Abstract
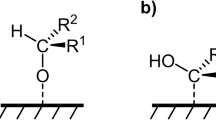
Catalytic hydrogenation of carbonyl compounds is widely used in chemical manufacturing and biomass refining, but current thermocatalytic processes require elevated temperatures, high-pressure H2 and expensive catalysts. Here we demonstrate an electroreduction of carbonyl compounds over Fe/Fe2O3 interfaces in Fe/Fe2O3 nanoarrays (Fe/Fe2O3 NAs), where Fe and Fe2O3 species synergistically accelerate the kinetics of acetone hydrogenation by promoting acetone adsorption and H* formation. With acetone as the probe molecule, an isopropanol partial current density of 1.6 A cm−2 and ~100% selectivity are achieved in 1 M KOH aqueous solution. Even in a large-scale, two-electrode electrolyser, Fe/Fe2O3 NAs stably deliver an acetone conversion of >99%, an isopropanol selectivity of 100%, and an isopropanol production rate of 21.6 g gcat−1 h−1 at 0.2 A cm−2 over a 1,000-h operation. Moreover, Fe/Fe2O3 NAs were applied in the electrochemical hydrogenation of various carbonyl compounds to corresponding alcohols with high conversion rates and selectivities.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the finding of this work are available in the main text and Supplementary Information. Additional data related to this work may be requested from the corresponding authors. Source data are provided with this paper.
References
Zhang, F.-H., Zhang, F.-J., Li, M.-L., Xie, J.-H. & Zhou, Q.-L. Enantioselective hydrogenation of dialkyl ketones. Nat. Catal. 3, 621–627 (2020).
Zhou, H. et al. Organocatalytic stereoselective cyanosilylation of small ketones. Nature 605, 84–89 (2022).
Zhao, M. et al. Metal–organic frameworks as selectivity regulators for hydrogenation reactions. Nature 539, 76–80 (2016).
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–800 (2016).
Bordet, A. et al. Selectivity control in hydrogenation through adaptive catalysis using ruthenium nanoparticles on a CO2-responsive support. Nat. Chem. 13, 916–922 (2021).
Ozkar, S. & Finke, R. G. Iridium(0) nanocluster, acid-assisted catalysis of neat acetone hydrogenation at room temperature: exceptional activity, catalyst lifetime and selectivity at complete conversion. J. Am. Chem. Soc. 127, 4800–4808 (2005).
Wang, X. S., Jiao, Y., Li, L. Q., Zheng, Y. & Qiao, S. Z. Local environment determined reactant adsorption configuration for enhanced electrocatalytic acetone hydrogenation to propane. Angew. Chem. Int. Ed. 61, e202114253 (2022).
Waldie, K. M., Flajslik, K. R., McLoughlin, E., Chidsey, C. E. D. & Waymouth, R. M. Electrocatalytic alcohol oxidation with ruthenium transfer hydrogenation catalysts. J. Am. Chem. Soc. 139, 738–748 (2017).
Feng, R. et al. Reversible ketone hydrogenation and dehydrogenation for aqueous organic redox flow batteries. Science 372, 836–840 (2021).
Panjapakkul, W. & El-Halwagi, M. M. Technoeconomic analysis of alternative pathways of isopropanol production. ACS Sustain. Chem. Eng. 6, 10260–10272 (2018).
Shen, M. et al. Ni-foam-structured Ni-Al2O3 ensemble as an efficient catalyst for gas-phase acetone hydrogenation to isopropanol. ACS Appl. Mater. Interfaces 13, 28334–28347 (2021).
Rahman, A. Catalytic hydrogenation of acetone to isopropanol: an environmentally benign approach. Bull. Chem. React. Eng. 5, 113–126 (2010).
Meng, N., Shinoda, S. & Saito, Y. Improvements on thermal efficiency of chemical heat pump involving the reaction couple of 2-propanol dehydrogenation and acetone hydrogenation. Int. J. Hydrogen Energ. 22, 361–367 (1997).
Balouch, A., Ali Umar, A., Shah, A. A., Mat Salleh, M. & Oyama, M. Efficient heterogeneous catalytic hydrogenation of acetone to isopropanol on semihollow and porous palladium nanocatalyst. ACS Appl. Mater. Interfaces 5, 9843–9849 (2013).
Zhu, Q. et al. Enhanced CO2 utilization in dry reforming of methane achieved through nickel-mediated hydrogen spillover in zeolite crystals. Nat. Catal. 5, 1030–1037 (2022).
Ji, Y. et al. Porous bimetallic Pt-Fe nanocatalysts for highly efficient hydrogenation of acetone. Nano Res. 8, 2706–2713 (2015).
Ro, I. et al. Intrinsic activity of interfacial sites for Pt-Fe and Pt-Mo catalysts in the hydrogenation of carbonyl groups. Appl. Catal. B 231, 182–190 (2018).
Gao, X., Heyden, A., Abdelrahman, O. A. & Bond, J. Q. Microkinetic analysis of acetone hydrogenation over Pt/SiO2. J. Catal. 374, 183–198 (2019).
Basu, S. & Pradhan, N. C. Kinetics of acetone hydrogenation for synthesis of isopropyl alcohol over Cu-Al mixed oxide catalysts. Catal. Today 348, 118–126 (2020).
Lu, S., Wu, J., Peng, H. & Chen, Y. Carbon-supported Raney nickel catalyst for acetone hydrogenation with high selectivity. Molecules 25, 803 (2020).
Matanović, I. Selectivity control for electroreduction of ketones. Nat. Catal. 2, 186–187 (2019).
Song, Y. et al. Hydrogenation of benzaldehyde via electrocatalysis and thermal catalysis on carbon-supported metals. J. Catal. 359, 68–75 (2018).
Antonietta Casadei, M. & Pletcher, D. The influence of conditions on the electrocatalytic hydrogenation of organic molecules. Electrochim. Acta 33, 117–120 (1988).
Li, Z. et al. Aqueous electrocatalytic hydrogenation of furfural using a sacrificial anode. Electrochim. Acta 64, 87–93 (2012).
zhao, B., Chen, M., Guo, Q. & Fu, Y. Electrocatalytic hydrogenation of furfural to furfuryl alcohol using platinum supported on activated carbon fibers. Electrochim. Acta 135, 139–146 (2014).
Zhang, X. et al. Simultaneously high-rate furfural hydrogenation and oxidation upgrading on nanostructured transition metal phosphides through electrocatalytic conversion at ambient conditions. Appl. Catal. B 244, 899–908 (2019).
Zhang, W., Shi, Y., Yang, Y., Tan, J. & Gao, Q. Facet dependence of electrocatalytic furfural hydrogenation on palladium nanocrystals. Chin. J. Catal. 43, 3116–3125 (2022).
Bondue, C. J., Calle-Vallejo, F., Figueiredo, M. C. & Koper, M. T. M. Structural principles to steer the selectivity of the electrocatalytic reduction of aliphatic ketones on platinum. Nat. Catal. 2, 243–250 (2019).
Wu, Y., Guo, Z., Sun, C., Ren, X. & Li, Q. High-efficiency electrochemical hydrogenation of biomass-derived benzaldehyde compounds via a durable and versatile dendritic-like Pd/Cu-CF electrocatalyst. Fuel Process. Technol. 237, 107436 (2022).
Anibal, J., Malkani, A. & Xu, B. Stability of the ketyl radical as a descriptor in the electrochemical coupling of benzaldehyde. Catal. Sci. Technol. 10, 3181–3194 (2020).
Koh, K. et al. Electrochemically tunable proton-coupled electron transfer in Pd-catalyzed benzaldehyde hydrogenation. Angew. Chem. Int. Ed. 59, 1501–1505 (2020).
Lopez-Ruiz, J. A. et al. Understanding the role of metal and molecular structure on the electrocatalytic hydrogenation of oxygenated organic compounds. ACS Catal. 9, 9964–9972 (2019).
Luo, Y., Zhang, Z., Chhowalla, M. & Liu, B. Recent advances in design of electrocatalysts for high-current-density water splitting. Adv. Mater. 34, 2108133 (2022).
Liu, K. et al. Intensified gas-phase hydrogenation of acetone to isopropanol catalyzed at metal-oxide interfacial sites. Chem. Eng. J. 454, 140059 (2023).
Wang, X. et al. Insights into the shape effect of H2 self-selective Ni catalysts for efficient acetone hydrogenation. Appl. Surf. Sci. 536, 147844 (2021).
Tao, K., Li, W., Li, H. & Qi, X. Effect of modified industrial zeolite beta on one-step catalytic hydration of propene to isopropanol. Appl. Catal. A Gen. 139, 43–49 (1996).
Ivanov, A. V., Zausa, E., Taârit, Y. B. & Essayem, N. Mechanism of propene hydration over heteropolyacid catalysts. Appl. Catal. A Gen. 256, 225–242 (2003).
Shin, H., Hansen, K. U. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat. Sustain. 4, 911–919 (2021).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Zhao, B.-H. et al. Economically viable electrocatalytic ethylene production with high yield and selectivity. Nat. Sustain. 6, 827–837 (2023).
Massaro, M. C. & Monteverde, A. H. A. Techno-economic analysis of FDCA production through electrocatalytic processes. J. Electrochem. Soc. 169, 054515 (2022).
Acknowledgements
This work was supported by the Key Projects of Intergovernmental International Cooperation in Key R&D Programs of the Ministry of Science and Technology of China (2021YFE0115800), the National Key Research and Development Program of China (2024YFA1510100), the National Natural Science Foundation of China NSF (52373308, 22005245, 52173224 and 51821002), the Key Research and Development of Shaanxi Province (2023-YBGY-284), the Fundamental Research Funds for the Central Universities (G2022KY0606) and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.
Author information
Authors and Affiliations
Contributions
J.Z. proposed and supervised the project. J.L. synthesized the catalysts. J.L., Z.L. and J.B. carried out the electrochemical experiments and related data processing. J.L., Z.W. and G.W. conducted materials characterization. H.W. and P.L. performed HRTEM characterization. Y.W. conducted the technoeconomic analysis. J.Z., Z.L. and J.L. cowrote the paper. P.L. and J.W. reviewed the paper. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
J.Z. and J.L. are inventors on a patent application submitted by Northwestern Polytechnical University (CN116555785A), which covers the electrocatalytic hydrogenation of carbonyl compounds for the production of corresponding alcohols. The other authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Alessandro Hugo, Feng Jiao, Antonio Monteverde and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–81, Tables 1 and 2, Notes 1–10 and references 1–11.
Supplementary Video 1
The distillation process of isopropanol after electrocatalytic acetone hydrogenation.
Supplementary Video 2
The solar-driven electrocatalytic acetone hydrogenation under sunlight irradiation. The solar panel has an area of 1.45 m2, an output voltage of 7.85 V and a power density of 35.3 W. The reactor consists of three tandem H-type batch electrolysers.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, J., Liu, Z., Wu, H. et al. Efficient electroreduction of carbonyl compounds to alcohols over Fe/Fe2O3 interfaces. Nat Catal 8, 338–347 (2025). https://doi.org/10.1038/s41929-025-01316-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01316-7
This article is cited by
-
Interfacial water regulation on Ru single atoms doped Co3O4 toward efficient electrochemical hydrogenation of quinoxaline
Nature Communications (2026)
-
Two-Dimensional Metastable-Phase Nickel Hexagonal Nanosheets for Highly-Performance Electrochemical Acetone Hydrogenation
Nature Communications (2025)