Abstract
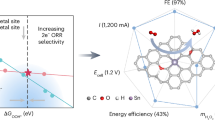
Hydrogen peroxide (H2O2) is a vital industrial chemical and sustainable energy carrier. However, achieving a simple, efficient and cost-effective synthesis under mild conditions remains an important challenge. Here we show that SnSe nanosheets with Sn vacancies can directly catalyse H2O2 production from H2O and O2 under ambient conditions, without additional energy inputs (for example, light and electricity), cocatalysts or sacrificial reagents. This approach achieves an optimal H2O2 production rate of ~2.6 mmol g−1 h−1 at 40 °C and maintains long-term stable production (~0.3 mmol l−1) in a continuous-flow reactor for over 50 h at room temperature. Experimental and theoretical analyses reveal that this unique thermocatalytic effect arises from a dynamic process involving Sn vacancy defect-induced sequential dissociation of H2O and activation of O2 molecules, along with reversible surface restructuring of the SnSe nanosheets to release H2O2. Our findings offer a notably simple, highly efficient and entirely green strategy for H2O2 production, with broader implications in other catalytic reactions involving water activation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information. All other data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Ciriminna, R. et al. Hydrogen peroxide: a key chemical for today’s sustainable development. ChemSusChem 9, 3374–3381 (2016).
Freese, T. et al. An organic perspective on photocatalytic production of hydrogen peroxide. Nat. Catal. 6, 553–558 (2023).
Zhang, Y. et al. H2O2 generation from O2 and H2O on a near-infrared absorbing porphyrin supramolecular photocatalyst. Nat. Energy 8, 361–371 (2023).
Disselkamp, R. S. Can aqueous hydrogen peroxide be used as a stand-alone energy source? Int. J. Hydrog. Energ. 35, 1049–1053 (2010).
Mousavi Shaegh, S. A. et al. A membraneless hydrogen peroxide fuel cell using Prussian Blue as cathode material. Energ. Environ. Sci. 5, 8225–8228 (2012).
Campos-Martin, J. M. et al. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 45, 6962–6984 (2006).
Freakley, S. J. et al. Palladium-tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 351, 965–968 (2016).
Zhang, J. et al. Photocatalytic phosphine-mediated water activation for radical hydrogenation. Nature 619, 506–513 (2023).
Liu, R. et al. Linkage-engineered donor–acceptor covalent organic frameworks for optimal photosynthesis of hydrogen peroxide from water and air. Nat. Catal. 7, 195–206 (2024).
Teng, Z. et al. Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide. Nat. Catal. 4, 374–384 (2021).
Tan, H. et al. Photocatalysis of water into hydrogen peroxide over an atomic Ga-N5 site. Nat. Synth. 2, 557–563 (2023).
Shiraishi, Y. et al. Resorcinol–formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion. Nat. Mater. 18, 985–993 (2019).
Yan, B. et al. Laser direct overall water splitting for H2 and H2O2 production. Proc. Natl Acad. Sci. USA 121, e2319286121 (2024).
Lu, Z. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 1, 156–162 (2018).
Xia, C. et al. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 366, 226–231 (2019).
Jung, E. et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020).
Siahrostami, S. et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 12, 1137–1143 (2013).
Zhao, J. et al. Contact-electro-catalysis for direct synthesis of H2O2 under ambient conditions. Angew. Chem. Int. Ed. 62, e202300604 (2023).
Ran, M. et al. Dynamic defects boost in-situ H2O2 piezocatalysis for water cleanup. Proc. Natl Acad. Sci. USA 121, e2317435121 (2024).
Zhao, L.-D. et al. Ultrahigh power factor and thermoelectric performance in hole-doped single-crystal SnSe. Science 351, 141–144 (2016).
Li, F. et al. Recent advances in SnSe nanostructures beyond thermoelectricity. Adv. Funct. Mater. 32, 2200516 (2022).
Wei, Z. et al. Efficient visible-light-driven selective oxygen reduction to hydrogen peroxide by oxygen-enriched graphitic carbon nitride polymers. Energ. Environ. Sci. 11, 2581–2589 (2018).
Peng, H. et al. Defective ZnIn2S4 nanosheets for visible-light and sacrificial-agent-free H2O2 photosynthesis via O2/H2O redox. J. Am. Chem. Soc. 145, 27757–27766 (2023).
Liu, T. et al. Overall photosynthesis of H2O2 by an inorganic semiconductor. Nat. Commun. 13, 1034 (2022).
Yue, J.-Y. et al. Thiophene-containing covalent organic frameworks for overall photocatalytic H2O2 synthesis in water and seawater. Angew. Chem. Int. Ed. 62, e202309624 (2023).
Qin, C. et al. Dual donor–acceptor covalent organic frameworks for hydrogen peroxide photosynthesis. Nat. Commun. 14, 5238 (2023).
Zhang, X. et al. Keto-anthraquinone covalent organic framework for H2O2 photosynthesis with oxygen and alkaline water. Nat. Commun. 15, 2649 (2024).
Richards, T. et al. A residue-free approach to water disinfection using catalytic in situ generation of reactive oxygen species. Nat. Catal. 4, 575–585 (2021).
Edwards, J. K. & Hutchings, G. J. Palladium and gold–palladium catalysts for the direct synthesis of hydrogen peroxide. Angew. Chem. Int. Ed. 47, 9192–9198 (2008).
Li, Q. et al. Shear stress triggers ultrathin-nanosheet carbon nitride assembly for photocatalytic H2O2 production coupled with selective alcohol oxidation. J. Am. Chem. Soc. 145, 20837–20848 (2023).
Zhao, L.-D. et al. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 508, 373–377 (2014).
Liu, D. et al. Lattice plainification advances highly effective SnSe crystalline thermoelectrics. Science 380, 841–846 (2023).
Huang, J. et al. Oxyhydroxide nanosheets with highly efficient electron–hole pair separation for hydrogen evolution. Angew. Chem. Int. Ed. 55, 2137–2141 (2016).
Sun, Y. et al. Pits confined in ultrathin cerium(IV) oxide for studying catalytic centers in carbon monoxide oxidation. Nat. Commun. 4, 2899 (2013).
Duvjir, G. et al. Origin of p-type characteristics in a SnSe single crystal. Appl. Phys. Lett. 110, 262106 (2017).
Yuan, S. et al. Surfactant-free aqueous synthesis of pure single-crystalline SnSe nanosheet clusters as anode for high energy- and power-density sodium-ion batteries. Adv. Mater. 29, 1602469 (2017).
Zhong, W. et al. Coupled vacancy pairs in Ni-doped CoSe for improved electrocatalytic hydrogen production through topochemical deintercalation. Angew. Chem. Int. Ed. 59, 22743–22748 (2020).
Liao, Q. et al. Regulating relative nitrogen locations of diazine functionalized covalent organic frameworks for overall H2O2 photosynthesis. Angew. Chem. Int. Ed. 62, e202310556 (2023).
Hou, Y. et al. Efficient photosynthesis of hydrogen peroxide by cyano‐containing covalent organic frameworks from water, air and sunlight. Angew. Chem. Int. Ed. 63, e202318562 (2024).
Derkosch, J. et al. Raman spectroscopic and X-ray diffraction study of Na2Se·9(H, D)2O and comparison between O-H(D)∙∙∙Se and O-H(D)∙∙∙S hydrogen bonds. J. Raman Spectrosc. 17, 75–78 (1986).
Vijayarangamuthu, K. & Rath, S. Nanoparticle size, oxidation state, and sensing response of tin oxide nanopowders using Raman spectroscopy. J. Alloy. Compd. 610, 706–712 (2014).
Adams, J. S. et al. Unifying concepts in electro- and thermocatalysis toward hydrogen peroxide production. J. Am. Chem. Soc. 143, 7940–7957 (2021).
Dronskowski, R. & Bloechl, P. E. Crystal orbital Hamilton populations (COHP): energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 97, 8617–8624 (1993).
Mohsenzadeh, A., Bolton, K. & Richards, T. DFT study of the adsorption and dissociation of water on Ni(111), Ni(110) and Ni(100) surfaces. Surf. Sci. 627, 1–10 (2014).
Mäkinen, M. & Laasonen, K. Density functional theory study of trends in water dissociation on oxygen-preadsorbed and pure transition metal surfaces. Surf. Sci. 734, 122305 (2023).
Sraitrova, K. et al. Vacancies in SnSe single crystals in a near-equilibrium state. Phys. Rev. B 99, 035306 (2019).
Zhang, S. et al. Formation and migration of vacancy defects in GeSe and SnSe. J. Phys. B 54, 035003 (2021).
Zhang, P. et al. Effects of four-phonon interaction and vacancy defects on the thermal conductivity of the low-temperature phase of SnSe. Phys. Rev. Appl. 21, 024043 (2024).
Chen, S. et al. In situ/operando analysis of surface reconstruction of transition metal-based oxygen evolution electrocatalysts. Cell Rep. Phys. Sci. 3, 100729 (2022).
Wu, Y.-j et al. Evolution of cationic vacancy defects: a motif for surface restructuration of OER precatalyst. Angew. Chem. Int. Ed. 60, 26829–26836 (2021).
Pang, H. et al. Realizing n-type SnTe thermoelectrics with competitive performance through suppressing Sn vacancies. J. Am. Chem. Soc. 143, 8538–8542 (2021).
Zhong, Y. et al. Large scale self-assembly of SnSe nanosheets prepared by the hot-injection method for photodetector and capacitor applications. Mater. Today Energy 12, 418–425 (2019).
Tomita, O. et al. Partial oxidation of alcohols on visible-light-responsive WO3 photocatalysts loaded with palladium oxide cocatalyst. ACS Catal. 6, 1134–1144 (2016).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Rehr, J. J. et al. Parameter-free calculations of X-ray spectra with FEFF9. Phys. Chem. Chem. Phys. 12, 5503–5513 (2010).
Berbille, A. et al. Mechanism for generating H2O2 at water–solid interface by contact-electrification. Adv. Mater. 35, 2304387 (2023).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S. et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Tao, J. et al. Climbing the density functional ladder: nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys. Rev. Lett. 91, 146401 (2003).
Nørskov, J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23 (2005).
Johnson, R. D. NIST Computational Chemistry Comparison and Benchmark Database 22nd edn, NIST standard reference database number 101 (NIST, 2022).
Jarzynski, C. Nonequilibrium equality for free energy differences. Phys. Rev. Lett. 78, 2690 (1997).
Sprik, M. & Ciccotti, G. Free energy from constrained molecular dynamics. J. Chem. Phys. 109, 7737–7744 (1998).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2024YFA1210400), the National Natural Science Foundation of China (grant nos. 22075126, 22209061, 52202242, 52402221 and 52172187; S.L., Y. Wan, Y. Zhang, Y. Zhu and Y.L.), the National Science Fund for Distinguished Young Scholars (grant no. 51925101; L.-D.Z), the Tencent Xplorer Prize (L.-D.Z), the Start-up Fund for Senior Talents in Jiangsu University (grant nos. 5501310030, 21JDG060 and 5501310015; S.L., Y. Wan and Y. Zhang) and the Jiangsu Provincial Dengfeng Program. We thank the Shanghai Synchrotron Radiation Facility of BL11B (https://cstr.cn/31124.02.SSRF.BL11B) for the assistance on XAFS measurements and Renishaw (Shanghai) for in situ Raman spectroscopy support. We also acknowledge the Hefei Advanced Computing Center for supporting the theoretical calculations.
Author information
Authors and Affiliations
Contributions
S.L. and L.-D.Z. conceived the idea and designed the study. X.Z. synthesized the catalysts and conducted catalytic performance tests. Y. Wan, J.Q., S.B. and Z.S. performed theoretical calculations. Y. Wen and X.G. conducted the TEM characterizations. Y. Zhu carried out the XAFS measurements and analysis. X.Z., H.L. and Z.Z. performed materials characterizations. X.Z., Y. Zhang and L.Z. conducted in situ experiments. S.L. and Y. Wan analysed the mechanisms. X.Y., J.Z. and Y.L. provided valuable discussions. S.L., Y. Wan and L.-D.Z. wrote the manuscript. All authors reviewed and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
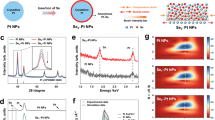
Extended Data Fig. 1 Characterization of Sn vacancy defects.
a, Se K-edge k2χ(k) EXAFS oscillation functions for SnSe NSs and bulk crystals. The reduced oscillation amplitudes in the 4 − 10 Å−1 range for SnSe NSs indicate structural disruptions associated with Sn vacancy defects. b, Corresponding Fourier transforms of the EXAFS spectra. c, EPR spectra of Sn1−xSe NSs and SnSe bulk crystals. d, Calculated defect concentration derived from the EPR spectra. e, Raman spectra of Sn1−xSe NSs and SnSe bulk crystals, exhibiting four characteristic peaks at 68.5, 103.1, 127.5 and 149.4 cm–1, correspongding to the Ag(1), B3g, Ag(2) and Ag(3) vibration modes of SnSe, respectively. The Sn1−xSe NSs exhibit broadened and diminished peaks compared to bulk crystals, indicating their ultrathin structure and the presence of defects. f, Calculated B3g/Ag(1) intensity ratios, demonstrating an increase in Sn vacancy defects with higher x values.
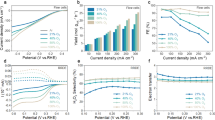
Extended Data Fig. 2 Thermocatalytic H2O2 production and mechanism analysis.
a, Time-dependent H2O2 production over SnSe NSs under different gas environment at 40˚C. Error bars represent the standard deviations (SD) of three replicate tests. b and c, Isotopic labelling experiments using both H218O and 18O2. The labeled product C7H6O218O was identified by LC-MS, characterized by a difference of charge to mass ratio (m/z) of +2. The LC-MS spectra show both oxidation of labeled water (b) and the reduction of 18O2 (c). d and e, In situ EPR spectra of DMPO-•O2− (d) and DMPO-•OH (e) at various temperatures. f, Quantitative analysis of radical concentrations for Sn0.9Se and SnSe NSs.
Supplementary information
Supplementary Information
Supplementary Notes 1–4, Figs. 1–34, Tables 1–8 and References 1–50.
Supplementary Video 1
The experimental set-up for hydrogen peroxide production using a flow reactor at room temperature. The catalyst is loaded into a commercial stainless-steel column, effectively shielding the system from any light exposure. The flow reactor operates under room temperature, with water and oxygen introduced into the system. The process relies solely on thermal energy to drive the production of hydrogen peroxide.
Supplementary Data 1
The atomic coordinates of the optimized computational models in this study.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Extended Data Fig./Table 1
Source data for Extended Data Fig. 1.
Source Data Extended Data Fig./Table 2
Source data for Extended Data Fig. 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Wan, Y., Wen, Y. et al. SnSe nanosheets with Sn vacancies catalyse H2O2 production from water and oxygen at ambient conditions. Nat Catal 8, 465–475 (2025). https://doi.org/10.1038/s41929-025-01335-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01335-4
This article is cited by
-
Multifunctional flexible thermoelectric devices for next-generation wearable and integrated systems
Science China Materials (2026)
-
Ketyl radical-mediated exfoliation and electron storage for solar hydrogen peroxide production
Nature Communications (2025)
-
Ultrafast energy-neutral molecular oxygen activation via atomically-adjacent bimetallic catalytic sites
Nature Communications (2025)