Abstract
Vestibular motion perception declines with age, increasing the risk of falling substantially. We performed a two-week perceptual learning intervention using a self-motion direction discrimination task (2800 training trials per person) on a 6 degrees of freedom motion platform in healthy older adults (n = 40, aged 70–88 yr). Linear inter-aural and angular roll tilt vestibular thresholds improved with training (95% credible interval for pre/post difference), suggesting altered sensitivity post-training. Moreover, improved perceptual abilities transfer to actual posture (reduced sway) and gait parameters. Passive self-motion discrimination training provides a new and promising way to counteract age-related sensory decline. It can reduce the risk of falling, and thereby maintain individual autonomy and quality of life.
Similar content being viewed by others
Introduction
Perceptual learning is the most basic form of learning. It is preserved throughout life, and it improves the ability to detect and recognize sensory stimuli. It occurs in all sensory modalities1, and numerous studies have provided compelling demonstrations that perceptual abilities improve after an intense training. This means that subtle differences in sensory stimuli that remained unnoticed before training can be successfully discriminated after training. Thus, the extraction of information from sensory input improved2,3.
Interestingly, perceptual learning opens new possibilities to counteract declining sensory abilities as a function of age. A case in point is the vestibular organ in the inner ear, which is required for postural stability, secure stance, and locomotion. Its roughly 100,000 receptors have a motion direction sensitivity, and the ascending afferent information reaches a network of subcortical and cortical areas4.
Just like any other sensory modality, the vestibular system suffers a structural decline with age. The number of hair cells decreases steadily throughout life, while the decline of the regular and irregular vestibular afferents and central neurons begins around mid-life5. Functional vestibular decline manifests itself in various tests of vestibular function6. Vestibular evoked myogenic potentials (VEMPs) start to decrease after the age of 50-60, and reflexive eye movements during the head impulse test (HIT) decline after the age of 70-90. On the perceptual level, vestibular thresholds are stable in young participants7, and they start to increase at the age of 408,9. This age-related vestibular decline is correlated with impaired cognitive functions [e.g., spatial navigation, memory, attention;10,11,12], an increased risk of dementia13,14, and an increased risk of falling15,16. Vice versa, there is evidence of impaired cognitive abilities in vestibular patients17,18,19,20, emphasizing the relevance of vestibular information to cognitive functions.
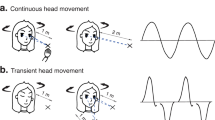
Changes in perceptual thresholds are the hallmark of perceptual learning, which has been demonstrated in the vestibular domain in younger participants (age < 35 years) for roll tilt motions [for a description of the motion profiles used in this study, see Fig. 2 and section Vestibular stimulation;21,22]. Vestibular perceptual learning leads to a better discrimination of self-motion direction. Proper detection of self-motion is crucial for everyday locomotion23,24,25.
We investigate vestibular perceptual learning in older adults to counteract age-related sensory impairment. To our knowledge, no attempt has been made to improve vestibular perceptual thresholds in a large sample of older adults. However, visual perceptual learning has been shown successfully in vision tests in college baseball players26 and older adults27.
The rate of fall-related death or serious injury is highest in people over 60 years of age15. While there are wide-ranging procedures and campaigns to improve balance and prevent falls in older adults, no intervention directly targets the vestibular component of postural control. Sensory information of vestibular origin plays a crucial role in the control of posture and balance28,29. For example, vestibular dysfunction in a posturographic measurement is linked to the risk of falling30. Moreover, mid-frequency (i.e., 0.5 Hz) roll tilt vestibular thresholds31, and inter-aural vestibular thresholds32 are correlated with quiet stance postural sway in young adults. Similarly, vestibular roll tilt thresholds have been shown to correlate with the likelihood of being able to successfully complete (i.e., stand for 30 s) a balance task in a sample of adults over the age of 408. This correlation is strongest when participants are standing on foam with eyes closed during the posturographic measurement25,32,33, thus further emphasizing the contribution of vestibular over visual and proprioceptive cues. Perceptual training of 0.5 Hz roll tilts was associated with a decrease in body sway, when standing on foam22.
We investigated whether improvements of vestibular sensitivity via perceptual learning transfers to balance performance, thus showing a causal relation between the distinct metrics. Indeed, seemingly distant metrics such as vestibular perceptual thresholds, postural sway, subjective visual verticality, or the vestibular ocular reflex have been shown to be closely related. They all estimate the standard deviation of a Gaussian distribution describing the underlying neural noise34.
In addition to posturography, we include gait measures. Vestibular loss or hypofunction is associated with clinical and functional deficits in gait, such as lateral gait deviation and tendency to fall, insecure gait with postural instability or severely slowed gait with fear of falling35,36. Gait also changes with age37 and impairments are an early indicator of the onset of mild cognitive impairment and Alzheimer’s disease38,39. It is therefore important to better understand the relationship between gait and vestibular thresholds, since impaired vestibular responses are also associated with the onset of mild cognitive impairment14.
Taken together, increased roll tilt thresholds in older age are correlated with a decline in balance test performance8. Head acceleration during roll tilt motion is interesting because it resembles head acceleration during the onset of a fall to the side. In this study, we want to go beyond correlation and alter roll tilt thresholds via vestibular perceptual learning, and assess balance abilities and gait before and after training. Declining balance skills are a serious health threat for older adults. To date, vestibular perceptual learning in older adults has not yet been investigated with a large sample. We expect an improvement in vestibular roll tilt thresholds, posturography and gait measures as a result of roll tilt training. Inter-aural translations did not yield altered thresholds in young adults21, and thus, changes due to inter-aural translation training are not expected.
Results
We computed vestibular perceptual thresholds (VPT, roll tilt and inter-aural translation), posturography, and gait before, during, and after training vestibular self-motion discrimination (Fig. 1).
A Visualization of the training and assessment process. Assessments of vestibular perceptual threshold (VPT) measurements, posturography, and gait analysis were conducted on day 1, day 5, and day 10. At all other days, participants performed a 1 h training session. B Motion platform used for assessment of vestibular perceptual thresholds and training during seven sessions. C Participant standing on force plate (eyes closed, on foam). D Participant walking across the sensor carpet (GAITRite®). The pictograms in (B–D) are published on Zenodo68.
Half of the participants were randomly assigned to the roll tilt training condition and the other half to the inter-aural training condition. Roll tilt and inter-aural thresholds were estimated for all participants. The vestibular training consisted of seven sessions, each of which had 400 trials of self-motion discrimination with feedback. Response bias and vestibular threshold are derived from the psychometric function in all conditions40,41. Posterior distributions of the estimated parameters and differences between them are described by their median and the 95% quantile interval (QI). The estimates are credibly smaller or larger than 0 when its entire 95% QI falls below or above zero. If zero is included in the interval, no difference can be inferred.
A full account of the estimated parameter values can be found in Supplementary Table 1.1, all differences between measurement time points (pre/post, pre/mid) in Supplementary Table 1.2, and estimated pre-post differences in posturography and gait in Supplementary Table 2.1, Supplementary Table 2.2, and Supplementary Table 3.1, Supplementary Table 3.2, respectively.
Vestibular perceptual thresholds
Data were excluded when the psychometric function could not be estimated. This was the case in five participants, because the predetermined fixed intensities used for the estimation of inter-aural translation thresholds did not contain stimuli with sufficiently large peak velocities. The same was the case for the estimation of roll tilt thresholds for one participant. One additional dataset was excluded due to an inconsistent usage of the response format. Roll tilt threshold estimation was successful for all 20 participants in the roll tilt training group, and for 19 participants in the inter-aural training group (transfer condition). Inter-aural threshold estimation was successful for 18 participants in the roll tilt training group (transfer condition), and for 17 in inter-aural training group. Figure 2A,C show the estimated psychometric functions with the corresponding threshold values in Fig. 2B, D before and after training. All included participants were able to perform the perceptual task. There is no evidence for a response bias in any of the conditions (all 50% QIs contain 0).
A Psychometric functions pre (green) and post (orange) training for the inter-aural translation group. B Individual (grey) pre and post thresholds for the inter-aural translation training group. Population-level thresholds are shown in color. C Psychometric functions pre (green) and post (orange) training for the roll tilt group. D Individual (grey) pre and post thresholds for the roll tilt training group. Population-level thresholds are shown in color. The motion profile pictograms were created using a 3D head model published on Zenodo69?.
The impact of training is shown in Fig. 3. After completion of seven sessions, inter-aural vestibular thresholds decreased with inter-aural discrimination training (pre/post = −1.74 cm/s, 95% QI [−3.29; −0.58]), and roll tilt thresholds decreased with roll tilt discrimination training (pre/post = −0.2 °/s, 95% QI [−0.41; 0.0]; Fig. 3: B). Hence, vestibular perceptual learning was successful.
Quantile intervals not containing zero are colored black, while those containing zero are colored grey. A Differences after three training sessions. B Differences after the full training (seven sessions). For posterior distribution of threshold differences for both motion profiles in the untrained condition see Supplementary Fig. 1.
After only three sessions (day 5), however, there was no reduction in thresholds (roll tilt pre/mid = −0.05 °/s, 95% QI [−0.25; 0.13]; inter-aural pre/mid = −0.97 cm/s, 95% QI [−2.55; 0.31]; Fig. 3: A). Thus, vestibular perceptual learning did not lead to noticeable changes in perceptual thresholds after three sessions. Only after seven sessions of training were we able to measure substantial changes in thresholds; Fig. 3: B)
Moreover, after completion of the seven training sessions, there was no substantial transfer from inter-aural discrimination training to roll tilt perception thresholds (pre/post = −0.05 °/s, 95% QI [−0.25; 0.13]), and, likewise, no transfer was found from roll tilt perception training to inter-aural translation thresholds (pre/post = −1.04 cm/s, 95% QI [−2.41; 0.12]). This suggests that vestibular perceptual learning is specific to the trained task.
Posturography and gait analysis
Posturographic and gait measurements were considered for all participants who completed the perception training. Posturographic measurements from one participant were excluded due to obvious measurement error.
To quantify a participant’s quiet standing balance, the trajectory of the center of pressure (COP) over time was analyzed42. The perceptual roll tilt training reduced the distance traveled by the COP (pre/post = −40.73 cm, 95% QI [−66.74; −15.13]; Fig. 4A) during the 30 s measurement interval in the difficult condition where the participants stood on foam with the eyes closed and gaze to the front. Training inter-aural translation discrimination did not change the length of the COP trajectory.
Quantile intervals not containing zero are colored black, while those containing zero are colored grey. A Change in path length of the center of pressure with participants standing on foam, eyes closed (see. Fig. 1 C). B–E Change in gait parameters.
Six gait parameters were measured (see Supplementary Table 3.1 for complete list) during six walking conditions: walking self-paced, slower or faster walking while looking forward and walking self-paced with the head reclined. Overall, gait was influenced by inter-aural training, but not by roll tilt training. Most affected was stride velocity with increased velocity in the slow (pre/post = 15.44 cm/s, 95% QI [8.49; 22.41]; Fig. 4 B), and head back (pre/post = 7.18 cm/s, 95% QI [0.39; 14.05]; Fig. 4 B) conditions, suggesting an effect of interaural training on walking speed. In the fast (pre/post = −8.09 cm/s, 95% QI [−14.98; −1.33]; Fig. 4B) condition, however, a decreased velocity was found. Stride length (pre/post = 10.35 cm, 95% QI [5.83; 14.92]; Fig. 4C), stride time (pre/post = −0.18 s, 95% QI [−0.23; −0.13]; Fig. 4D) and swing-% (pre/post = −1.63%, 95% QI [0.93; 2.34]; Fig. 4E) were changed only in the slow condition, with longer strides, a shorter stride time and a greater proportion of the gait cycle spent in the swing phase. From the increased proportion of the gait cycle spent in the swing phase follows a reduced proportion spent in the double support phase (not shown in Fig. 4, see Supplementary Table 3.2). Stride width was not influenced by either of the two training conditions.
Discussion
This study provides first evidence of vestibular perceptual learning in people above the age of 70 years. In line with our hypothesis, vestibular perceptual thresholds decreased in the roll tilt training group. Unexpectably, thresholds also improved in the inter-aural group. In fact, vestibular perceptual training led to a substantial improvement of perceptual thresholds by 33.7% for the inter-aural translation and by 21.3% for the roll tilt training group. This is in the same order of magnitude as the improvement reported by Klaus et al.21 for roll tilt at 0.2 Hz, thus exceeding other approaches like noisy galvanic vestibular stimulation, which led to a reduction of vestibular thresholds by 14% [roll tilt at 0.5 Hz, ref. 43].
The training also reduced postural sway, which is in line with previous findings showing improvements in a young cohort (20–32 years) using the same frequency of stimulus motion (0.5 Hz) for vestibular perceptual learning22,31. A participant in our study reported after the training that s/he was able to more easily put on their socks while standing on one foot. While this is just an anecdote, it is exactly the type of improvement that we are trying to achieve in real life (even though we do not recommend this type of behavior for adults older than 70). Improved vestibular perception enables to efficiently counteract slight body sway, which could ultimately result in a fall.
Postural improvements were found with eyes-closed, and standing on foam. This is the condition, which relies most on vestibular signals, as vision is blocked and proprioception is disturbed by unstable stance. All other conditions allow for strong contributions of non-vestibular signals, and posturography measures were not altered by vestibular perceptual learning.
Our study is among the first investigating the relationship between vestibular perceptual thresholds and gait parameters. The analysis of the walking conditions (slow, normal, normal with head tilted back, and fast) showed relevant changes in the slow-walking condition, with four improved parameters (stride velocity, stride length, stride time, swing-%). Overall, participants took larger steps and walked faster, which might be due to increased confidence in their balance capabilities while walking. This resulted in a more dynamic gait pattern after inter-aural training. In addition to this, we found increased stride velocity in the head-back condition and a reduced stride velocity in the fast-walking condition. This is consistent with the pattern observed in patients with visual, proprioceptive and vestibular deficits, where the strongest increase in gait variability is observed during slow walking and normalizes during fast walking44. Interestingly, gait parameters only changed in the inter-aural training group, but parameters were shifted in the same directions in the roll tilt training group.
The pre-/post-training assessments indicated that participants increased their walking speed after the training (longer stride length, shorter stride time, increased swing phase and stride velocity). Cautious gait (shorter stride length, decreased swing phase and increased stride width) is typically known in older adults with frontal gait disorder45 and patients with severe bilateral vestibulopathy44. While there were changes in both training groups, we can only claim with a high degree of certainty an improvement of gait parameters for the inter-aural training group. This group primarily trained the perception of signals generated by the otolith organs. So far, studies investigating the relation between vestibular function and gait parameters attribute gait stability to canal rather than otolith function46,47. It has to be pointed out, however, that vestibular evoked myogenic potentials (VEMPs), the method commonly used for assessing otolith function is influenced by a variety of factors such as muscle activity and electrode placement, and VEMPs show a large interpersonal variability48. Therefore, the absence of changes in otolith function in previous studies could be due to the method used. The advantage of perceptual thresholds is their direct relation to individual perceptual performance, which is not the case for reflexive responses like VEMPs. Using perceptual thresholds also provides the possibility to selectively target a certain axis of motion along which the discrimination deviates from the norm values.
More research on vestibular thresholds in older adults is needed. There are no vestibular threshold data available for older adults at the frequency used in this study [0.5 Hz; see review: ref. 9]. Bermúdez Rey et al.8 reported the best comparable inter-aural vestibular threshold for older adults (age range: 60–80 years) of 1.15 cm/s at 1 Hz. The same study reported roll tilt thresholds in the same participants as 0.67 and 1.74 deg/s at 0.2 and 1.0 Hz, respectively. Since vestibular thresholds change as a function of frequency9,49, a direct comparison of these values to our thresholds is not possible. Inter-aural thresholds in older adults before training (5.14 and 4.69 cm/s) were increased by about a factor of 2 compared to the threshold (2.3 cm/s) of a younger cohort [mean age: 36.8 years; ref. 9]. In line with Bermúdez Rey et al.8, this suggests that sensitivity to translations, sensed mainly by the otolith organs, is more impacted by age related decline than rotations, which are detected by the semicircular canals. The average pre-training threshold for roll tilt was 0.94°/s respectively 0.81°/s which is, as expected, a slight increase compared to the median of 0.7°/s reported for a younger population (mean age: 27.8 years). This is in line with some clinical assessments of age effects on the peripheral vestibular organ, which show an earlier onset of decline for the otolith organs compared to the canal function6. Others argued that central compensation processes make up for sensory decline of the semicircular canals50. However, systematic assessment of vestibular function of all five subcomponents of the vestibular organ showed inconsistent results. While one study found a higher prevalence of canal rather than otolith dysfunction in people of 70 years or older51, another study reported stronger age effects (decline) for the otolith organs52. It is important to note that clinical tests use reflex-based metrics like the VOR to assess vestibular function, while vestibular perceptual thresholds also reflect cognitive processes, as they require decision-making, which might explain different findings between reflex- and perception-based methods.
In fact, we found in a previous study21, just like in this study, an improvement in roll tilt perception via vestibular perceptual learning in young participants. Contrary to our expectations we also found an improvement in inter-aural translation perception due to perceptual learning. In young participants, the age-related decline in inter-aural translation perception did not yet occur, so that no compensation by vestibular perceptual learning could take place. In addition to this, the training protocols differed. In the current study, participants performed a total of seven training sessions, each lasting about 60 min, during which they were exposed to a total of 2800 motion stimuli with 0.5 Hz frequency. In the earlier study by Klaus et al.21, the training consisted of six sessions of about 90 min and a total of 1800 trials with 0.2 Hz motions. The comparison of the two studies yields important information about the training regime31. Klaus et al.21 trained participants for 540 min, compared to the 420 min in this study, but did not observe improvements in all movement conditions, and we argue that the number of trials should be preferred over training duration in future studies. A shorter motion duration (e.g. 0.5 Hz in this study) allows for a substantially higher number of stimuli per session and is probably suitable for fall prevention, given that transfer effects from 0.5 Hz profiles to lower frequencies have been demonstrated for the same motion axis31.
Contrary to a previous study22, reporting training effects already after 1300 trials performed over a 5-day period, we could not detect an improvement in thresholds for older adults at the intermediate assessment which was performed after 1200 trials. This discrepancy is likely due to different age groups; older adults might need a more intense training for perceptual improvement, which can be caused by reduced brain plasticity53. It is also possible that declining attentional capacities can impair the learning gain, as shown in visual perceptual learning54.
The practicability of vestibular perceptual training for fall prevention depends on precise knowledge about the required training regime. At this point in time, the evidence provided by this study is promising because not only do we see lower thresholds for detecting the direction of motion stimuli, but it also leads to an improvement in posture and gait. In future studies, however, it will be important to compare the perceptual training to standard interventions, and to assess the frequency of falls in real life. More research is also needed to fine tune the training regime, to determine how much training is necessary to preserve the training efffects for longer than 24 h, and to eventually implement the training for patients (e.g., vestibular hypofunction, stroke rehabilitation).
Unlike other countermeasures to prevent falls, vestibular perceptual learning is targeting selectively the sensory processing level. A correlation of perceptual sensitivity with posture has been shown in previous studies31,32,34, but the underlying mechanism is still unknown. We assume that improved vestibular sensitivity allows for faster evidence accumulation. This will help to prevent a fall at the earliest stage by precisely detecting slight displacements of the body earlier. This leads to faster and more accurate planning and coordination of the motor execution of countermeasures and thus allowing for an earlier interception of a potential fall, before its trajectory unfolds.
Vestibular perceptual learning has the advantage for targeted interventions. The increase in sensitivity is expected for the trained motion direction, since transfer effects are rather limited21,55,56. The available studies on vestibular perceptual learning point towards lacking or small transfer across motion types (translations, rotations) and planes (roll, pitch, yaw) but transfer effects between motion frequencies in close vicinity (e.g., from 0.2 Hz to 0.5 HZ) have been reported22. Vestibular perceptual learning does not replace existing and recommended activities such as gait, balance and functional training, and Tai Chi15, but it rather complements them because it targets specifically the perceptual discrimination abilities at the individual threshold, which is hard to achieve with any other method. Moreover, it can be used in older adults who are lacking the motivation for sports like Tai Chi or for persons with a reduced mobility range. Concrete implementations of targeted vestibular interventions therefore need more careful consideration of fall prevention and balance training programs.
No data is available on the persistence of performance improvements due to vestibular perceptual learning, but a study on subliminal conditioning of vestibular perception reported that the initial positive effects lasted for less than 20 min57. However, we conducted the final assessment one day after the last training, and it is therefore safe to claim, that the positive training effects last at least for 24 h. Longer lasting effects have been reported in visual perceptual learning, where the retention of performance improvements can last up to 18 months58.
In this study, we demonstrated that vestibular perceptual training can be used to lower perceptual thresholds, while improving posture and gait parameters. We argue that improved training protocols can provide a unique and novel intervention for fall prevention. Measuring thresholds can help to identify particularly vulnerable individuals, which can be targeted by an individualized vestibular perceptual training. Thereby, vestibular perceptual training can help to reduce fall related health costs.
Methods
Participants
Forty community-dwelling adults aged 70–88 years old (13 female) without impaired mobility participated in this study. This convenience sample was recruited through newspaper advertisements, in senior university or various local senior groups. Participants with a history of neurological or psychiatric condition, with an ear or vestibular disorder, with a balance disorder, with balance-related medication or with a severe cold were excluded from participation. Participation was compensated with 200 CHF. All participants gave informed consent prior to the study. The study was carried out in accordance with the Declaration of Helsinki and ethical approval was obtained from the Ethics Committee of the Human Sciences Faculty of the University of Bern (2020-04-00004).
Vestibular stimulation
A six degrees of freedom motion platform (6DOF2000E, MOOG Inc., East Aurora, NY, USA) was used for the vestibular perceptual training as well as for estimating the vestibular perceptual thresholds. While on the platform, participants were blindfolded and seated on a padded chair (5-point harness). The head was firmly secured to the motion platform and white noise was presented via noise-cancelling headphones (Sony WH-1000XM3, Japan) to mask the sound generated by the motion platform. The participants’ responses were recorded by means of button presses on a game controller (Logitech G F310, Switzerland). The motion platform was controlled by PlatformCommander59 an open source software for interfacing motion platforms. Roll tilt consisting of a combination of linear acceleration and angular acceleration in the roll plane (rotation axis at the level of the participant’s hips), and inter-aural translation consisting of acceleration along the inter-aural axis were used. All motion stimuli consisted of single cycles of sinusoidal acceleration with a frequency of 0.5 Hz. The peak velocities were predefined based on a pilot study (see 4).
Posturography
The posturography measurement was performed in upright stance on a force plate (Type 9286BA; Kistler, Winterthur, Switzerland) with hanging arms under six conditions. The gaze was either directed straight ahead or towards the ceiling, with eyes open or closed. The same test with gaze straight-ahead was performed while standing on foam rubber (AIREX Balance-pad Elite, Switzerland), eyes closed and eyes open. Balance had to be maintained for 30 s per stance condition. Path length and mean distance of the center of pressure (Cop) from the center of the trajectory were extracted from the force plate data. These calculations were performed according to Quijoux et al.42 implemented in Julia (https://gitlab.com/dr_e/forceplate).
Gait analysis
Participants’ gait was recorded under four conditions using a pressure-sensitive carpet (GAITRite®, CIR System, Sparta, NJ, USA). In each condition, the walk started 1.5 m in front of the carpet and stopped 1.5 m after the carpet’s end. The carpet was crossed twice at a self-selected pace, with the gaze directed forward or toward the ceiling. Crossings at slower and faster than self-selected pace were performed once facing forward. Stride length, stride time, stride velocity, stride width, the percentage of the gait cycle spent in the swing phase, and the percentage of the gait cycle spent in the total double support phase were calculated by the PKMAS ProtoKinetics Movement Analysis Software (version 5.08C2i4).
Procedure
Each participant spent 3 performance assessment days, and 7 training days in the lab. No appointments were scheduled over the weekend (between day 5 and 6 in Fig. 1D).
Performance assessment
Vestibular perceptual thresholds, posturography parameters, and gait parameters were measured before (pre; day 1), during (mid; day 8), and after completion (post; day 10) of the training program (see fig 1D). Roll tilt and inter-aural translation thresholds were measured in all participants using the method of constant stimuli. The predefined peak velocities for inter-aural translations were 1.0 cm/s, 4.4 cm/s, 7.8 cm/s, 11.2 cm/s, 14.6 cm/s, 18.0 cm/s, 21.4 cm/s and 0.5 °/s, 1.75 °/s, 3.0 °/s, 4.25 °/s, 5.5 °/s, 6.75 °/s, 8.0 °/s for roll tilts. All stimulus intensities were presented in random order with 20 repetitions each. Before each threshold measurement block, the participants were able to (re)accustom themselves with the task in a practice block with supra-threshold stimuli.
Vestibular perceptual training
All participant were assigned to one of two training conditions alternating according to recruiting order. The conditions differed exclusively in the trained stimulus type: roll tilt or inter-aural translation. Roll tilts involve the otoliths and semicircular canals, while the inter-aural translation involves exclusively the otoliths because of the absence of any rotatory component. Training consisted of a two alternative one-interval forced choice discrimination task. This means, participants were instructed to report the perceived direction (left or right) of a self-motion stimulus. For visual perceptual learning, it has been shown that task difficulty influences learning in older individuals60. Therefore, the intensity of the training stimulus was maintained at a level at which participants responded 65% correct. Based on the first threshold measurement, peak velocity was adjusted to keep the participants’ discrimination performance within ± 10% from 65% correct, by monitoring the rolling mean over the last 60 trials. Each training session consisted of 400 trials and lasted approximately 60 min. Auditory feedback on the correctness of the response was provided at the end of each trial.
Data analysis
The psychometric function relates an observers’ performance to stimulus intensity (peak velocity) and has two free parameters40. These two parameters are the bias and threshold. The bias is the stimulus intensity that yields the percentage correct midway between the lower and upper bounds of the psychometric function. The threshold refers to the width of the function’s transition (slope)41. Perceptual sensitivity is reflected in the slope of the psychometric function. For parameter estimation, the psychometric function can be formulated as a type of generalized linear model (GLM). This regression-like approach permits the inclusion of categorical predictors (e.g. time: pre, mid, post) in the basic statistical model in order to estimate the psychometric functions of different conditions simultaneously61,62. A Bayesian Hierarchical Generalized Linear Model with a logit link function was used to analyze the responses from the threshold measurement sessions. The probability of a rightward response was predicted by the stimulus (peak velocity; positive = rightward), the time (pre, mid, post) and the training status (trained Roll, trained I-A). Cell means coding was used for the categorical predictors ‘time‘ and ‘trained`. This model was fit to the vestibular data from all participants simultaneously. By using a hierarchical GLM, we account for the fact that the data is based on many participants62,63. Random effects were estimated for the within-subject variables (stimulus, time). Varying effects were estimated for the within-subject variables (stimulus, time). The vestibular bias and thresholds were calculated based on the regression coefficients (bias = − b0/b1, threshold = 1/b1). Therefore, the results are in the same units as the motion stimuli. Perceptual learning is defined as a decreased threshold (increased slope of the psychometric function) after training. The posterior distributions for the threshold comparisons between the measurement times resulted from computing the difference of the respective posterior samples. For the posturography and gait parameters, the pre/post difference for each parameter was predicted by condition. Cell means coding was used for the categorical predictors ‘time‘ and ‘axis trained`. To evaluate these differences, the 95% credibility interval (95% CrI) of the posterior distribution was used. When the entire 95% CrI of a comparison was below 0, it was interpreted as strong evidence for perceptual learning64,65. Bayesian inference was performed using brms and cmdstanr66,67. Weakly informative priors were used for model estimation. For the population-level intercepts a normal prior (mean = 0, SD = 1) and for population-level slopes a student-t distribution (df = 3, mean = 0, spread = 5) was used. Default priors provided by brms were used for all other parameters. We obtained parameter estimates by sampling four independent Markov Chains (MCMC) of 1000 warm-up samples and 1000 samples from the posterior distribution. The chains were visually checked and R-hat statistics were calculated to ensure that the samples from the chains converged to the same posterior distribution.
This study was preregistered on the Open Science Framework (OSF: https://osf.io/3tahv/). The data is available on the same repository. In the analysis, we had to deviate from the registered statistical model. We were unable to recover the parameters of the registered model and therefore opted for the model described above.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Vestibular response data, posturography, and gait data is available on OSF.
Code availability
R code to reproduce the statistical analyses, plots, and tables are available on OSF.
References
Seitz, A. R. Perceptual learning. Curr. Biol. 27, R631–R636 (2017).
Kellman, P. J. & Garrigan, P. Perceptual learning and human expertise. Phys. Life Rev. 6, 53–84 (2009).
Gold, J. I. & Watanabe, T. Perceptual learning. Curr. Biol. 20, R46–R48 (2010).
Lopez, C., Blanke, O. & Mast, F. W. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience 212, 159–179 (2012).
Jahn, K. The Aging Vestibular System: Dizziness and Imbalance in the Elderly. In Lea, J. & Pothier, D. (eds.) Advances in Oto-Rhino-Laryngology, vol. 82, 143–149 (S. Karger AG, 2019). https://www.karger.com/Article/FullText/490283.
Ji, L. & Zhai, S. Aging and the peripheral vestibular system. J. Otol. 13, 138–140 (2018).
Clark, T. K., Galvan-Garza, R. C. & Merfeld, D. M. Intra-individual consistency of vestibular perceptual thresholds. Attention, Percep., Psychophys.https://doi.org/10.3758/s13414-024-02886-7 (2024).
Bermúdez Rey, M. C. et al. Vestibular Perceptual Thresholds Increase above the Age of 40. Front. Neurol. 7, 162 (2016).
Fitze, D. C., Mast, F. W. & Ertl, M. Human vestibular perceptual thresholds - A systematic review of passive motion perception. Gait Posture 107, 83–95 (2023).
Guo, J. et al. Vestibular dysfunction leads to cognitive impairments: State of knowledge in the field and clinical perspectives (Review). Int. J. Mol. Med. 53, 36 (2024).
Smith, L. J., Wilkinson, D., Bodani, M. & Surenthiran, S. S. Cognition in vestibular disorders: state of the field, challenges, and priorities for the future. Front. Neurol. 15, 1159174 (2024).
Semenov, Y. R., Bigelow, R. T., Xue, Q.-L., du Lac, S. & Agrawal, Y. Association Between Vestibular and Cognitive Function in U.S. Adults: Data From the National Health and Nutrition Examination Survey. J. Gerontol. Ser. A, Biol. Sci. Med. Sci. 71, 243–250 (2016).
Smith, P. F. Aging of the vestibular system and its relationship to dementia. Curr. Opin. Neurol.https://doi.org/10.1097/WCO.0000000000001231 (2023).
Agrawal, Y., Smith, P. F. & Rosenberg, P. B. Vestibular impairment, cognitive decline and Alzheimer’s disease: balancing the evidence. Aging Ment. Health 24, 705–708 (2020).
WHO. Step safely: strategies for preventing and managing falls across the life-course. Tech. Rep., World Health Organization https://www.who.int/publications-detail-redirect/978924002191-4 (2021).
Montero-Odasso, M. et al. World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing 51, afac205 (2022).
Bigelow, R. T., Semenov, Y. R., du Lac, S., Hoffman, H. J. & Agrawal, Y. Vestibular vertigo and comorbid cognitive and psychiatric impairment: the 2008 National Health Interview Survey. J. Neurol., Neurosurg., Psychiatry 87, 367–372 (2016).
Yardley, L. et al. Interference between postural control and mental task performance in patients with vestibular disorder and healthy controls. J. Neurol., Neurosurg., Psychiatry 71, 48–52 (2001).
Schöne, C. G., Vibert, D. & Mast, F. W. Executive functions in patients with bilateral and unilateral peripheral vestibular dysfunction. J. Neurol. 271, 3291–3308 (2024).
Grabherr, L., Cuffel, C., Guyot, J.-P. & Mast, F. W. Mental transformation abilities in patients with unilateral and bilateral vestibular loss. Exp. Brain Res. 209, 205–214 (2011).
Klaus, M. P. et al. Roll tilt self-motion direction discrimination training: First evidence for perceptual learning. Atten., Percept., Psychophys. 82, 1987–1999 (2020).
Wagner, A. R., Kobel, M. J., Tajino, J. & Merfeld, D. M. Improving self-motion perception and balance through roll tilt perceptual training. J. Neurophysiol. 128, 619–633 (2022).
Bent, L. R., McFadyen, B. J. & Inglis, J. T. Vestibular Contributions during Human Locomotor Tasks. Exerc. Sport Sci. Rev. 33, 107 (2005).
Magnani, R. M., Bruijn, S. M., van Dieën, J. H. & Forbes, P. A. Stabilization demands of walking modulate the vestibular contributions to gait. Sci. Rep. 11, 13736 (2021).
Karmali, F., Bermúdez Rey, M. C., Clark, T. K., Wang, W. & Merfeld, D. M. Multivariate analyses of balance test performance, vestibular thresholds, and age. Front. Neurol. 8, 578 (2017).
Deveau, J., Ozer, D. J. & Seitz, A. R. Improved vision and on-field performance in baseball through perceptual learning. Curr. Biol. 24, R146–R147 (2014).
Erbes, S. & Michelson, G. Stereoscopic Visual Perceptual Learning in Seniors. Geriatrics (Basel, Switz.) 6, 94 (2021).
Forbes, P. A. et al. Transformation of Vestibular Signals for the Control of Standing in Humans. J. Neurosci. 36, 11510–11520 (2016).
Cohen, H. S., Mulavara, A. P., Peters, B. T., Sangi-Haghpeykar, H. & Bloomberg, J. J. Tests of walking balance for screening vestibular disorders. J. Vestib. Res. 22, 95–104 (2012).
Agrawal, Y., Carey, J. P., Della Santina, C. C., Schubert, M. C. & Minor, L. B. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Arch. Intern. Med. 169, 938–944 (2009).
Wagner, A. R., Kobel, M. J. & Merfeld, D. M. Impact of Canal-Otolith Integration on Postural Control. Front. Integr. Neurosci. 15, 773008 (2021).
Karmali, F. et al. The role of vestibular cues in postural sway. J. Neurophysiol. 125, 672–686 (2021).
Ahmadi, S.-A. et al. Towards computerized diagnosis of neurological stance disorders: data mining and machine learning of posturography and sway. J. Neurol. 266, 108–117 (2019).
Diaz-Artiles, A. & Karmali, F. Vestibular Precision at the Level of Perception, Eye Movements, Posture, and Neurons. Neuroscience 468, 282–320 (2021).
Grove, C. R., Whitney, S. L., Pyle, G. M. & Heiderscheit, B. C. Instrumented Gait Analysis to Identify Persistent Deficits in Gait Stability in Adults With Chronic Vestibular Loss. JAMA Otolaryngol.-Head. Neck Surg. 147, 729 (2021).
Schniepp, R., Möhwald, K. & Wuehr, M. Gangstörungen bei Schwindelerkrankungen: Übersicht zu Diagnostik und Therapie. Nervenheilkunde 42, 59–65 (2023).
Jahn, K., Zwergal, A. & Schniepp, R. Gait Disturbances in Old Age. Deutsches Ärzteblatt internationalhttps://www.aerzteblatt.de/10.3238/arztebl.2010.0306 (2010).
Buracchio, T., Dodge, H. H., Howieson, D., Wasserman, D. & Kaye, J. The Trajectory of Gait Speed Preceding Mild Cognitive Impairment. Arch. Neurol. 67, 980–986 (2010).
Valkanova, V. & Ebmeier, K. P. What can gait tell us about dementia? Review of epidemiological and neuropsychological evidence. Gait Posture 53, 215–223 (2017).
Wichmann, F. A. & Hill, N. J. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept. Psychophys. 63, 1293–1313 (2001).
Merfeld, D. M. Signal detection theory and vestibular thresholds: I. Basic theory and practical considerations. Exp. Brain Res. 210, 389–405 (2011).
Quijoux, F. et al. A review of center of pressure (COP) variables to quantify standing balance in elderly people: Algorithms and open access code*. Physiological Rep. 9, e15067 (2021).
Keywan, A., Wuehr, M., Pradhan, C. & Jahn, K. Noisy Galvanic Stimulation Improves Roll-Tilt Vestibular Perception in Healthy Subjects. Front. Neurol. 9, 83 (2018).
Schniepp, R., Möhwald, K. & Wuehr, M. Gait ataxia in humans: vestibular and cerebellar control of dynamic stability. J. Neurol. 264, 87–92 (2017).
Danoudis, M., Ganesvaran, G. & Iansek, R. Disturbances of automatic gait control mechanisms in higher level gait disorder. Gait Posture 48, 47–51 (2016).
Agrawal, Y., Davalos-Bichara, M., Zuniga, M. G. & Carey, J. P. Head Impulse Test Abnormalities and Influence on Gait Speed and Falls in Older Individuals. Otol. Neurotol. 34, 1729 (2013).
Anson, E., Pineault, K., Bair, W., Studenski, S. & Agrawal, Y. Reduced vestibular function is associated with longer, slower steps in healthy adults during normal speed walking. Gait Posture 68, 340–345 (2019).
Ertl, M., Boegle, R., Kirsch, V. & Dieterich, M. On the impact of examiners on latencies and amplitudes in cervical and ocular vestibular-evoked myogenic potentials evaluated over a large sample (N = 1038). Eur. Arch. Oto-Rhino-Laryngol. 273, 317–323 (2015).
Grabherr, L., Nicoucar, K., Mast, F. W. & Merfeld, D. M. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp. Brain Res. 186, 677–681 (2008).
Karmali, F., Whitman, G. T. & Lewis, R. F. Bayesian optimal adaptation explains age-related human sensorimotor changes. J. Neurophysiol. 119, 509–520 (2018).
Agrawal, Y. et al. Decline in semicircular canal and otolith function with age. Otol. Neurotol.: Off. Publ. Am. Otological Soc., Am. Neurotol. Soc. [and] Eur. and Acad. Otol. Neurotol. 33, 832–839 (2012).
Maes, L. et al. The Effect of Age on the Sinusoidal Harmonic Acceleration Test, Pseudorandom Rotation Test, Velocity Step Test, Caloric Test, and Vestibular-evoked Myogenic Potential Test. Ear Hearing 31, 84 (2010).
Burke, S. N. & Barnes, C. A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 7, 30–40 (2006).
Seitz, A. R. Perceptual Learning: Changes across the Lifespan. Curr. Biol. 31, R69–R72 (2021).
Schoups, A. A., Vogels, R. & Orban, G. A. Human perceptual learning in identifying the oblique orientation: retinotopy, orientation specificity and monocularity. J. Physiol. 483, 797–810 (1995).
Karni, A. & Sagi, D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc. Natl Acad. Sci. 88, 4966–4970 (1991).
Keywan, A., Yassin, G., Jahn, K. & Wuehr, M. Subliminal conditioning of vestibular perception generalizes within otolith organs. J. Neurol. 269, 5258–5261 (2022).
Zhou, Y. et al. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vis. Res. 46, 739–750 (2006).
Ertl, M., Prelz, C., Fitze, D. C., Wyssen, G. & Mast, F. W. PlatformCommander - An open source software for an easy integration of motion platforms in research laboratories. SoftwareX 17, 100945 (2022).
DeLoss, D. J., Watanabe, T. & Andersen, G. J. Optimization of perceptual learning: Effects of task difficulty and external noise in older adults. Vis. Res. 99, 37–45 (2014).
DeCarlo, L. T. Signal detection theory and generalized linear models. Psychol. Methods 3, 186–205 (1998).
Knoblauch, K. & Maloney, L. T.Modeling Psychophysical Data in R (Springer New York, New York, NY, 2012). https://doi.org/10.1007/978-1-4614-4475-6.
Moscatelli, A., Mezzetti, M. & Lacquaniti, F. Modeling psychophysical data at the population-level: the generalized linear mixed model. J. Vis. 12, 26 (2012).
Kruschke, J. K. Bayesian estimation supersedes the t test. J. Exp. Psychol.: Gen. 142, 573–603 (2013).
Nicenboim, B. & Vasishth, S. Statistical methods for linguistic research: Foundational Ideas - Part II. Lang. Linguist. Compass 10, 591–613 (2016).
Bürkner, P.-C. brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 80, 1–28 (2017).
Gabry, J., Češnovar, R. & Johnson, A. cmdstanr: R Interface to ’CmdStan’ https://mc-stan.org/cmdstanr/ (2023).
Steuri, R. A., Fitze, D., Borer, R., Ertl, M. & Mast, F. Illustrations 6 DoF-Platform, Gait, Stance https://zenodo.org/records/12582584 (2024).
Rihs, R., Fitze, D., Ertl, M., Wyssen, G. & Mast, F. 3D models of 6dof motion. Zenodo https://doi.org/10.5281/zenodo.6035612 (2022).
Acknowledgements
We would like to thank the participants; R. Borer, A. Szukics, F. Leuzinger, C. Berther, and J. Beck for assistance in data collection; C. Prelz (Technology Platform of the Human Sciences Faculty, University of Bern), and P. Eichelberger (Bern Movement Lab, Berner Fachhochschule) for technical support; M. Wühr for constructive criticism on an early version of the manuscript; and R. Steuri for the graphical illustrations.
Author information
Authors and Affiliations
Contributions
These authors contributed equally: D.C.F. and M.E. D.C.F., M.E., and F.W.M. conceived, designed, and performed the project. D.C.F. and M.E. performed the data analyses. All authors contributed to interpreting the results. L.R. contributed gait and posture-related materials and analysis resources. D.C.F., M.E., and F.W.M. led the writing of the manuscript with input from L.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Faisal Karmali, Yong Gu and Sun-Young Oh for their contribution to the peer review of this work. A peer review file is available. Primary Handling Editors: Joao Valente.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fitze, D.C., Ertl, M., Radlinger, L. et al. Vestibular perceptual learning improves self-motion perception, posture, and gait in older adults. Commun Biol 7, 1087 (2024). https://doi.org/10.1038/s42003-024-06802-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-06802-5
This article is cited by
-
The effect of vibrotactile feedback on performance, perception and trust when balancing in different analog g-levels
Experimental Brain Research (2025)