2021).
Gu, Y., Lu, C., Gu, Y. & Shen, Q. Ligand-controlled copper-catalyzed highly regioselective difluoromethylation of allylic chlorides/bromides and propargyl bromides. Chin. J. Chem. 36, 55–58 (2018).
Endo, Y., Ishii, K. & Mikami, K. Chiral copper-catalyzed enantioselective Michael difluoromethylation of arylidene Meldrum’s acids with (difluoromethyl)zinc reagents. Tetrahedron 75, 4099–4103 (2019).
Gao, X., Cheng, R., Xiao, Y.-L., Wan, X.-L. & Zhang, X. Copper-catalyzed highly enantioselective difluoroalkylation of secondary propargyl sulfonates with difluoroenoxysilanes. Chem 5, 2987–2999 (2019).
Rong, M.-Y., Li, J.-S., Zhou, Y., Zhang, F.-G. & Ma, J.-A. Catalytic enantioselective synthesis of difluoromethylated tetrasubstituted stereocenters in isoindolones enabled by a multiple-fluorine system. Org. Lett. 22, 9010–9015 (2020).
Wang, Y. et al. Asymmetric α-electrophilic difluoromethylation of β-keto esters by phase transfer catalysis. Org. Biomol. Chem. 19, 4788–4795 (2021).
Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl–alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Wang, F., Chen, P. & Liu, G. Copper-catalyzed radical relay for asymmetric radical transformations. Acc. Chem. Res. 51, 2036–2046 (2018).
Gu, Q.-S., Li, Z.-L. & Liu, X.-Y. Copper(I)-catalyzed asymmetric reactions involving radicals. Acc. Chem. Res. 53, 170–181 (2020).
Chen, C. & Fu, G. C. Copper-catalysed enantioconvergent alkylation of oxygen nucleophiles. Nature 618, 301–307 (2023).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C–N cross-couplings induced by visible light. Science 351, 681–684 (2016).
Chen, C., Peters, J. C. & Fu, G. C. Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity. Nature 596, 250–256 (2021).
Chen, J.-J. et al. Enantioconvergent Cu-catalysed N-alkylation of aliphatic amines. Nature 618, 294–300 (2023).
Zhao, X. & MacMillan, D. W. C. Metallaphotoredox perfluoroalkylation of organobromides. J. Am. Chem. Soc. 142, 19480–19486 (2020).
Kornfilt, D. J. P. & MacMillan, D. W. C. Copper-catalyzed trifluoromethylation of alkyl bromides. J. Am. Chem. Soc. 141, 6853–6858 (2019).
Shen, H. et al. Trifluoromethylation of alkyl radicals in aqueous solution. J. Am. Chem. Soc. 139, 9843–9846 (2017).
Zeng, X. J. et al. Copper-catalyzed decarboxylative difluoromethylation. J. Am. Chem. Soc. 141, 11398–11403 (2019).
Cai, A., Yan, W. & Liu, W. Aryl radical activation of C–O bonds: copper-catalyzed deoxygenative difluoromethylation of alcohols. J. Am. Chem. Soc. 143, 9952–9960 (2021).
Zeng, X. et al. Copper-catalyzed deaminative difluoromethylation. Angew. Chem. Int. Ed. 59, 16398–16403 (2020).
Cai, A., Yan, W., Wang, C. & Liu, W. Copper-catalyzed difluoromethylation of alkyl iodides enabled by aryl radical activation of carbon–iodine bonds. Angew. Chem. Int. Ed. 60, 27070–27077 (2021).
Mao, E., Prieto Kullmer, C. N., Sakai, H. A. & MacMillan, D. W. C. Direct bioisostere replacement enabled by metallaphotoredox deoxydifluoromethylation. J. Am. Chem. Soc. 146, 5067–5073 (2024).
Fier, P. S. & Hartwig, J. F. Copper-mediated difluoromethylation of aryl and vinyl iodides. J. Am. Chem. Soc. 134, 5524–5527 (2012).
Bour, J. R., Kariofillis, S. K. & Sanford, M. S. Synthesis, reactivity, and catalytic applications of isolable (NHC)Cu(CHF2) complexes. Organometallics 36, 1220–1223 (2017).
Zhao, H., Leng, X. B., Zhang, W. & Shen, Q. [Ph4P]+[Cu(CF2H)2]−: a powerful difluoromethylating reagent inspired by mechanistic investigation. Angew. Chem. Int. Ed. 61, e202210151 (2022).
Jiang, C. et al. Enantioselective copper-catalyzed trifluoromethylation of benzylic radicals via ring opening of cyclopropanols. Chem 6, 2407–2419 (2020).
Xu, P., Fan, W., Chen, P. & Liu, G. Enantioselective radical trifluoromethylation of benzylic C–H bonds via cooperative photoredox and copper catalysis. J. Am. Chem. Soc. 144, 13468–13474 (2022).
Xu, L. & Vicic, D. A. Direct difluoromethylation of aryl halides via base metal catalysis at room temperature. J. Am. Chem. Soc. 138, 2536–2539 (2016).
Serizawa, H., Ishii, K., Aikawa, K. & Mikami, K. Copper-catalyzed difluoromethylation of aryl iodides with (difluoromethyl)zinc reagent. Org. Lett. 18, 3686–3689 (2016).
Chidambaram, R. et al. A practical synthesis of the RARγ agonist, BMS-270394. Org. Process. Res. Dev. 6, 632–636 (2002).
Klaholz, B. P., Mitschler, A., Belema, M., Zusi, C. & Moras, D. Enantiomer discrimination illustrated by high-resolution crystal structures of the human nuclear receptor hRARγ. Proc. Natl Acad. Sci. USA 97, 6322–6327 (2000).
Ogawa, Y., Tokunaga, E., Kobayashi, O., Hirai, K. & Shibata, N. Current contributions of organofluorine compounds to the agrochemical industry. iScience 23, 101467 (2020).
Foster, R. J. & Carr, R. A. in Analytical Profiles of Drug Substances Vol. 19 (ed. Florey, K.) 1–26 (Academic Press,1990).
Dighiero, G. et al. Chlorambucil in indolent chronic lymphocytic leukemia. N. Engl. J. Med. 338, 1506–1514 (1998).
Jacquelynn, J. C. et al. Acute γ-secretase inhibition of nonhuman primate CNS shifts amyloid precursor protein (APP) metabolism from amyloid-β production to alternative APP fragments without amyloid-β rebound. J. Neurosci. 30, 6743–6750 (2010).
Bhat, V., Welin, E. R., Guo, X. & Stoltz, B. M. Advances in stereoconvergent catalysis from 2005 to 2015: transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations. Chem. Rev. 117, 4528–4561 (2017).
Luo, Y. et al. Oxidative addition of an alkyl halide to form a stable Cu(III) product. Science 381, 1072–1079 (2023).
Yan, W. et al. Catalytically relevant organocopper(III) complexes formed through aryl-radical-enabled oxidative addition. J. Am. Chem. Soc. 146, 15176–15185 (2024).
Liu, S. et al. C(sp3)-CF3 reductive elimination from a five-coordinate neutral copper(III) complex. J. Am. Chem. Soc. 142, 9785–9791 (2020).
Paeth, M. et al. Csp3–Csp3 bond-forming reductive elimination from well-defined copper(III) complexes. J. Am. Chem. Soc. 141, 3153–3159 (2019).
Liu, J.-R., Xu, G.-X., Liu, L.-G., Zhang, S.-Q. & Hong, X. Recent advances in theoretical studies on Cu-mediated bond formation mechanisms involving radicals. ACS Catal. 14, 2429–2454 (2024).
Lu, T. & Chen, Q. Interaction region indicator: a simple real space function clearly revealing both chemical bonds and weak interactions. Chem. Methods 1, 231–239 (2021).
Acknowledgements
This work was supported by the National Institute of General Medical Science (R35GM146765). Mechanistic studies were supported by National Science Foundation under grant CHE-2237757. W.L. also thanks the ACS Herman Frasch Foundation (926-HF22) for the financial support. NMR experiments were performed using a Bruker AVANCE NEO 400 MHz NMR spectrometer, funded by NSF-MRI grant CHE-1726092. Funding for the D8 Venture diffractometer was through NSF-MRI grant CHE-1625737.
Author information
Authors and Affiliations
Contributions
D.D. and W.L. designed experiments. D.D. and L.Y. performed the synthetic experiments and prepared the supplementary information. A.T.P., S.C.Y., R.M.K. and S.T. performed EPR experiments. M.-J.C., Y.-H.C. and C.-T.H. conducted DFT calculations. J.A.K. performed X-ray diffraction analysis. W.L. conceived and supervised the project. W.L. wrote this manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Al Postigo, Nigam Rath, Qilong Shen, Beatrice Tuccio-Lauricella and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–396, Tables 1–17, Notes, Methods and References.
Supplementary Data 1
Crystallographic data for compound 24.
Supplementary Data 2
Crystallographic Information File check report for compound 24.
Supplementary Data 3
Crystallographic data for compound L*Cu(OAc)2.
Supplementary Data 4
Crystallographic Information File check report for compound compound L*Cu(OAc)2.
Supplementary Data 5
Tables of energy and coordinates for optimized structures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, D., Yin, L., Poore, A.T. et al. Enantioconvergent copper-catalysed difluoromethylation of alkyl halides. Nat Catal 7, 1372–1381 (2024). https://doi.org/10.1038/s41929-024-01253-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-024-01253-x
This article is cited by
-
Dual transition metal electrocatalysis for difluoromethylation of Aryl halides using potassium difluoroacetate
Nature Communications (2025)
-
Synthesis of chiral difluoromethyl cyclopropanes through desymmetric difluoromethylation of cyclopropenes enabled by enantioselective copper catalysis
Nature Synthesis (2025)