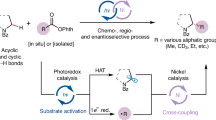
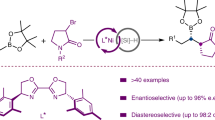
Abstract
Catalytic sp3–sp3 bond-forming reactions have been the subject of considerable interest in both academic and pharmaceutical laboratories. This is largely due to the observation that a higher content of sp3-hybridized carbons has recently been shown to improve several molecular attributes that contribute to clinical success. Although the ready availability of unactivated olefins and aziridines makes them ideal precursors to forge enantioenriched sp3–sp3 architectures with added-value amine functions, an enantioconvergent catalytic scenario of these counterparts has not yet been realized. Here we describe a nickel-catalysed blueprint that enables the enantioselective construction of amine-containing sp3–sp3 architectures via photoinduced enantioconvergent coupling of racemic aziridines with alkylzirconium reagents generated in situ from unactivated terminal and even internal olefins. The broad applicability of this protocol is illustrated in a series of late-stage diversification of advanced synthetic intermediates.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Experimental procedures and characterization data for the synthesized compounds are included in the Supplementary Information. Crystallographic data are available from the Cambridge Crystallographic Data Centre with the following codes: 3w (CCDC 2360556) and 3ah (CCDC 2360555). Other data are available from the corresponding authors upon reasonable request.
References
Geist, E., Kirschning, A. & Schmidt, T. sp3–sp3 coupling reactions in the synthesis of natural products and biologically active molecules. Nat. Prod. Rep. 31, 441–448 (2014).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Enantioselective and enantiospecific transition metal-catalyzed cross-coupling reactions of organometallic reagents to construct C-C bonds. Chem. Rev. 115, 9587–9652 (2015).
Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl–alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Yus, M., Nájera, C., Foubelo, F. & Sansano, J. M. Metal-catalyzed enantioconvergent transformations. Chem. Rev. 123, 11817–11893 (2023).
Zhou, J., Wang, D., Xu, W., Hu, Z. & Xu, T. Enantioselective C(sp3)–C(sp3) reductive cross-electrophile coupling of unactivated alkyl halides with α-chloroboronates via dual nickel/photoredox catalysis. J. Am. Chem. Soc. 145, 2081–2087 (2023).
Zhao, W.-T. & Shu, W. Enantioselective Csp3–Csp3 formation by nickel-catalyzed enantioconvergent cross-electrophile alkyl–alkyl coupling of unactivated alkyl halides. Sci. Adv. 9, eadg9898 (2023).
Zhao, W.-T., Zhang, J.-X., Chen, B.-H. & Shu, W. Ligand-enabled Ni-catalysed enantioconvergent intermolecular alkyl–alkyl cross-coupling between distinct alkyl halides. Nat. Commun. 14, 2938 (2023).
Fu, H. et al. An asymmetric sp3–sp3 cross-electrophile coupling using ‘ene’-reductases. Nature 610, 302–307 (2022).
Nguyen, K. D. et al. Metal-catalyzed reductive coupling of olefin-derived nucleophiles: reinventing carbonyl addition. Science 354, aah5133 (2016).
Wang, Y., He, Y. & Zhu, S. NiH-catalyzed functionalization of remote and proximal olefins: new reactions and innovative strategies. Acc. Chem. Res. 55, 3519–3536 (2022).
Zhang, Z., Bera, S., Fan, C. & Hu, X. Streamlined alkylation via nickel-hydride-catalyzed hydrocarbonation of alkenes. J. Am. Chem. Soc. 144, 7015–7029 (2022).
Yang, P.-F. & Shu, W. Asymmetric alkyl–alkyl cross-coupling enabled by earth-abundant metal-catalyzed hydroalkylations of olefins. Chem. Catal. 3, 100508 (2023).
Vasseur, A., Bruffaerts, J. & Marek, I. Remote functionalization through alkene isomerization. Nat. Chem. 8, 209–219 (2016).
Sommer, H., Juliá-Hernández, F., Martin, R. & Marek, I. Walking metals for remote functionalization. ACS Cent. Sci. 4, 153–165 (2018).
Wang, Y., He, Y. & Zhu, S. Nickel-catalyzed migratory cross-coupling reactions: new opportunities for selective C–H functionalization. Acc. Chem. Res. 56, 3475–3491 (2023).
Rodrigalvarez, J., Haut, F.-L. & Martin, R. Regiodivergent sp3 C–H functionalization via Ni-catalyzed chain-walking reactions. JACS Au 3, 3270–3282 (2023).
Li, J. et al. Enantioselective alkylation of α-amino C(sp3)–H bonds via photoredox and nickel catalysis. Nat. Catal. 7, 889–899 (2024).
Yudin, A. K. Aziridines and Epoxides in Organic Synthesis (Wiley, 2006).
Huang, C. Y. & Doyle, A. G. The chemistry of transition metals with three-membered ring heterocycles. Chem. Rev. 114, 8153–8198 (2014).
Sabir, S., Kumar, G., Verma, V. P. & Jat, J. L. Aziridine ring opening: an overview of sustainable methods. ChemistrySelect 3, 3702–3711 (2018).
Watson, I. D. G., Yu, L. & Yudin, A. K. Advances in nitrogen transfer reactions involving aziridines. Acc. Chem. Res. 39, 194–206 (2006).
Holst, D. E., Wang, D. J., Kim, M. J., Guzei, I. A. & Wickens, Z. K. Aziridine synthesis by coupling amines and alkenes via an electrogenerated dication. Nature 596, 74–79 (2021).
Mitchell, J. K., Hussain, W. A., Bansode, A. H., O’Connor, R. M. & Parasram, M. Aziridination via nitrogen-atom transfer to olefins from photoexcited azoxy-triazenes. J. Am. Chem. Soc. 146, 9499–9505 (2024).
Lawrence, S. A. Amines: Synthesis Properties and Applications (Cambridge Univ. Press, 2004).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Ouyang, K., Hao, W., Zhang, W.-X. & Xi, Z. Transition metal-catalyzed cleavage of C-N single bonds. Chem. Rev. 115, 12045–12090 (2015).
Kong, D., Moon, P. J. & Lundgren, R. J. Radical coupling from alkyl amines. Nat. Catal. 2, 473–476 (2019).
Woods, B. P., Orlandi, M., Huang, C. Y., Sigman, M. S. & Doyle, A. G. Nickel-catalyzed enantioselective reductive cross-coupling of styrenyl aziridines. J. Am. Chem. Soc. 139, 5688–5691 (2017).
Lau, S. H. et al. Ni/photoredox-catalyzed enantioselective cross-electrophile coupling of styrene oxides with aryl iodides. J. Am. Chem. Soc. 143, 15873–15881 (2021).
Wang, Y.-Z. et al. Nickel/biimidazole-catalyzed electrochemical enantioselective reductive cross-coupling of aryl aziridines with aryl iodides. Nat. Commun. 14, 2322 (2023).
Hu, X., Cheng-Sánchez, I., Cuesta-Galisteo, S. & Nevado, C. Nickel-catalyzed enantioselective electrochemical reductive cross-coupling of aryl aziridines with alkenyl bromides. J. Am. Chem. Soc. 145, 6270–6279 (2023).
Huang, C.-Y. & Doyle, A. G. Electron-deficient olefin ligands enable generation of quaternary carbons by Ni-catalyzed cross-coupling. J. Am. Chem. Soc. 137, 5638–5641 (2015).
Trowbridge, A., Walton, S. M. & Gaunt, M. J. New strategies for the transition metal-catalyzed synthesis of aliphatic amines. Chem. Rev. 120, 2613–2692 (2020).
Marek, I., Chinkov, N. & Levin, A. A zirconium promenade—an efficient tool in organic synthesis. Synlett 4, 501–514 (2006).
Vasseur, A., Perrin, L., Eisenstein, O. & Marek, I. Remote functionalization of hydrocarbons with reversibility enhanced stereocontrol. Chem. Sci. 6, 2770–2776 (2015).
Gao, Y. et al. Visible-light-induced nickel-catalyzed cross-coupling with alkylzirconocenes from unactivated alkenes. Chem 6, 675–688 (2020).
Yang, C., Gao, Y., Bai, S., Jiang, C. & Qi, X. Chemoselective cross-coupling of gem-borazirconocene alkanes with aryl halides. J. Am. Chem. Soc. 142, 11506–11513 (2020).
Hirao, Y., Katayama, Y., Mitsunuma, H. & Kanai, M. Chromium-catalyzed linear selective alkylation of aldehydes with alkenes. Org. Lett. 22, 8584–8588 (2020).
Ren, X., Gao, X., Min, Q.-Q., Zhang, S. & Zhang, X. (Fluoro)alkylation of alkenes promoted by photolysis of alkylzirconocenes. Chem. Sci. 13, 3454–3460 (2022).
Yang, C., Bai, S., Gao, Y., Wu, Q. & Qi, X. Visible-light-induced enantioselective radical cross-coupling of C(sp3)-borazirconocene. Chem 9, 2222–2236 (2023).
Fan, P., Jin, Y., Liu, J., Wang, R. & Wang, C. Nickel/photo-cocatalyzed regioselective ring opening of N-tosyl styrenyl aziridines with aldehydes. Org. Lett. 23, 7364–7369 (2021).
Lan, Y. et al. Nickel-catalyzed enantioselective C(sp3)–C(sp3) cross-electrophile coupling of N‑sulfonyl styrenyl aziridines with alkyl bromides. J. Am. Chem. Soc. 146, 25426–25432 (2024).
Meijere, A. D., Bräse, S. & Oestreich, M. Metal-Catalyzed Cross-Coupling Reactions and More (Wiley, 2014).
Gao, Y. F. et al. Recent advances in intensified ethylene production—a review. ACS Catal. 9, 8592–8621 (2019).
Lin, B. L., Clough, C. R. & Hillhouse, G. L. Interactions of aziridines with nickel complexes: oxidative-addition and reductive-elimination reactions that break and make C–N bonds. J. Am. Chem. Soc. 124, 2890–2891 (2002).
Chen, J., Zhu, G. & Wu, Jie Recent advances in nickel-catalyzed ring opening cross-coupling of aziridines. Acta Chim. Sin. 82, 190–212 (2024).
Somerville, R. et al. Ni(I)–alkyl complexes bearing phenanthroline ligands: experimental evidence for CO2 insertion at Ni(I) centers. J. Am. Chem. Soc. 142, 10936–10941 (2020).
Lin, Q., Spielvogel, E. H. & Diao, T. Carbon-centered radical capture at nickel(II) complexes: spectroscopic evidence, rates, and selectivity. Chem 9, 1295–1308 (2023).
Acknowledgements
We thank ICIQ and FEDER/MCI PID2021-123801NB-I00 and MCI/AIE (Secero Ochoa Excellence Accreditation 2002-2023 CEX2019-000925-S) for financial support. L.Z. thanks the Deutsche Forschungsgemeinschaft (DFG) for a postdoctoral fellowship and H.W. and W.-J.Y. thank the China Scholarship Council (CSC) for a predoctoral fellowship. We also thank the ICIQ X-ray diffraction, NMR and mass spectrometry units.
Author information
Authors and Affiliations
Contributions
L.Z. and R.M. conceived the project. L.Z. designed and performed experiments and analysed the data unless otherwise stated. R.M. supervised the project. H.W. performed parts of the late-stage functionalization experiments. T.G.S. performed a series of mechanistic experiments. W.-J.Y. performed parts of the synthetic application experiments. H.W., T.G.S. and W.-J.Y. contributed equally. L.Z. and R.M. wrote the paper. All authors participated in the discussion and preparation of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Haohua Huo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13, discussion and Tables 1–17.
Supplementary Tables 1–17
Supplementary Tables 1–17.
Supplementary Data 1
Crystallographic Information File (CIF) of compound 3w.
Supplementary Data 2
Crystallographic Information File (CIF) of compound 3ah.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Wang, H., Santiago, T.G. et al. Photoinduced nickel-catalysed enantioconvergent sp3–sp3 coupling of unactivated olefins and aziridines. Nat Catal 8, 348–356 (2025). https://doi.org/10.1038/s41929-025-01319-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01319-4
This article is cited by
-
Illuminating enantioselective alkyl−alkyl coupling
Nature Catalysis (2025)