Abstract
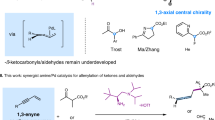
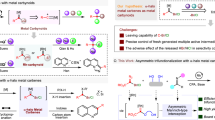
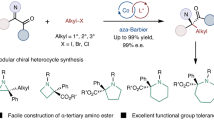
The catalytic asymmetric Michael addition of α,β-unsaturated carbonyl compounds is one of the most valuable methods for constructing the β-carbon chirality centre because of its atom economy and efficiency. However, the catalytic asymmetric reverse α-addition of a nucleophile to an α,β-unsaturated carbonyl compound is much less common. Here we realize a palladium-catalysed asymmetric α-carboranylation of α,β-unsaturated carboxylic acids via an inverse electron-demand nucleophilic addition. The reaction features good B(9)-site selectivity of o/m-carboranes, precise α-regioselectivity towards α,β-unsaturated carboxylic acids, wide functional group tolerance and excellent enantioselectivities. A detailed reaction mechanism is proposed based on experimental and computational results that elucidates the origin of the enantioselectivity and α-selectivity. This finding has a guiding significance for the catalytic asymmetric anti-Michael-type addition of α,β-unsaturated carbonyl compounds and provides a different avenue for synthesizing α-chiral carboxylic acids.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Crystallographic data for compounds 1, 36, (S)-48, (R)-48, 66 and 72 have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2380830-2380835. Additional optimization, experimental procedures, characterization of compounds and all other data supporting the findings are available in the Supplementary Information and from the corresponding author upon request.
References
Grimes, R. N. Carboranes 3rd edn (Elsevier, 2016).
Marfavi, A., Kavianpour, P. & Rendina, L. M. Carboranes in drug discovery, chemical biology and molecular imaging. Nat. Rev. Chem. 6, 486–504 (2022).
Stockmann, P., Gozzi, M., Kuhnert, R., Sárosi, M. B. & Hey-Hawkins, E. New keys for old locks: carborane-containing drugs as platforms for mechanism-based therapies. Chem. Soc. Rev. 48, 3497–3512 (2019).
Ochi, J., Tanaka, K. & Chujo, Y. Recent progress in the development of solid-state luminescent o-carboranes with stimuli responsivity. Angew. Chem. Int. Ed. 59, 9841–9855 (2020).
Ready, A. D., Nelson, Y. A., Pomares, D. F. T. & Spokoyny, A. M. Redox-active boron clusters. Acc. Chem. Res. 57, 1310–1324 (2024).
He, T., Klare, H. F. T. & Oestreich, M. Arenium-ion-catalysed halodealkylation of fully alkylated silanes. Nature 623, 538–543 (2023).
Kona, C. N. et al. Aromatic halogenation using carborane catalyst. Chem. 10, 402–413 (2024).
Hawthorne, M. F. & Maderna, A. Applications of radiolabeled boron clusters to the diagnosis and treatment of cancer. Chem. Rev. 99, 3421–3434 (1999).
Issa, F., Kassiou, M. & Rendina, L. M. Boron in drug discovery: carboranes as unique pharmacophores in biologically active compounds. Chem. Rev. 111, 5701–5722 (2011).
Xu, Y., Yang, Y., Liu, Y., Li, Z. & Wang, H. Boron-catalysed hydrogenolysis of unactivated C(aryl)–C(alkyl) bonds. Nat. Catal. 6, 16–22 (2023).
Keener, M. et al. Redox-switchable carboranes for uranium capture and release. Nature 577, 652–655 (2020).
Bawari, D., Toami, D., Jaiswal, K. & Dobrovetsky, R. Hydrogen splitting at a single phosphorus centre and its use for hydrogenation. Nat. Chem. 16, 1261–1266 (2024).
Ma, W. et al. Luminescence modulation in boron-cluster-based luminogens via boron isotope effects. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202410430 (2024).
Qiu, Z. & Xie, Z. Functionalization of o-carboranes via carboryne intermediates. Chem. Soc. Rev. 51, 3164–3180 (2022).
Liu, Q. et al. Visible-light-induced photoreduction of carborane phosphonium salts: efficient synthesis of carborane-oxindole-pharmaceutical hybrids. Angew. Chem. Int. Ed. 62, e202305088 (2023).
Spokoyny, A. M. et al. A coordination chemistry dichotomy for icosahedral carborane-based ligands. Nat. Chem. 3, 590–596 (2011).
Yu, W.-B., Cui, P.-F., Gao, W.-X. & Jin, G.-X. B–H activation of carboranes induced by late transition metals. Coord. Chem. Rev. 350, 300–319 (2017).
Quan, Y. & Xie, Z. Controlled functionalization of o-carborane via transition metal catalyzed B–H activation. Chem. Soc. Rev. 48, 3660–3673 (2019).
Zhang, X. & Yan, H. Transition metal-induced B–H functionalization of o-carborane. Coord. Chem. Rev. 378, 466–482 (2019).
Qiu, Z. & Xie, Z. A strategy for selective catalytic B–H functionalization of o-carboranes. Acc. Chem. Res. 54, 4065–4079 (2021).
Yang, L., Zhang, Z.-J., Jei, B. B. & Ackermann, L. Electrochemical cage activation of carboranes. Angew. Chem. Int. Ed. 61, e202200323 (2022).
Ren, H. et al. Dative bonding activation enables precise functionalization of the remote B–H bond of nido-carborane clusters. J. Am. Chem. Soc. 146, 26543–26555 (2024).
Xu, S. et al. Photoinduced selective B–H activation of nido-carboranes. J. Am. Chem. Soc. 146, 7791–7802 (2024).
Guo, C., Zhang, J., Ge, Y., Qiu, Z. & Xie, Z. Asymmetric palladium migration for synthesis of chiral-at-cage o-carboranes. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202416987 (2024).
Cheng, R., Zhang, J., Zhang, H., Qiu, Z. & Xie, Z. Ir-catalyzed enantioselective B–H alkenylation for asymmetric synthesis of chiral-at-cage o-carboranes. Nat. Commun. 12, 7146 (2021).
Cheng, R. et al. Enantioselective Synthesis of chiral-at-cage o-carboranes via Pd-catalyzed asymmetric B–H substitution. J. Am. Chem. Soc. 140, 4508–4511 (2018).
Guo, W., Guo, C., Ma, Y.-N. & Chen, X. Practical synthesis of B(9)-halogenated carboranes with N-haloamides in hexafluoroisopropanol. Inorg. Chem. 61, 5326–5334 (2022).
Ma, Y.-N. et al. B(9)-OH-o-carboranes: synthesis, mechanism, and property exploration. J. Am. Chem. Soc. 145, 7331–7342 (2023).
Wang, Y. et al. Highly selective electrophilic B(9)-amination of o-carborane driven by HOTf and HFIP. Org. Chem. Front. 9, 4975–4980 (2022).
Wang, Y., Li, Y.-G., Chen, F., Ma, Y.-N. & Chen, X. HSAB theory guiding electrophilic substitution reactions of o-carborane. Org. Chem. Front. https://doi.org/10.1039/d4qo01546k (2024).
Ma, Y.-N. et al. Palladium-catalyzed regioselective B(9)-amination of o-carboranes and m-carboranes in HFIP with broad nitrogen sources. J. Am. Chem. Soc. 144, 8371–8378 (2022).
Schnitzer, T., Budinská, A. & Wennemers, H. Organocatalysed conjugate addition reactions of aldehydes to nitroolefins with anti selectivity. Nat. Catal. 3, 143–147 (2020).
Deng, T. et al. Organocatalytic asymmetric α-C–H functionalization of alkyl amines. Nat. Catal. 7, 1076–1085 (2024).
Zhao, M. et al. Cobalt-catalyzed enantioselective reductive arylation, heteroarylation, and alkenylation of michael acceptors via an elementary mechanism of 1,4-addition. J. Am. Chem. Soc. 146, 20477–20493 (2024).
Vastakaite, G., Budinská, A., Bögli, C. L., Boll, L. B. & Wennemers, H. Kinetic resolution of β-branched aldehydes through peptide-catalyzed conjugate addition reactions. J. Am. Chem. Soc. 146, 19101–19107 (2024).
Chen, L. et al. Asymmetric nucleophilic additions promoted by quaternary phosphonium ion-pair catalysts. CCS Chem. 6, 2110–2130 (2024).
Su, W. et al. Copper-catalysed asymmetric hydroboration of alkenes with 1,2-benzazaborines to access chiral naphthalene isosteres. Nat. Chem. 16, 1312–1319 (2024).
Rossiter, B. E. & Swingle, N. M. Asymmetric conjugate addition. Chem. Rev. 92, 771–806 (1992).
Jerphagnon, T., Pizzuti, M. G., Minnaard, A. J. & Feringa, B. L. Recent advances in enantioselective copper-catalyzed 1,4-addition. Chem. Soc. Rev. 38, 1039–1075 (2009).
Berger, M., Ma, D., Baumgartner, Y., Wong, T. H.-F. & Melchiorre, P. Stereoselective conjugate cyanation of enals by combining photoredox and organocatalysis. Nat. Catal. 6, 332–338 (2023).
Schnurr, M., Rackl, J. W. & Wennemers, H. Overcoming deactivation of amine-based catalysts: access to fluoroalkylated γ-nitroaldehydes. J. Am. Chem. Soc. 145, 23275–23280 (2023).
Suzuki, H., Moro, R. & Matsuda, T. Palladium-catalyzed anti-Michael-type (Hetero)arylation of acrylamides. J. Am. Chem. Soc. 146, 13697–13702 (2024).
Kang, G., Strassfeld, D. A., Sheng, T., Chen, C.-Y. & Yu, J.-Q. Transannular C–H functionalization of cycloalkane carboxylic acids. Nature 618, 519–525 (2023).
Zhang, T. et al. Enantioselective remote methylene C−H (hetero)arylation of cycloalkane carboxylic acids. Science 384, 793–798 (2024).
Wang, T.-C. et al. Stereoselective amino acid synthesis by photobiocatalytic oxidative coupling. Nature 629, 98–104 (2024).
Ren, H. et al. Direct B–H functionalization of icosahedral carboranes via hydrogen atom transfer. J. Am. Chem. Soc. 145, 7638–7647 (2023).
Zhang, Q., Wu, L.-S. & Shi, B.-F. Forging C−heteroatom bonds by transition-metal-catalyzed enantioselective C–H functionalization. Chem. 8, 384–413 (2022).
Zhang, Q. & Shi, B.-F. 2-(Pyridin-2-yl)isopropyl (PIP) amine: an enabling directing group for divergent and asymmetric functionalization of unactivated methylene C(sp3)-H bonds. Acc. Chem. Res. 54, 2750–2763 (2021).
Wakchaure, V. N. et al. Catalytic asymmetric cationic shifts of aliphatic hydrocarbons. Nature 625, 287–292 (2024).
Wagen, C. C., McMinn, S. E., Kwan, E. E. & Jacobsen, E. N. Screening for generality in asymmetric catalysis. Nature 610, 680–686 (2022).
Singh, V. K. et al. Taming secondary benzylic cations in catalytic asymmetric SN1 reactions. Science 382, 325–329 (2023).
Dziedzic, R. M. et al. Cage-walking: vertex differentiation by palladium-catalyzed isomerization of B(9)-bromo-meta-carborane. J. Am. Chem. Soc. 139, 7729–7732 (2017).
Frisch, M. J. et al. Gaussian 16, Revision B.01 (Gaussian Inc., 2016).
Gao, T. et al. Stereodivergent synthesis through catalytic asymmetric reversed hydroboration. J. Am. Chem. Soc. 141, 4670–4677 (2019).
Bader, R. F. W. A quantum theory of molecular structure and its applications. Chem. Rev. 91, 893–928 (1991).
Zhang, X. et al. B–H···π interaction: a new type of nonclassical hydrogen bonding. J. Am. Chem. Soc. 138, 4334–4337 (2016).
Acknowledgements
This work was supported by National Natural Science Foundation of China (grant nos. 22271256 to Y.-N.M.; U23A2078 and 22171246 to X.C.; 22473100 to D.W.), Natural Science Foundation of Henan Province (grant nos. 232301420046 and 232300421088 to Y.-N.M.) and the Key Project of the Joint Fund for Science and Technology Research and Development in Henan Province (grant no. 232301420008 to D.W.).
Author information
Authors and Affiliations
Contributions
Y.-N.M. and X.C. conceived and designed the study. D.W. directed the DFT calculations and mechanism analysis. C.L. and W.L. conducted most experiments on the asymmetric reactions. T.S. conducted the DFT calculations. Y.-X.W. conducted the experiments on the synthesis of ligands. M.H. conducted the experiments on the synthesis of substrates. Y.-N.M. and X.C. prepared the manuscript. C.L. prepared the Supplementary Information.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–49, Tables 1–19 and Spectra of NMR and HPLC.
Supplementary Data
CIF files of compounds 1, 36, (S)-48, 66, 72 and (R)-48.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lei, C., Lu, W., Shen, T. et al. Palladium-catalysed asymmetric anti-Michael-type addition of α,β-unsaturated carboxylic acids with carboranes. Nat Catal (2026). https://doi.org/10.1038/s41929-026-01480-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41929-026-01480-4