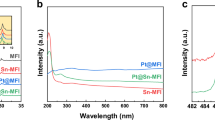
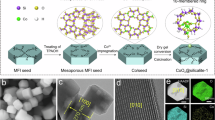
Abstract
Propane dehydrogenation has been used industrially as a non-oil-based propylene production process, but it strongly depends on precious-metal catalysts such as supported Pt materials, which dominate most propane dehydrogenation processes currently used in industry. Catalysts with earth-abundant metals have been explored with a view to replacing Pt, but their performances remain inadequate. Here we report a cobaltosilicate zeolite catalyst, which has solely tetrahedral cobalt sites and none of the unstable cobalt species in the zeolite crystals that are characteristic of conventional cobaltosilicate materials. This catalyst exhibits properties that could be attractive for industrial application, including sufficient propylene productivity, high stability and facile regenerability. Moreover, this system outperforms the benchmark supported Pt–Sn catalysts under equivalent conditions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data that led us to understand the results presented here are available with the paper or from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Sattler, J. J. H. B., Ruiz-Martinez, J., Santillan-Jimenez, E. & Weckhuysen, B. M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 114, 10613–10653 (2014).
Chen, S. et al. Propane dehydrogenation: catalyst development, new chemistry and emerging technologies. Chem. Soc. Rev. 50, 3315–3354 (2021).
Yan, W., Sun, Q. & Yu, J. Dehydrogenation of propane marches on. Matter 4, 2642–2644 (2021).
Otroshchenko, T., Jiang, G., Kondratenko, V. A., Rodemerck, U. & Kondratenko, E. V. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts. Chem. Soc. Rev. 50, 473–527 (2021).
Hannagan, R. T. et al. First-principles design of a single-atom-alloy propane dehydrogenation catalyst. Science 372, 1444–1447 (2021).
Yan, H. et al. Tandem In2O3-Pt/Al2O3 catalyst for coupling of propane dehydrogenation to selective H2 combustion. Science 371, 1257–1260 (2021).
Gomez, E., Yan, B., Kattel, S. & Chen, J. G. Carbon dioxide reduction in tandem with light-alkane dehydrogenation. Nat. Rev. Chem. 3, 638–649 (2019).
Martino, M., Meloni, E., Festa, G. & Palma, V. Propylene synthesis: recent advances in the use of Pt-based catalysts for propane dehydrogenation reaction. Catalysts 11, 1070 (2021).
Liu, L. et al. Structural modulation and direct measurement of subnanometric bimetallic PtSn clusters confined in zeolites. Nat. Catal. 3, 628–638 (2020).
Motagamwala, A. H., Almallahi, R., Wortman, J., Igenegbai, V. O. & Linic, S. Stable, selective catalysts for propane dehydrogenation operating at thermodynamic limit. Science 373, 217–222 (2021).
Shi, L., Deng, G.-M., Li, W.-C., Miao, S. & Lu, A.-H. Al2O3 nanosheets rich in pentacoordinate Al3+ ions stabilize Pt-Sn clusters for propane dehydrogenation. Angew. Chem. Int. Ed. 54, 13994–13998 (2015).
Ryoo, R. et al. Rare-earth–platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature 585, 221–224 (2020).
Nakaya, Y., Xing, F., Ham, H., Shimizu, K. & Furukawa, S. Doubly decorated platinum–gallium intermetallics as stable catalysts for propane dehydrogenation. Angew. Chem. Int. Ed. 60, 19715–19719 (2021).
Sun, Q. et al. Subnanometer bimetallic platinum–zinc clusters in zeolites for propane dehydrogenation. Angew. Chem. Int. Ed. 59, 19450–19459 (2020).
Searles, K. et al. Highly productive propane dehydrogenation catalyst using silica-supported Ga–Pt nanoparticles generated from single-sites. J. Am. Chem. Soc. 140, 11674–11679 (2018).
Chen, S. et al. Propane dehydrogenation on single-site [PtZn4] intermetallic catalysts. Chem 7, 387–405 (2021).
Qi, L. et al. Propane dehydrogenation catalyzed by isolated Pt atoms in ≡SiOZn−OH nests in dealuminated zeolite beta. J. Am. Chem. Soc. 143, 21364–21378 (2021).
Zhao, D. et al. In situ formation of ZnOx species for efficient propane dehydrogenation. Nature 599, 234–238 (2021).
Cai, W. et al. Subsurface catalysis-mediated selectivity of dehydrogenation reaction. Sci. Adv. 4, eaar5418 (2018).
Schreiber, M. W. et al. Lewis–Brønsted acid pairs in Ga/H-ZSM-5 to catalyze dehydrogenation of light alkanes. J. Am. Chem. Soc. 140, 4849–4859 (2018).
Lin, G., Su, Y., Duan, X. & Xie, K. High-density Lewis acid sites in porous single-crystalline monoliths to enhance propane dehydrogenation at reduced temperatures. Angew. Chem. Int. Ed. 60, 9311–9315 (2021).
Song, S. et al. In situ encapsulated subnanometric CoO clusters within silicalite-1 zeolite for efficient propane dehydrogenation. AIChE J. 68, e17451 (2022).
Bian, Z. et al. Mesoporous-silica-stabilized cobalt (II) oxide nanoclusters for propane dehydrogenation. ACS Appl. Nano Mater. 4, 1112–1125 (2021).
Wang, Y., Suo, Y., Ren, J.-T., Wang, Z. & Yuan, Z.-Y. Spatially isolated cobalt oxide sites derived from MOFs for direct propane dehydrogenation. J. Colloid Interface Sci. 594, 113–121 (2021).
Hu, Z.-P. et al. Atomic insight into the local structure and microenvironment of isolated Co-motifs in MFI zeolite frameworks for propane dehydrogenation. J. Am. Chem. Soc. 147, 12127–12137 (2022).
Chen, C., Zhang, S., Wang, Z. & Yuan, Z.-Y. Ultrasmall Co confined in the silanols of dealuminated beta zeolite: a highly active and selective catalyst for direct dehydrogenation of propane to propylene. J. Catal. 383, 77–87 (2020).
Wu, L. et al. Atomically dispersed Co2+ sites incorporated into a silicalite‑1 zeolite framework as a high-performance and coking-resistant catalyst for propane nonoxidative dehydrogenation to propylene. ACS Appl. Mater. Interfaces 13, 48934–48948 (2021).
Liu, L. et al. Rivet of cobalt in siliceous zeolite for catalytic ethane dehydrogenation. Chem 9, 637–649 (2023).
Lin, L., Zhang, T., Zang, J. & Xu, Z. Dynamic process of carbon deposition on Pt and Pt-Sn catalysts for alkane dehydrogenation. Appl. Catal. 67, 11–23 (1990).
Otroshchenko, T. et al. ZrO2-based alternatives to conventional propane dehydrogenation catalysts: active sites, design and performance. Angew. Chem. Int. Ed. 54, 15880–15883 (2015).
Romeo, E., Saeys, M., Monzón, A. & Borgna, A. Carbon nanotube formation during propane decomposition on boron-modified Co/Al2O3 catalysts: a kinetic study. Int. J. Hydrog. Energy 39, 18016–18026 (2014).
Bethune, D. S. et al. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 363, 605–607 (1993).
Shen, B. et al. A single-molecule van der Waals compass. Nature 592, 541–544 (2021).
Jiang, H. et al. Atomic-resolution characterization on the structure of strontium doped barium titanate nanoparticles. Nano Res. 14, 4802–4807 (2021).
Li, S. et al. Composite Si-O-metal network catalysts with uneven electron distribution: enhanced activity and electron transfer for catalytic ozonation of carbamazepine. Appl. Catal. B Environ. 263, 118311 (2020).
Schwartz, V., Prins, R., Wang, X. & Sachtler, W. M. H. Characterization by EXAFS of Co/MFI catalysts prepared by sublimation. J. Phys. Chem. B 106, 7210–7217 (2002).
Ahn, H. S., Yano, J. & Tilley, T. D. Photocatalytic water oxidation by very small cobalt domains on a silica surface. Energy Environ. Sci. 6, 3080–3087 (2013).
Yang, M.-L., Zhu, Y.-A., Zhou, X.-G., Sui, Z.-J. & Chen, D. First-principles calculations of propane dehydrogenation over PtSn catalysts. ACS Catal. 2, 1247–1258 (2012).
Wang, J. et al. On the role of Sn segregation of Pt-Sn catalysts for propane dehydrogenation. ACS Catal. 11, 4401–4410 (2021).
Stegelmann, C., Andreasen, A. & Campbell, C. T. Degree of rate control: how much the energies of intermediates and transition states control rates. J. Am. Chem. Soc. 131, 8077–8082 (2009).
Nykänen, L. & Honkala, K. Selectivity in propene dehydrogenation on Pt and Pt3Sn surfaces from first principles. ACS Catal. 3, 3026–3030 (2013).
Jiao, Y. et al. Creation of Al-enriched mesoporous ZSM-5 nanoboxes with high catalytic activity: converting tetrahedral extra-framework Al into framework sites by post treatment. Angew. Chem. Int. Ed. 59, 19478–19486 (2020).
Wang, C. et al. Extra-framework aluminum-assisted initial C-C bond formation in methanol-to-olefins conversion on zeolite H-ZSM-5. Angew. Chem. Int. Ed. 130, 10354–10358 (2018).
Ravi, M., Sushkevich, V. L. & van Bokhoven, J. A. Towards a better understanding of Lewis acidic aluminium in zeolites. Nat. Mater. 19, 1047–1056 (2020).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Blöchl, P. E., Jepsen, O. & Andersen, O. K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 49, 16223–16233 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Selcuk, S. & Selloni, A. DFT + U study of the surface structure and stability of Co3O4 (110): dependence on U. J. Phys. Chem. C 119, 9973–9979 (2015).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A 31, 1695 (1985).
Woo, T. K., Margl, P. M., Blöchl, P. E. & Ziegler, T. A. Combined Car−Parrinello QM/MM implementation for ab initio molecular dynamics simulations of extended systems: application to transition metal catalysis. J. Phys. Chem. B 101, 7877–7880 (1997).
Jarzynski, C. Nonequilibrium equality for free energy differences. Phys. Rev. Lett. 78, 2690–2693 (1997).
Sprik, M. & Ciccotti, G. Free energy from constrained molecular dynamics. J. Chem. Phys. 109, 7737–7744 (1998).
Ryckaert, J.-P., Ciccotti, G. & Berendsen, H. J. C. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977).
Andersen, H. Rattle: a ‘velocity’ version of the shake algorithm for molecular dynamics calculations. J. Comput. Phys. 52, 24–34 (1983).
Searle, M. S. & Williams, D. H. The cost of conformational order: entropy changes in molecular associations. J. Am. Chem. Soc. 114, 10690–10697 (1992).
Kemball, C. Entropy of adsorption. Adv. Catal. 2, 233–250 (1950).
Carlsson, J. & Åqvist, J. Absolute and relative entropies from computer simulation with applications to ligand binding. J. Phys. Chem. B 109, 6448–6456 (2005).
Zhang, Y. & Maginn, E. J. A simple AIMD approach to derive atomic charges for condensed phase simulation of ionic liquids. J. Phys. Chem. B 116, 10036–10048 (2012).
Pham, T. D. & Lobo, R. F. Adsorption equilibria of CO2 and small hydrocarbons in AEI-, CHA-, STT- and RRO-type siliceous zeolites. Micropor. Mesopor. Mater. 236, 100–108 (2016).
Kadam, S. A., Li, H., Wormsbecher, R. F. & Travert, A. Impact of zeolite structure on entropic–enthalpic contributions to alkane monomolecular cracking: an IR operando study. Chem. Eur. J. 24, 5489–5492 (2018).
Medford, A. J. et al. Assessing the reliability of calculated catalytic ammonia synthesis rates. Science 345, 197–200 (2014).
Baerlocher, C. & McCusker, L. B. Database of Zeolite Structures http://www.iza-structure.org/databases/ (IZA Structure Commission, 2016).
Chen, J., Jia, M., Hu, P. & Wang, H. CATKINAS: a large-scale catalytic microkinetic analysis software for mechanism auto-analysis and catalyst screening. J. Comput. Chem. 42, 379–391 (2021).
Acknowledgements
We acknowledge beamtime at Beijing Synchrotron Radiation Facility (beamline 1W1B) and Shanghai Synchrotron Radiation Facility (SSRF, beamline BL14 W1) and are grateful for help with XANES and EXAFS characterization. We thank F. Chen for helping with SEM characterization. This work was supported by the National Key Research and Development Program of China (2022YFA1504500, 2021YFA1500700 and 2022YFE0108000), the National Natural Science Foundation of China (22288101, 22241801, 22321002, 22425207 and 22202177) and a Start-up Grant from Ningbo Innovation Center, Zhejiang University (NBHG2023X012). This work was also supported by the User Experiment Assist System of SSRF.
Author information
Authors and Affiliations
Contributions
H.Z. performed the catalyst preparation, characterization and catalytic tests. H.L. and J.X. performed the theoretical simulation and wrote the corresponding section. S.C., X.T. and L.L. performed the XANES and EXAFS characterization and analysed the data. J.Q., Z.R., A.C., X.L. and L.C. performed TEM characterization and identified the isolated cobalt. Lujie Liu, Lu Liu, X.Q., J.S., J.H. and S.X. participated in the characterization, catalysis and discussion. Y.H., Y.Q., L.S., Q.Y. and B.H. provided help with discussion on the catalyst structure. Y.D. and J.M. performed the ToF-MS characterization. L.W. and F.-S.X. designed the study, analysed the data and wrote the paper. All authors discussed the results and improved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–68, Tables 1–6 and References.
Supplementary Data
Atomic coordinate details.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, H., Li, H., Wang, L. et al. Cobaltosilicate zeolite beyond platinum catalysts for propane dehydrogenation. Nat Catal 8, 357–367 (2025). https://doi.org/10.1038/s41929-025-01320-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01320-x
This article is cited by
-
Electronically tailored metal-ion-chelation strategy promotes ionic liquid catalysis at near-ambient condition
Nature Communications (2025)
-
Ethane dehydrogenation over CaCO3-mediated tandem catalysts
Nature Communications (2025)