Abstract
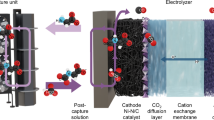
Post-combustion CO2 capture with amines offers an almost ready-to-use capture technology to assist in the transition towards net-zero carbon emission. However, the technology suffers from a high regeneration cost due to the high process temperatures involved. Utilization of catalysts in the regeneration process was reported to be an elegant solution to lower process temperatures while maintaining high reaction kinetics. Earlier studies were performed under batch conditions and therefore lack practical validation, and a deeper mechanistic understanding of the catalysis is also missing. This study introduces a practical-to-synthesize, highly efficient, stable and recyclable ZrOxHy solid catalyst, showing high catalytic CO2 desorption rates for most common aqueous amine solutions. Kinetic and ex situ/in situ spectroscopic data reveal a proximity-independent acid–base synergistic mechanism between two catalytic cycles. The approach was validated in a fixed-bed continuous reactor, demonstrating sensible contact time shortening (up to 85%), suggesting considerable potential savings in regeneration energy, reactor construction and amine solvent cost.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting findings of this study are available within the paper and its Supplementary Information, or from the authors on reasonable request.
References
United Nations Framework Convention on Climate Change. The Paris Agreement. https://unfccc.int/process-and-meetings/the-paris-agreement (UM, 2015).
Energy Technology Perspectives 2020. https://www.iea.org/reports/energy-technology-perspectives-2020 (IEA, 2020).
Further Assessment of Emerging CO2 Capture Technologies for the Power Sector and their Potential to Reduce Costs (IEAGHG, 2019).
Danaci, D., Bui, M., Petit, C. & MacDowell, N. En route to zero emissions for power and industry with amine-based post-combustion capture. Environ. Sci. Technol. 55, 10619–10632 (2021).
Plaza, M. G., Martínez, S. & Rubiera, F. CO2 capture, use, and storage in the cement industry: state of the art and expectations. Energies 13, 5692 (2020).
Rao, A. B. & Rubin, E. S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 36, 4467–4475 (2002).
Yu, C. H., Huang, C. H. & Tan, C. S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 12, 745–769 (2012).
Rao, A. B., Rubin, E. S., Keith, D. W. & Granger Morgan, M. Evaluation of potential cost reductions from improved amine-based CO2 capture systems. Energy Policy 34, 3765–3772 (2006).
Aghel, B., Janati, S., Wongwises, S. & Safdari, M. Review on CO2 capture by blended amine solutions. Int. J. Greenh. Gas Control 119, 103715 (2022).
Rezazadeh, F., Gale, W. F., Lin, Y. J. & Rochelle, G. T. Energy performance of advanced reboiled and flash stripper configurations for CO2 capture using monoethanolamine. Ind. Eng. Chem. Res. 55, 4622–4631 (2016).
Rochelle, G. T. et al. Pilot plant demonstration of piperazine with the advanced flash stripper. Int. J. Greenh. Gas Control 84, 72–81 (2019).
Oyenekan, B. A. & Rochelle, G. T. Performance of Innovative Stripper Options for CO2 Capture (American Institute of Chemical Engineers, 2005).
Khalifa, O. et al. Modifying absorption process configurations to improve their performance for post-combustion CO2 capture—what have we learned and what is still missing? Chem. Eng. J. 430, 133096 (2022).
Wang, Z., Fang, M., Pan, Y., Yan, S. & Luo, Z. Amine-based absorbents selection for CO2 membrane vacuum regeneration technology by combined absorption–desorption analysis. Chem. Eng. Sci. 93, 238–249 (2013).
Machida, H., Esaki, T., Esaki, T., Yamaguchi, T. & Norinaga, K. Energy-saving CO2 capture by H2 gas stripping for integrating CO2 separation and conversion processes. ACS Sustain. Chem. Eng. 8, 8732–8740 (2020).
Chowdhury, F. A., Okabe, H., Shimizu, S., Onoda, M. & Fujioka, Y. Development of novel tertiary amine absorbents for CO2 capture. Energy Procedia 1, 1241–1248 (2009).
Chowdhury, F. A., Okabe, H., Yamada, H., Onoda, M. & Fujioka, Y. Synthesis and selection of hindered new amine absorbents for CO2 capture. Energy Procedia 4, 201–208 (2011).
Singto, S. et al. Synthesis of new amines for enhanced carbon dioxide (CO2) capture performance: the effect of chemical structure on equilibrium solubility, cyclic capacity, kinetics of absorption and regeneration, and heats of absorption and regeneration. Sep. Purif. Technol. 167, 97–107 (2016).
Alivand, M. S. et al. Catalytic solvent regeneration for energy-efficient CO2 capture. ACS Sustain. Chem. Eng. 8, 18755–18788 (2020).
Idem, R., Shi, H., Gelowitz, D. & Tontiwachwuthikul, P. Catalytic method and apparatus for separating a gas component from an incoming gas stream. WO patent 2011/120138 (2011).
Zhou, C. et al. A critical revisit of zeolites for CO2 desorption in primary amine solution argues its genuine catalytic function. ACS Catal. 12, 11485–11493 (2022).
Liu, H. et al. Investigation of CO2 regeneration in single and blended amine solvents with and without catalyst. Ind. Eng. Chem. Res. 56, 7656–7664 (2017).
Zhang, X. et al. Reducing energy penalty of CO2 capture using Fe promoted SO42-/ZrO2/MCM-41 catalyst. Environ. Sci. Technol. 53, 6094–6102 (2019).
Prasongthum, N., Natewong, P., Reubroycharoen, P. & Idem, R. Solvent regeneration of a CO2-loaded BEA-AMP bi-blend amine solvent with the aid of a solid Brønsted Ce(SO4)2/ZrO2 superacid catalyst. Energy Fuels 33, 1334–1343 (2019).
Bairq, Z. A. S., Gao, H., Murshed, F. A. M., Tontiwachwuthikul, P. & Liang, Z. Modified heterogeneous catalyst-aided regeneration of CO2 capture amines: a promising perspective for a drastic reduction in energy consumption. ACS Sustain. Chem. Eng. 8, 9526–9536 (2020).
Gao, H. et al. Catalytic performance and mechanism of SO42−/ZrO2/SBA-15 catalyst for CO2 desorption in CO2-loaded monoethanolamine solution. Appl. Energy 259, 114179 (2020).
Tan, Z. et al. Attapulgite as a cost-effective catalyst for low-energy consumption amine-based CO2 capture. Sep. Purif. Technol. 298, 121577 (2022).
Alivand, M. S. et al. Engineered assembly of water-dispersible nanocatalysts enables low-cost and green CO2 capture. Nat. Commun. 13, 1–11 (2022).
Bhatti, U. H. et al. Effects of transition metal oxide catalysts on MEA solvent regeneration for the post-combustion carbon capture process. ACS Sustain. Chem. Eng. 5, 5862–5868 (2017).
Bhatti, U. H., Sivanesan, D., Nam, S., Park, S. Y. & Baek, I. H. Efficient Ag2O–Ag2CO3 catalytic cycle and its role in minimizing the energy requirement of amine solvent regeneration for CO2 capture. ACS Sustain. Chem. Eng. 7, 10234–10240 (2019).
Ji, L. et al. Metal oxyhydroxide catalysts promoted CO2 absorption and desorption in amine-based carbon capture: a feasibility study. ACS Omega 7, 44620–44630 (2022).
Lai, Q. et al. Catalyst-TiO(OH)2 could drastically reduce the energy consumption of CO2 capture. Nat. Commun. 9, 1–7 (2018).
Danckwerts, P. V. The reaction of CO2 with ethanolamines. Chem. Eng. Sci. 34, 443–446 (1979).
Srisang, W. et al. Evaluation of the heat duty of catalyst-aided amine-based post combustion CO2 capture. Chem. Eng. Sci. 170, 48–57 (2017).
Mondal, A. & Ram, S. Monolithic t-ZrO2 nanopowder through a ZrO(OH)2·xH2O polymer precursor. J. Am. Ceram. Soc. 87, 2187–2194 (2004).
Chen, J. et al. Interactions of oxide surfaces with water revealed with solid-state NMR spectroscopy. J. Am. Chem. Soc. 142, 11173–11182 (2020).
Boehm, H. P. Acidic and basic properties of hydroxylated metal oxide surfaces. Discuss. Faraday Soc. 52, 264–275 (1971).
Hohl, H., Sigg, L. & Stumm, W. Characterization of surface chemical properties of oxides in natural waters. Adv. Chemistry 189, 1–31 (1980).
Sun, C. & Berg, J. C. A review of the different techniques for solid surface acid–base characterization. Adv. Colloid Interface Sci. 105, 151–175 (2003).
Rose, J. et al. Aqueous zirconium complexes for gelling polymers. A combined X-ray absorption spectroscopy and quantum mechanical study. J. Phys. Chem. B 107, 2910–2920 (2003).
Clearfield, A. The mechanism of hydrolytic polymerization of zirconyl solutions. J. Mater. Res. 5, 161–162 (1990).
Chung, S. et al. Origin of active sites on silica-magnesia catalysts and control of reactive environment in the one-step ethanol-to-butadiene process. Nat. Catal. 6, 363–376 (2023).
Zhang, R. et al. CuO modified KIT-6 as a high-efficiency catalyst for energy-efficient amine solvent regeneration. Sep. Purif. Technol. 300, 121702 (2022).
Richner, G. Promoting CO2 absorption in aqueous amines with benzylamine. Energy Procedia 37, 423–430 (2013).
Richner, G. & Puxty, G. Assessing the chemical speciation during CO2 absorption by aqueous amines using in situ FTIR. Ind. Eng. Chem. Res. 51, 14317–14324 (2012).
Choi, W. J., Seo, J. B., Jang, S. Y., Jung, J. H. & Oh, K. J. Removal characteristics of CO2 using aqueous MEA/AMP solutions in the absorption and regeneration process. J. Environ. Sci. 21, 907–913 (2009).
D’Alessandro, D. M., Smit, B. & Long, J. R. Carbon dioxide capture: prospects for new materials. Angew. Chem. Int. Ed. 49, 6058–6082 (2010).
Aronu, U. E. et al. Solubility of CO2 in 15, 30, 45 and 60 mass% MEA from 40 to 120 °C and model representation using the extended UNIQUAC framework. Chem. Eng. Sci. 66, 6393–6406 (2011).
Mathias, P. M. & Gilmartin, J. P. Effect of uncertainty in property models on the simulated performance of solvent-based CO2-capture—study of aqueous AMP as solvent. Int. J. Greenh. Gas Control 109, 103334 (2021).
Huang, B. et al. Industrial test and techno-economic analysis of CO2 capture in Huaneng Beijing coal-fired power station. Appl. Energy 87, 3347–3354 (2010).
Acknowledgements
We thank W. Vermandel and L. Utiu for kind technical support. Y.L. thanks the National Natural Science Foundation of China (52176213), C.Z. thanks the Chinese Scholarship Council (201906310137) for financial support, M.D. acknowledges FWO infrastructure projects (AKUL13/19 and I000920N) and B.F.S. acknowledges TotalEnergies for financial support.
Author information
Authors and Affiliations
Contributions
C.Z., Y.L. and B.F.S. designed the experiments. C.Z., M.T.B. and F.R. performed the experiments. C.Z., M.T.B., Y.L. and B.F.S. analysed the data. C.Z. wrote the paper with guidance from B.F.S., Y.L., P.K., M.L., W.V. and M.D. provided valuable input in data analysis and comments on the paper.
Corresponding authors
Ethics declarations
Competing interests
This work is partly funded by TotalEnergies.
Peer review
Peer review information
Nature Catalysis thanks Maohong Fan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–22, Tables 1–4 and Refs. 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, C., Beydokhti, M.T., Rammal, F. et al. Proximity-independent acid–base synergy in a solid ZrOxHy catalyst for amine regeneration in post-combustion CO2 capture. Nat Catal 8, 270–281 (2025). https://doi.org/10.1038/s41929-025-01307-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01307-8