Abstract
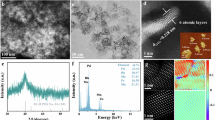
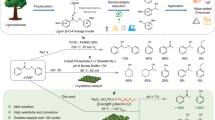
Metal single-atom catalysts offer improved activity and selectivity due to their unique electronic and coordination properties compared with bulk metals. However, many single-atom catalysts suffer from randomly dispersed active sites and limited electron-donating ability due to bonding with electronegative elements or less reactive metals. Here we demonstrate that Mg-rich intermetallic Mg29TM4 (TM = Pd, Rh, Ir, Pt) nanocatalysts overcome these limitations. These materials feature periodically dispersed, electron-rich single-atom sites of noble metals within a uniform chemical environment. Mg29TM4 exhibits high activity and selectivity in C2H2 semihydrogenation (Mg29Pd4) and olefin hydroformylation (Mg29Rh4), with Mg29Rh4 achieving high regioselectivity for branched aldehydes (branched:linear > 200:1). Kinetic and density functional theory studies suggest that the Mg–TM ensemble enables precise control over carbon–carbon multiple bond adsorption and activation, enhancing both activity and selectivity. Furthermore, the ternary Mg29Pd1.3Rh2.7 catalyst, with its synergistic Mg–Pd and Mg–Rh dual single-atom sites, efficiently catalyses a cascade reaction involving phenylacetylene hydrogenation followed by hydroformylation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this article are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Guo, Y., Wang, M., Zhu, Q., Xiao, D. & Ma, D. Ensemble effect for single-atom, small cluster and nanoparticle catalysts. Nat. Catal. 5, 766–776 (2022).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Kyriakou, G. et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 335, 1209–1212 (2012).
Lucci, F. et al. Selective hydrogenation of 1,3-butadiene on platinum–copper alloys at the single-atom limit. Nat. Commun. 6, 8550 (2015).
Hannagan, R. T. et al. First-principles design of a single-atom–alloy propane dehydrogenation catalyst. Science 372, 1444–1447 (2021).
Réocreux, R. & Stamatakis, M. One decade of computational studies on single-atom alloys: is in silico design within reach? Acc. Chem. Res. 55, 87–97 (2022).
Thirumalai, H. & Kitchin, J. R. Investigating the reactivity of single atom alloys using density functional theory. Top. Catal. 61, 462–474 (2018).
Gu, J. et al. Synergizing metal–support interactions and spatial confinement boosts dynamics of atomic nickel for hydrogenations. Nat. Nanotechnol. 16, 1141–1149 (2021).
Liu, P., Huang, X., Mance, D. & Copéret, C. Atomically dispersed iridium on MgO(111) nanosheets catalyses benzene–ethylene coupling towards styrene. Nat. Catal. 4, 968–975 (2021).
Liu, W. et al. Discriminating catalytically active FeNx species of atomically dispersed Fe–N–C catalyst for selective oxidation of the C–H bond. J. Am. Chem. Soc. 139, 10790–10798 (2017).
Sun, K. et al. Co(CN)3 catalysts with well-defined coordination structure for the oxygen reduction reaction. Nat. Catal. 6, 1164–1173 (2023).
Chen, M. et al. Intermetallic nanocatalyst for highly active heterogeneous hydroformylation. J. Am. Chem. Soc. 143, 20907–20915 (2021).
Gross, N., Kotzyba, G., Künnen, B. & Jeitschko, W. Binary compounds of rhodium and zinc: RhZn, Rh2Zn11, and RhZn13. Z. Anorg. Allg. Chem. 627, 155–163 (2001).
Dasgupta, A., Zimmerer, E. K., Meyer, R. J. & Rioux, R. M. Generalized approach for the synthesis of silica supported Pd–Zn, Cu–Zn and Ni–Zn gamma brass phase nanoparticles. Catal. Today 334, 231–242 (2019).
Dasgupta, A. et al. Atomic control of active-site ensembles in ordered alloys to enhance hydrogenation selectivity. Nat. Chem. 14, 523–529 (2022).
Ji, Y. et al. Selective CO-to-acetate electroreduction via intermediate adsorption tuning on ordered Cu–Pd sites. Nat. Catal. 5, 251–258 (2022).
Han, A. et al. Isolating contiguous Pt atoms and forming Pt–Zn intermetallic nanoparticles to regulate selectivity in 4-nitrophenylacetylene hydrogenation. Nat. Commun. 10, 3787 (2019).
Niu, Y. et al. Patterning the consecutive Pd3 to Pd1 on Pd2Ga surface via temperature-promoted reactive metal–support interaction. Sci. Adv. 8, eabq5751 (2022).
Ye, T.-N. et al. Palladium-bearing intermetallic electride as an efficient and stable catalyst for Suzuki cross-coupling reactions. Nat. Commun. 10, 5653 (2019).
Agnarelli, L. et al. Mg29−xPt4+y: chemical bonding inhomogeneity and structural complexity. Inorg. Chem. 61, 16148–16155 (2022).
Ye, T.-N. et al. Copper-based intermetallic electride catalyst for chemoselective hydrogenation reactions. J. Am. Chem. Soc. 139, 17089–17097 (2017).
Biggins, J. S., Yazdi, S. & Ringe, E. Magnesium nanoparticle plasmonics. Nano Lett. 18, 3752–3758 (2018).
Cao, Y. et al. Adsorption site regulation to guide atomic design of Ni–Ga catalysts for acetylene semi-hydrogenation. Angew. Chem. Int. Ed. 59, 11647–11652 (2020).
Fang, Q. et al. Isolated single-atom Pd sites in intermetallic nanostructures: high catalytic selectivity for semihydrogenation of alkynes. J. Am. Chem. Soc. 139, 7294–7301 (2017).
Muravev, V. et al. Interface dynamics of Pd–CeO2 single-atom catalysts during CO oxidation. Nat. Catal. 4, 469–478 (2021).
Huang, F. et al. Atomically dispersed Pd on nanodiamond/graphene hybrid for selective hydrogenation of acetylene. J. Am. Chem. Soc. 140, 13142–13146 (2018).
Huang, F. et al. Low-temperature acetylene semi-hydrogenation over the Pd1–Cu1 dual-atom catalyst. J. Am. Chem. Soc. 144, 18485–18493 (2022).
Kim, H., Park, J. & Jung, Y. The binding nature of light hydrocarbons on Fe/MOF-74 for gas separation. Phys. Chem. Chem. Phys. 15, 19644–19650 (2013).
Ma, S., Jin, Y. & Si, Y. Adsorption behavior of Pd-doped SnS2 monolayer upon H2 and C2H2 for dissolved gas analysis in transformer oil. Adsorption 25, 1587–1594 (2019).
Guo, Q. et al. Enabling semihydrogenation of alkynes to alkenes by using a calcium palladium complex hydride. J. Am. Chem. Soc. 143, 20891–20897 (2021).
Abu-Reziq, R., Alper, H., Wang, D. & Post, M. L. Metal supported on dendronized magnetic nanoparticles: highly selective hydroformylation catalysts. J. Am. Chem. Soc. 128, 5279–5282 (2006).
Liu, B., Huang, N., Wang, Y., Lan, X. & Wang, T. Promotion of inorganic phosphorus on Rh catalysts in styrene hydroformylation: geometric and electronic effects. ACS Catal. 11, 1787–1796 (2021).
Liu, B., Wang, Y., Huang, N., Lan, X. & Wang, T. Activity promotion of Rh8−xCoxP4 bimetallic phosphides in styrene hydroformylation: dual influence of adsorption and surface reaction. ACS Catal. 11, 9850–9859 (2021).
Amsler, J. et al. Prospects of heterogeneous hydroformylation with supported single atom catalysts. J. Am. Chem. Soc. 142, 5087–5096 (2020).
Lang, R. et al. Hydroformylation of olefins by a rhodium single-atom catalyst with activity comparable to RhCl(PPh3)3. Angew. Chem. Int. Ed. 55, 16054–16058 (2016).
Li, T. et al. Styrene hydroformylation with in situ hydrogen: regioselectivity control by coupling with the low-temperature water–gas shift reaction. Angew. Chem. Int. Ed. 59, 7430–7434 (2020).
Gao, P. et al. Phosphorus coordinated Rh single-atom sites on nanodiamond as highly regioselective catalyst for hydroformylation of olefins. Nat. Commun. 12, 4698 (2021).
Sun, Q. et al. Highly efficient heterogeneous hydroformylation over Rh-metalated porous organic polymers: synergistic effect of high ligand concentration and flexible framework. J. Am. Chem. Soc. 137, 5204–5209 (2015).
Xiong, Y. et al. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat. Nanotechnol. 15, 390–397 (2020).
Ferrari, P. et al. Controlling the adsorption of carbon monoxide on platinum clusters by dopant-induced electronic structure modification. Angew. Chem. Int. Ed. 55, 11059–11063 (2016).
Kress, P. L. et al. A priori design of dual-atom alloy sites and experimental demonstration of ethanol dehydrogenation and dehydration on PtCrAg. J. Am. Chem. Soc. 145, 8401–8407 (2023).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Rehr, J. J., Kas, J. J., Vila, F. D., Prange, M. P. & Jorissen, K. Parameter-free calculations of X-ray spectra with FEFF9. Phys. Chem. Chem. Phys. 12, 5503–5513 (2010).
Muñoz, M., Argoul, P. & Farges, F. Continuous Cauchy wavelet transform analyses of EXAFS spectra: a qualitative approach. Am. Mineral. 88, 694–700 (2003).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dronskowski, R. & Bloechl, P. E. Crystal orbital Hamilton populations (COHP): energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 97, 8617–8624 (1993).
Maintz, S., Deringer, V. L., Tchougréeff, A. L. & Dronskowski, R. LOBSTER: a tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem. 37, 1030–1035 (2016).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-PU. J. Chem. Phys. 132, 154104 (2010).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Henkelman, G. & Jónsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 111, 7010–7022 (1999).
Rahm, J. M. & Erhart, P. WulffPack: a Python package for Wulff constructions. J. Open Source Softw. 5, 1944 (2020).
Wang, V., Xu, N., Liu, J.-C., Tang, G. & Geng, W.-T. VASPKIT: a user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033 (2021).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22275121, 21931005, 22105122 and 52272265), the National Key R&D Program of China (2023YFA1506300 and 2023YFB3809101) and the Shanghai Municipal Science and Technology Major Project. We also thank the Fundamental Research Funds for the Central Universities (23X010301599, 24X010301678), the project of Jiangxi Academy of Sciences (2023YSTZX01), Liuchuang Program of Chongqing Municipality (cx2022038), Guangdong Provincial University Science and Technology Program (2023KTSCX123) from the Department of Education of Guangdong Province, and Shenzhen Fundamental Research funding (JCYJ20220530114616036). We also thank the User Experiment Assist System of the Shanghai Synchrotron Radiation Facility (SSRF).
Author information
Authors and Affiliations
Contributions
T.-N.Y. conceived the idea and supervised the project. X.L., J.W., Y. Lu, W.L., J.L., M.X., Y. Liu, F.P. and T.-N.Y. performed the synthesis, characterization and catalytic measurements. X.H., Z.L. and T.K. conducted the model construction and DFT calculations. Y.Q. and Q.Z. helped with the STEM measurements. M.D. helped with the CO-DRIFT measurements. X.L., H.H., J.-S.C. and T.-N.Y. co-wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Romain Réocreux and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–59, Tables 1–19, Notes 1 and 2 and references.
Supplementary Video 1
In situ TEM images.
Supplementary Data 1
Compressed zip file containing the crystal structures of Mg29Pd4, Mg29Rh4, Mg29Ir4 and Mg29Pt4.
Supplementary Data 2
Compressed zip file containing the geometric configurations of the intermediates of C2H2 hydrogenation on Mg29Pd4.
Supplementary Data 3
Compressed zip file containing the geometric configurations of the intermediates of styrene hydroformylation on Mg29Rh4.
Supplementary Data 4
Compressed zip file containing the geometric configurations for the insertion of H adatoms at the α- and β-sites of the vinyl group of styrene on Mg29Rh4.
Source data
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Fig. 5
Source data for Fig. 5.
Source Data Fig. 6
Source data for Fig. 6.
Source Data Fig. 7
Source data for Fig. 7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, X., Wu, J., He, X. et al. Ordered single active sites for cascade hydrogenation and hydroformylation reactions. Nat Catal 8, 536–547 (2025). https://doi.org/10.1038/s41929-025-01346-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01346-1
This article is cited by
-
Triangular-ordered Co atoms activate substrates for ampere-level durable hydrogen production
Nature Communications (2025)
-
From chaos to order: electron-rich single-atom intermetallic catalysts
Science China Chemistry (2025)