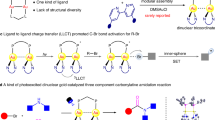
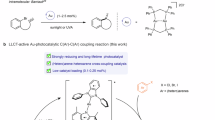
Abstract
Radical ligand transfer (RLT), a process in which alkyl radicals capture ligands from high-valent metal species, has emerged as a powerful synthetic approach in organic chemistry. While RLT processes mediated by 3d transition metals have been developed, the application of 5d transition metals remains underexplored due to the limited flexibility in their oxidation states. Here we present a catalytic approach leveraging sequential photoinduced electron transfer in dinuclear gold complexes to achieve an efficient RLT process. This strategy facilitates formal additions of gem-dichloroalkanes and Freon-22 bearing unactivated C(sp3)–Cl bonds to different kinds of alkenes, with high reactivity, excellent atom economy and broad scope. Combined mechanistic and theoretical investigations reveal a latent AuIIAuII pathway. The success originates from sequential excitation of dinuclear gold complexes for the formation of a covalent Au–Au bond, which can markedly weaken the Au–Cl bond and facilitate ligand transfer.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in the paper and its Supplementary Information or from the corresponding authors upon request.
References
Che, C.-M., Lo, V. K.-Y., Zhou, C.-Y. & Huang, J.-S. Selective functionalisation of saturated C–H bonds with metalloporphyrin catalysts. Chem. Soc. Rev. 40, 1950–1975 (2011).
Zhang, Z., Chen, P. & Liu, G. Copper-catalyzed radical relay in C(sp3)–H functionalization. Chem. Soc. Rev. 51, 1640–1658 (2022).
Li, Z.-L., Fang, G.-C., Gu, Q.-S. & Liu, X.-Y. Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 49, 32–48 (2020).
Nemoto, D. T., Bian, K.-J., Kao, S.-C. & West, J. G. Radical ligand transfer: a general strategy for radical functionalization. Beilstein J. Org. Chem. 19, 1225–1233 (2023).
Zhang, Y.-F. et al. Asymmetric amination of alkyl radicals with two minimally different alkyl substituents. Science 388, 283–291 (2025).
Zhong, F., Li, R., Mai, B. K., Liu, P. & Fu, G.-C. Photoinduced copper-catalysed deracemization of alkyl halides. Nature 640, 107–113 (2025).
Zhou, Q., Chin, M., Fu, Y., Liu, P. & Yang, Y. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450. Science 374, 1612–1616 (2021).
Li, J. et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature 574, 516–521 (2019).
Ortiz de Montellano, P. R. Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem. Rev. 110, 932–948 (2009).
Williams, T. M. & Stephenson, C. R. J. in Visible Light Photocatalysis in Organic Chemistry (eds Stephenson, C. R. J. et al.) 73–92 (Wiley-Blackwell, 2018).
Bian, K. J., Kao, S. C., Nemoto, D., Chen, X. W. & West, J. G. Photochemical diazidation of alkenes enabled by ligand-to-metal charge transfer and radical ligand transfer. Nat. Commun. 13, 7881 (2022).
Bian, K.-J. et al. Modular difunctionalization of unactivated alkenes through bio-inspired radical ligand transfer catalysis. J. Am. Chem. Soc. 144, 11810–11821 (2022).
Fu, N., Sauer, G. S., Saha, A., Loo, A. & Lin, S. Metal-catalyzed electrochemical diazidation of alkenes. Science 357, 575–579 (2017).
Xu, P., Xie, J., Wang, D.-S. & Zhang, X. P. Metalloradical approach for concurrent control in intermolecular radical allylic C–H amination. Nat. Chem. 15, 498–507 (2023).
Kochi, J. K. The mechanism of oxidative decarboxylation with lead(IV) acetate. J. Am. Chem. Soc. 87, 1811–1812 (1967).
Nakanishi, W., Yamanaka, M. & Nakamura, E. Reactivity and stability of organocopper(I), silver(I), and gold(I) ate compounds and their trivalent derivatives. J. Am. Chem. Soc. 127, 1446–1453 (2005).
Wang, W. et al. Dinuclear gold catalysis. Chem. Soc. Rev. 50, 1874–1912 (2021).
Chintawar, C. C., Yadav, A. K., Kumar, A., Sancheti, S. P. & Patil, N. T. Divergent gold catalysis: unlocking molecular diversity through catalyst control. Chem. Rev. 121, 8478–8558 (2021).
Wu, J. et al. Photosensitized gold-catalyzed cross-couplings of aryl bromides. J. Am. Chem. Soc. 147, 5839–5850 (2025).
Zhang, S., Wunsch, J. F., Rudolph, M., Rominger, F. & Hashmi, A. S. K. Selective coupling of β-(borylvinyl)silanes by a gold-catalyzed Hiyama reaction—an easy way to diaryl-substituted vinyl boronates. Angew. Chem. Int. Ed. Engl. 63, e202406856 (2024).
Melder, J. J. et al. Easy access to functionalized indolines and tetrahydroquinolines via a photochemical cascade cyclization reaction. J. Am. Chem. Soc. 146, 14521–14527 (2024).
Revol, G., McCallum, T., Morin, M., Gagosz, F. & Barriault, L. Photoredox transformations with dimeric gold complexes. Angew. Chem. Int. Ed. Engl. 52, 13342–13345 (2013).
McCallum, T. & Barriault, L. Direct alkylation of heteroarenes with unactivated bromoalkanes using photoredox gold catalysis. Chem. Sci. 7, 4754–4758 (2016).
Pyykkö, P. Theoretical chemistry of gold. III. Chem. Soc. Rev. 37, 1967–1997 (2008).
Ji, C.-L. et al. Photoinduced gold-catalyzed divergent dechloroalkylation of gem-dichloroalkanes. Nat. Catal. 5, 1098–1109 (2022).
Ji, C.-L. et al. Dinuclear gold-catalyzed divergent dechlorinative radical borylation of gem-dichloroalkanes. Nat. Commun. 15, 3721 (2024).
Che, C.-M., Kwong, H.-L., Yam, V. W.-W. & Cho, K.-C. Spectroscopic properties and redox chemistry of the phosphorescent excited state of [Au2(dppm)2]2+[dppm = bis(diphenylphosphino)methane]. J. Chem. Soc. Chem. Commun. 13, 885−886 (1989).
Che, C.-M., Kwong, H.-L., Poon, C.-K. & Yam, V. W.-W. Spectroscopy and redox properties of the luminescent excited state of [Au2(dppm)2]2+(dppm = Ph2PCH2PPh2). J. Chem. Soc. Dalton Trans. 11, 3215–3219 (1990).
Li, D., Che, C.-M., Kwong, H.-L. & Yam, V. W.-W. Photoinduced C–C bond formation from alkyl halides catalysed by luminescent dinuclear gold(I) and copper(I) complexes. J. Chem. Soc. Dalton Trans. 23, 3325–3329 (1992).
Wang, K. & Bao, X. Computational insights into the photoinduced dimeric gold-catalyzed divergent dechloroalkylation of gem-dichloroalkanes with alkenes. J. Am. Chem. Soc. 146, 7679–7689 (2024).
Huang, G., Yu, J.-T. & Pan, C. Recent advances in polychloromethylation reactions. Adv. Synth. Catal. 363, 305–327 (2021).
Fang, Q.-Y. et al. Trinuclear gold-catalyzed 1,2-difunctionalization of alkenes. Angew. Chem. Int. Ed. Engl. 62, e202305121 (2023).
Fang, Q.-Y. et al. Trinuclear gold-catalyzed site-selective alkylation of peptides. Sci. China Chem. 68, 249–256 (2025).
Mageed, A. H., Skelton, B. W., Sobolev, A. N. & Baker, M. V. Formation of dinuclear AuII and AuI/AuIII mixed-valence complexes is directed by structural constraints imposed by cyclophane-NHC ligands. Eur. J. Inorg. Chem. 1, 109–120 (2018).
Ghosh, I., Ghosh, T., Bardagi, J. I. & König, B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science 346, 725–728 (2014).
Kancherla, R. et al. Photoexcitation of distinct divalent palladium complexes in cross-coupling amination under air. Angew. Chem. Int. Ed. Engl. 63, e202314508 (2023).
Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2, 73–78 (2011).
Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 161, 082503 (2024).
Zidan, M., McCallum, T., Swann, R. & Barriault, L. Formal bromine atom transfer radical addition of nonactivated bromoalkanes using photoredox gold catalysis. Org. Lett. 22, 8401–8406 (2020).
Zeng, X. et al. Copper difluorocarbene enables catalytic difluoromethylation. J. Am. Chem. Soc. 146, 16902–16911 (2024).
Zhang, X.-Y. et al. Reductive catalytic difluorocarbene transfer via palladium catalysis. Angew. Chem. Int. Ed. Engl. 62, e202306501 (2023).
Zhang, Z.-Q. et al. Difluoromethylation of unactivated alkenes using Freon-22 through tertiary amine-borane-triggered halogen atom transfer. J. Am. Chem. Soc. 144, 14288–14296 (2022).
Acknowledgements
We thank National Natural Science Foundation of China (22471121, 22471122, 22271144), National Key Research and Development Program of China (2022YFA150320), the Fundamental Research Funds for the Central Universities (grant no. 0020514380327) and the Open Project of State Key Laboratory of Natural Medicines (SKLNMKF202401) for financial support. All theoretical calculations were performed at the High-Performance Computing Center (HPCC) of Nanjing University. We thank W. Wang, Q. Fang, B. Ling and G. Dong at Nanjing University for reproduction of the experimental procedures for products 33, 18, 59 and 64.
Author information
Authors and Affiliations
Contributions
J.X. conceived the work and designed the experiments. Y. Cheng, Y. Chen, Y.T and C.Z. performed the experiments and analysed the experimental data. C.G. performed the density functional theory calculations and discussed the results with J.H., and J.X. co-wrote the paper with input from all the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Dominik Munz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–218 and Tables 1–4.
Supplementary Data 1
Cartesian coordinates of all the optimized geometries.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, Y., Gu, C., Han, J. et al. Radical ligand transfer catalysis of photoexcited dinuclear gold complexes. Nat Catal 9, 18–27 (2026). https://doi.org/10.1038/s41929-025-01462-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01462-y