Abstract
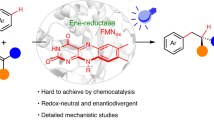
Established strategies for enantioselective hydroalkylation for C(sp3)–C(sp3) bond formation usually require prefunctionalized substrates as radical precursors in both transition-metal and photoenzymatic catalysis. Here, based on a sequential proton transfer/electron transfer strategy, we show a cooperative photoenzymatic system consisting of a flavin-dependent ‘ene’-reductase and an organophotoredox catalyst fluorescein (FI) to achieve atom-economic enantiodivergent hydroalkylation of electron-deficient C(sp3)–H with olefins. Mechanistic studies revealed a pathway for radical intermediate formation via excited-state FI*-induced single-electron oxidation of carbanions under alkaline conditions. The overall catalytic efficiency is enhanced by the electron transfer between FMNox and FI−•, while the stereoselectivity is controlled by ene-reductases through enantioselective hydrogen atom transfer. We anticipate that this mode of photoenzymatic catalysis will inspire new pathways for generating free radical intermediates and foster innovative strategies for achieving photoenzymatic new-to-nature reactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data relating to the materials and methods, experimental procedures, mechanistic studies and computational calculations, HPLC spectra and NMR spectra are available in the Supplementary Information or from the authors on reasonable request. The coordinates of QM/MM and DFT calculations and configurations for MD simulations are available via GitHub at https://github.com/calculations01/The-coordinates-of-QM-MM-and-DFT-calculations. Source data are provided with this paper.
References
Wang, J. Z., Lyon, W. L. & MacMillan, D. W. C. Alkene dialkylation by triple radical sorting. Nature 628, 104–109 (2024).
Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl–alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Zetzsche, L. E. & Narayan, A. R. H. Broadening the scope of biocatalytic C–C bond formation. Nat. Rev. Chem. 4, 334–346 (2020).
Denes, F., Perez-Luna, A. & Chemla, F. Addition of metal enolate derivatives to unactivated carbon–carbon multiple bonds. Chem. Rev. 110, 2366–2447 (2010).
Yamashita, Y., Ogasawara, Y., Banik, T. & Kobayashi, S. Photoinduced efficient catalytic α‑alkylation reactions of active methylene and methine compounds with nonactivated alkenes. J. Am. Chem. Soc. 145, 23160–23166 (2023).
Kranthikumar, R. Recent advances in C(sp3)–C(sp3) cross-coupling chemistry: a dominant performance of nickel catalysts. Organometallics 41, 667–679 (2022).
Le, C., Liang, Y., Evans, R. W., Li, X. & MacMillan, D. W. C. Selective sp3 C–H alkylation via polarity-match-based cross-coupling. Nature 547, 79–83 (2017).
Gao, D.-W. et al. Cascade CuH-catalysed conversion of alkynes into enantioenriched 1,1-disubstituted products. Nat. Catal. 3, 23–29 (2020).
Romano, C. & Martin, R. Ni-catalysed remote C(sp3)–H functionalization using chain-walking strategies. Nat. Rev. Chem. 8, 833–850 (2024).
Zhang, W. & Lin, S. Electroreductive carbofunctionalization of alkenes with alkyl bromides via a radical-polar crossover mechanism. J. Am. Chem. Soc. 142, 20661–20670 (2020).
Li, Y. et al. Enantioselective alkene hydroalkylation overcoming heteroatom constraints via cobalt catalysis. Nat. Synth. 3, 1134–1144 (2024).
Wang, Z., Yin, H. & Fu, G. C. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature 563, 379–383 (2018).
Seath, C. P., Vogt, D. B., Xu, Z., Boyington, A. J. & Jui, N. T. Radical hydroarylation of functionalized olefins and mechanistic investigation of photocatalytic pyridyl radical reactions. J. Am. Chem. Soc. 140, 15525–15534 (2018).
Su, Y.-L. et al. Radical-mediated strategies for the functionalization of alkenes with diazo compounds. J. Am. Chem. Soc. 142, 13846–13855 (2020).
Wheatley, E., Melnychenko, H. & Silvi, M. Iterative one-carbon homologation of unmodified carboxylic acids. J. Am. Chem. Soc. 146, 34285–34291 (2024).
Bian, K.-J. et al. Photocatalytic hydrofluoroalkylation of alkenes with carboxylic acids. Nat. Chem. 15, 1683–1692 (2023).
Zhu, Q. & Nocera, D. G. Photocatalytic hydromethylation and hydroalkylation of olefins enabled by titanium dioxide mediated decarboxylation. J. Am. Chem. Soc. 142, 17913–17918 (2020).
Yang, T. et al. Chemoselective union of olefins, organohalides, and redox-active esters enables regioselective alkene dialkylation. J. Am. Chem. Soc. 142, 21410–21419 (2020).
Chowdhury, R. et al. Decarboxylative alkyl coupling promoted by NADH and blue light. J. Am. Chem. Soc. 142, 20143–20151 (2020).
Wang, J.-X., Fu, M.-C., Yan, L.-Y., Lu, X. & Fu, Y. Photoinduced triphenylphosphine and iodide salt promoted reductive decarboxylative coupling. Adv. Acids. 11, 2307241 (2024).
Zhou, Z. et al. Direct stereoselective C(sp3)–H alkylation of saturated heterocycles using olefins. Nat. Chem. 17, 344–355 (2025).
Zhang, Z. et al. Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles. Nat. Chem. 16, 521–532 (2024).
Xu, P., Zhou, F., Zhu, L. & Zhou, J. Catalytic desymmetrization reactions to synthesize all-carbon quaternary stereocentres. Nat. Synth. 2, 1020–1036 (2023).
Kar, S., Sanderson, H., Roy, K., Benfenati, E. & Leszczynski, J. Green chemistry in the synthesis of pharmaceuticals. Chem. Rev. 122, 3637–3710 (2022).
Yi, H. et al. Recent advances in radical C–H activation/radical cross-coupling. Chem. Rev. 117, 9016–9085 (2017).
Rivas, M., Palchykov, V., Jia, X. & Gevorgyan, V. Recent advances in visible light-induced C(sp3)–N bond formation. Nat. Rev. Chem. 6, 544–561 (2022).
Biegasiewicz, K. F. et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization. Science 364, 1166–1169 (2019).
Page, C. G. et al. Quaternary charge-transfer complex enables photoenzymatic intermolecular hydroalkylation of olefins. J. Am. Chem. Soc. 143, 97–102 (2021).
Huang, X. et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation. Nature 584, 69–74 (2020).
Li, M., Harrison, W., Zhang, Z., Yuan, Y. & Zhao, H. Remote stereocontrol with azaarenes via enzymatic hydrogen atom transfer. Nat. Chem. 16, 277–284 (2024).
Li, M., Yuan, Y., Harrison, W., Zhang, Z. & Zhao, H. Asymmetric photoenzymatic incorporation of fluorinated motifs into olefins. Science 385, 416–421 (2024).
Bender, S. G. & Hyster, T. K. Pyridylmethyl radicals for enantioselective alkene hydroalkylation using “ene”-reductases. ACS Catal. 13, 14680–14684 (2023).
Huang, X. et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition. Nat. Catal. 5, 586–593 (2022).
Duan, X. et al. A photoenzymatic strategy for radical-mediated stereoselective hydroalkylation with diazo compounds. Angew. Chem. Int. Ed. Engl. 63, e202214135 (2023).
Sun, S.-Z. et al. Enantioselective decarboxylative alkylation using synergistic photoenzymatic catalysis. Nat. Catal. 7, 35–42 (2024).
Yu, J. et al. Repurposing visible-light-excited ene-reductases for diastereo-and enantioselective lactones synthesis. Angew. Chem. Int. Ed. Engl. 63, e202402673 (2024).
Zhao, B. et al. Direct visible-light-excited flavoproteins for redox-neutral asymmetric radical hydroarylation. Nat. Catal. 6, 996–1004 (2023).
Jiang, L. et al. Photoenzymatic redox-neutral radical hydrosulfonylation initiated by FMN. ACS Catal. 14, 6710–6716 (2024).
Shi, Q. et al. Single-electron oxidation-initiated enantioselective hydrosulfonylation of olefins enabled by photoenzymatic catalysis. J. Am. Chem. Soc. 146, 2748–2756 (2024).
Liang, Y.-F. et al. Carbon–carbon bond cleavage for late-stage functionalization. Chem. Rev. 123, 12313–12370 (2023).
O’Brien, T. E., Morris, A. O., Villela, L. F. & Barriault, L. Synthetic applications of photochemically generated radicals from protic C(sp3)–H bonds. ChemCatChem 15, e202300989 (2023).
Dong, M.-Y. et al. Hydrogen-evolution allylic C(sp3)–H alkylation with protic C(sp3)–H bonds via triplet synergistic Brønsted base/Cobalt/photoredox catalysis. ACS Catal. 12, 9533–9539 (2022).
Bas, S., Yamashita, Y. & Kobayashi, S. Development of Brønsted base-photocatalyst hybrid systems for highly efficient C–C bond formation reactions of malonates with styrenes. ACS Catal. 10, 10546–10550 (2020).
Palomo, C., Oiarbide, M. & Garcia, J. M. Current progress in the asymmetric aldol addition reaction. Chem. Soc. Rev. 33, 65–75 (2004).
Schetter, B. & Mahrwald, R. Modern aldol methods for the total synthesis of polyketides. Angew. Chem. Int. Ed. Engl. 45, 7506–7525 (2006).
Tian, J. & Zhou, L. Photoredox radical/polar crossover enables C–H gem-difunctionalization of 1,3-benzodioxoles for the synthesis of monofluorocyclohexenes. Chem. Sci. 14, 6045–6051 (2023).
Pitzer, L., Schwarz, J. L. & Glorius, F. Reductive radical-polar crossover: traditional electrophiles in modern radical reactions. Chem. Sci. 10, 8285–8291 (2019).
Black, M. J. et al. Asymmetric redox-neutral radical cyclization catalysed by flavin-dependent ‘ene’-reductases. Nat. Chem. 12, 71–75 (2020).
Evans, E. W. et. al. Magnetic field effects in flavoproteins and related systems. Interface Focus 3, 20130037 (2013).
Su, D., Kabir, M. P., Orozco-Gonzalez, Y., Gozem, S. & Gadda, G. Fluorescence properties of flavin semiquinone radicals in nitronate monooxygenase. ChemBioChem 20, 1646–1652 (2019).
Bowry, V. W. & Inlgold, K. U. Kinetics of nitroxide radical trapping. 2. Structural effects. J. Am. Chem. Soc. 114, 4992–4996 (1992).
Acknowledgements
This work was supported by the National Key R&D Program of China (grant no. 2022YFA0912000 to Y.W.), the Center of Synthetic Biology and Integrated Bioengineering (grant nos. WU2022A006, WU2022A007 and WU2023A009 to Y.W.), the Research Center for Industries of the Future (grant number WU2022C032 to Y.W.) and National Natural Science Foundation of China (grant no. 22121001 to B.W.). We thank the Instrumentation and Service Center for Molecular Sciences at Westlake University for the assistance in measurement and data interpretation. We thank Y. Chen for the assistance with HRMS, Y. Chen for the assistance with circular dichroism, X. Lou for the assistance with TAS and D. Gu for the assistance with EPR.
Author information
Authors and Affiliations
Contributions
J.Z. and Y.W. conceived and designed the overall project. J.Z. performed all synthetic experiments and wet mechanism experiments. T.G., X.L. and Mingzhe Ma created mutations. Q.Z. and X.W. carried out the computational studies and the calculation of absolute configuration (ECD) under the supervision of B.W. B.C. completed the calculation of redox potentials and Gibbs reaction energy (ΔG) for SET of Int. A. Mingjie Ma performed the purification of some GluER variants. J.Z., B.W. and Y.W. wrote the paper with input of all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Qi Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–14, Figs. 1–53 and Tables 1–17.
Supplementary Data 1
This data file corresponds to and provides the source data for Supplementary Figs. 10–16, 18–22, 24–28 and 46–51.
Source data
Source Data Fig. 1
Unprocessed statistical source data of Fig. 4a–c.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, J., Zhang, Q., Gu, T. et al. Atom-economic enantioselective photoenzymatic radical hydroalkylation via single-electron oxidation of carbanions. Nat Catal 8, 1188–1197 (2025). https://doi.org/10.1038/s41929-025-01434-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01434-2