Abstract
Amniote skulls are diverse in shape and skeletal composition, which is the basis of much adaptive diversification within this clade. Major differences in skull shape are established early in development, at a critical developmental interval spanning the initial outgrowth and fusion of the facial processes. In birds, this is orchestrated by domains of Shh and Fgf8 expression, known as the frontonasal ectodermal zone (FEZ). It is unclear whether this model of facial development applies to species with diverse facial skeletons, especially species possessing a skull morphology representative of early amniotes. By investigating facial morphogenesis in the lizard, Anolis sagrei, we show that reptilian skull development is driven by the same genes as mammals and birds, but the manner in which those genes regulate facial development is clade-specific. These genes are not expressed in the frontal-nasal prominence, the region of the avian FEZ. Downregulating Shh and Fgf8 signaling disrupts normal facial development, but in pathway-specific ways. Our results demonstrate that early facial morphogenesis in lizards does not conform to the FEZ model. Lizard skull development may be more representative of the ancestral amniote than other model species with highly derived facial skeletons suggesting that the FEZ may be an avian-specific novelty.
Similar content being viewed by others
Introduction
The amniote skull is remarkably diverse, varying both in facial proportions between closely-related species and the fundamental organization of its constituent bones among major amniote clades (Fig. 1). In spite of the diversity accrued over 318 million years of evolution, the developmental processes of facial prominence outgrowth and fusion are highly similar among amniotes1. These prominences grow anteriorly and medially around the nasal pits and stomodeum (the embryonic precursor to the oral cavity) as they fill with proliferating neural crest-derived mesenchyme, finally fusing together to form the upper and lower jaws2,3. The conserved nature of this process suggests a period of conserved genetic regulation, consistent with their shared common ancestry or the Developmental Hourglass model1,3. Prior research comparing chicken and mouse facial development has suggested that this conserved period of prominence outgrowth may be orchestrated by an epithelial signaling center, the frontonasal ectodermal zone (FEZ), which is identifiable as a broad region of Sonic hedgehog (Shh)-expressing ectoderm interacting with Fibroblast Growth Factor (FGF)-8 located at the dorsal lip of the stomodeum4,5. By regulating the growth of the facial prominences, the FEZ is thought to control the size and shape of the facial skeleton and, therefore, be a driver of adaptive diversification of facial morphology5,6,7,8. Similar Shh-Fgf8 expression domains are also present in the mandibular process, suggesting a shared mechanism of facial prominence growth and basis for developmental integration between the upper and lower jaw9,10,11,12.
Premaxilla bone (red), maxilla (blue) and nasal (yellow) highlighted to show variation in skeletal composition of the antorbital face. Skulls not to scale. Colored phylogenetic branches highlight the major amniote radiations: birds in orange, crocodilians in purple, chelonians in blue, squamates in yellow, and mammals in red. Species names and specimen numbers are provided in Table S1.
The possibility that the FEZ represents an amniote-wide driver of facial morphology is of particular interest in understanding the developmental basis for amniote facial diversity. Hedgehog signaling appears necessary for midline facial formation not only in birds13,14, but also in mouse5,15 and zebrafish16. Yet, whether a birdlike FEZ can be generalized across all amniotes remains unclear. The position and shape of Shh and Fgf8 expression domains differ between mouse and chicken. In chickens, Shh and Fgf8 are expressed in broad domains across the ectoderm of the fronto-nasal prominence. In mice, these genes are expressed as paired domains in the maxillo-nasal ectoderm5. Studying the expression and function of Shh and Fgf8 in clades other than mammals and birds may shed new light on the ancestral pattern of the FEZ and how these molecules contribute to the evolution of amniote facial diversity.
In addition to these questions about signaling within the facial prominences, recent research has raised new questions about the homology of facial skeleton, specifically the premaxilla. Despite a clear transitional sequence in the fossil record linking the mammalian premaxilla to the ancestral amniote premaxilla, Higashiyama et al.17,18 reported the presence of maxillary prominence contributions to the mammalian premaxilla. They hypothesized that the mammalian premaxilla is a novel bone consisting primarily of the ancestral amniote septomaxilla, uprooting traditional interpretations of skeletal homology. In that evolutionary scenario, the terminal premaxilla is lost and replaced with the rhinarium, the fleshy nose. Iyyanar et al.19 challenged this by showing contributions from the frontanasal prominence to the murine premaxilla, but a decisive resolution of this problem depends on a baseline understanding of tissue contributions to the ancestral amniote face, which can only be established by examining facial development in species from additional clades and in species with diverse facial skeletons.
Facial development in modern lizards, which constitute ~40% of modern amniote species20 and more closely approximate the skull of early amniotes and lepidiosaurs (i.e., terminal tooth-bearing premaxilla, an elongate, tooth-bearing maxilla, and dorsally placed nasal bones)21,22, can provide critical comparisons with chicken and mouse. Herein we characterize the expression and function of Shh and Fgf8 across early facial development of Anolis sagrei, an emerging model for reptilian developmental studies23,24,25,26. Our results add evolutionary polarity to prior studies of craniofacial development. We provide evidence that Shh and Fgf8 may regulate the relative proportion of the midline and lateral facial skeletons of amniotes, but in a way that is inconsistent with the FEZ model.
Results
Facial development in A. sagrei begins at or around the time of ovipositon (Sanger Stage 4). At these stages, there are no visible medial facial processes and the first branchial arch has just visibly separated into its maxillary and mandibulary processes. During the first 24–48 h of post-oviposition development (Sanger Stage 3–4) neural crest-derived mesenchyme is laterally restricted with very little neural-crest derived mesenchyme between the forebrain neurectoderm and oral epithelium. A thin layer of mesenchyme begins to appear at the midline by stage 5, but remains thin through stage 6 (Fig S1).
Shh expression and function in Anolis craniofacial development
We first asked where Shh is expressed in the face of A. sagrei during the period of facial prominence outgrowth (Sanger Stage 3 through Stage 6)27 using fluorescent hybridization chain reaction (HCR) in situ hybridization28,29 in whole embryos and sagittal tissue sections (Fig. 2A–D; Fig. S1). At the initial stage of facial prominence outgrowth (stage 3, oviposition), Shh is expressed ventrally along the midline of the ventricle, between the diencephalon (DI) and telencephalon (TE) and extending into the posteriormost neuroectoderm of the diencephalon (Fig. 2A, Fig. S1). Shh is also expressed in the midline endoderm of the dorsal pharynx (PE) posterior to Rathke’s pouch (RE) and in the oral ectoderm (OE) of the internal surface of the mandibular process. Expression of Shh in the mandibular process is present from stage 3 until stage 6 (Fig. S1). We did not observe Shh expression in the oral ectoderm adjacent to the neural ectoderm.
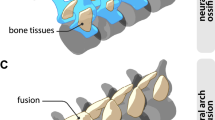
Shh expression (orange) in whole mount in situ hybridization in Anolis embryos at developmental Stage 3 (A), Stage 4 (B), Stage 5 (C), and Stage 6 (D). Fp frontal process, Fnp frontonasal process, Mxp maxillary process, Mp mandibular process. Scale bar = 200μm. Stage 18 embryos treated at oviposition with DMSO as control (E) and treated with 100 μM cyclopamine (F). Scale bar = 1 mm. Skull of Anolis Stage 18 in DMSO (G) and 100 μM cyclopamine (H) rendered from µCT, premaxilla in red, maxilla in blue, nasal in yellow, not to scale.
At stage 4, we see that expression is maintained medially in the diencephalon and in the posterior ventral neuroectoderm of the telencephalon (Fig. 2B, Fig.S1). We still see expression medially in the pharyngeal endoderm (PE) posterior to Rathke’s pouch (RE) (Fig. 2B, Fig.S1). At this stage, as in stage 3, there is no expression of Shh in the oral ectoderm adjacent to the neural ectoderm, although Shh is expressed more laterally in the oral ectoderm posterior to the nasal pit. This pattern of expression is largely maintained through the peak of facial prominence outgrowth (Sanger Stage 5–6). Expression remains strong in the ventral telencephalon. Additional sparse expression is observed in the oral epithelium anterior to Rathke’s pouch adjacent to neuroepithelial expression in the forebrain (Fig. 2C, D, Fig. S1).
Having established the time and place that Shh is expressed in the developing A. sagrei face, we asked whether Shh expression is necessary for facial prominence outgrowth. To accomplish this, we used Cyclopamine, an inhibitor of Smoothened, part of the Hedgehog signal transduction pathway30. Eggs were immersed in Cyclopamine on the day of oviposition and incubated until collection at stages 17–19 (post-oviposition days 22–24). At oviposition, embryos are most often Sanger Stage 4, but may be as early as Sanger Stage 2. In comparison with controls, embryos treated with cyclopamine have a shorter but wider body and limbs (Fig. 2E, F). Treated embryos exhibit a distinctive facial phenotype compared to the controls, characterized by a shorter snout with a mandible that is either highly reduced or completely absent. Changes in facial morphology result from the loss of the palatine and maxillary processes of the premaxilla and extreme reduction or loss of the entire mandibular ramus (Fig. 2G, H). A portion of the premaxilla facial process and egg tooth are sometimes retained, but greatly reduced in size. Additional changes to the skull include a reduced external naris, shortening of the maxilla with loss of the premaxillary process and maxillary tooth row, and rounder orbits. Despite these substantial changes in facial morphology, the nasal bone seems generally unaffected by a reduction in Hedgehog signaling. These functional results confirm that Hedgehog signaling is necessary for development of structures associated with the maxillary and mandibular processes but is dispensable in the frontonasal process.
Given these chemically induced phenotypes, we hypothesized that inhibiting Hedgehog would reduce proliferation of the neural-crest derived mesoderm within maxillary and mandibular prominences. We labeled proliferating cells in S phase of mitosis by injecting the thymidine analogue EdU into the egg (5-ethynyl-2’-deoxyuridine)31 to qualitatively compare the proliferation pattern in whole mount embryos treated with Cyclopamine. We found less EdU signal in the developing maxillary and mandibular processes in Cyclopamine-treated embryos, indicating a diffuse reduction in proliferation in these processes (Fig. S2-b, white arrows).
Fgf8 expression and function in Anolis
Given the close relationship between Shh and Fgf8 in avian embryos4, we also characterized the expression of Fgf8 across facial prominence outgrowth in whole embryos and sagittal tissue sections (Fig. 3A–D; Fig. S3). At stage 3, Fgf8 is expressed in the midline epithelium of the most anterior area of the frontal process. Fgf8 is also expressed in the neuroepithelium, but in contrast with Shh this expression is not adjacent to the frontal expression domain. Fgf8 is instead expressed more laterally within the neuroepithelium of the telencephalon. There is also a zone of strong expression on the posterior area of the maxillary process and on the most anterior area of the mandibular processes. The mandibular Fgf8 expression domain is located just anterior to the mandibular Shh expression domain.
Fgf8 expression (orange) in whole mount in situ hybridization in Anolis embryos at developmental Stage 3 (A), Stage 4 (B), Stage 5 (C), and Stage 6 (D). Fn frontal process, Fnp frontonasal process, Mxp maxillary process, Mp mandibular process. Scale bar = 200 μm. Stage 18 embryos treated at oviposition with DMSO as control (E) and treated with 100 μM Infigratinib (F). Scale bar = 1 mm. Skull of Anolis Stage 18 in DMSO (G) and 100 μM Infigratinib (H) rendered from µCT, premaxilla in red, maxilla in blue, nasal in yellow, not to scale.
By stage 4, Fgf8 expression becomes localized anteriorly at the midline of the telencephalon neuroepithelium. There is also weak expression of Fgf8 in the oral epithelium frontally and near the midline, but this disappears by stage 5. Expression of Fgf8 is particularly strong in the posterior area of the maxillary process. At this stage, some expression of Fgf8 is also visible in the nasal pit epithelium for the first time. Expression in the maxillary prominence, nasal pit epithelium, neuroepithelium of the telencephalon, and mandible persists through stage 6.
Given clear expression of Fgf8 in the facial prominences of A. sagrei, we asked whether FGF signaling is necessary for facial prominence outgrowth. We used the drug Infigratinib, an inhibitor of FGF receptors FGFR1, FGFR2, and FGFR3, to identify the effect of FGF inhibition on the morphology of the developing embryo. Embryos treated with Infigratinib show defects in the forelimbs, hindlimbs, and face (Fig. 3E, F) compared to the control embryos. Facial phenotypes include a shorter recumbent snout and reduced lower jaw. In treated embryos, the maxilla is substantially reduced or completely absent. When the maxilla is present but reduced, it is the posterior (suborbital) part of the maxilla which is missing. We also observe no effect to moderate reduction of the premaxilla in Infigratinib treated embryos. We observe no differences in the nasal bones between treated and control embryos.
Based on the observed phenotypes, we hypothesized that inhibiting the FGF pathway would change distribution of proliferating cells in the maxillary and mandibular processes. We therefore qualitatively compared the proliferation pattern between Infigratinib and control embryos using EdU. In contrast with Cyclopamine-treated embryos, we find that areas of proliferation in the maxillary and mandibular processes of Infigratinib-treated embryos are localized to the ventral area of the maxilla process and dorsal mandibular process (Fig. S2-d, white arrows), and a strong reduction in EdU signal between these processes (Fig. S2-d, red arrow). This is consistent with the understanding that FGF signaling in the facial processes sustains proliferation of the neural-crest derived mesoderm during early prominence outgrowth32,33 (Fig. S2).
Discussion
The FEZ model has served as the primary model of amniote craniofacial development for over two decades4,5,34. The FEZ is defined as the position of ectodermal Shh and Fgf8 expression, which establishes the dorsal-ventral axis and direction of outgrowth for the upper jaw4. Yet, most experimental studies of craniofacial development have focused on birds, a group where the face is dominated by a highly modified premaxilla (the beak) and a highly-reduced maxilla. Our data from Anolis, a species with a facial skeleton similar to that of early branching amniote lineages, demonstrates that the FEZ model does not readily apply to non-avian amniotes (Fig. 1).
Multiple observations from Anolis are inconsistent with the avian-derived FEZ model. We show that Hedgehog signaling is absent in the medial nasal process of Anolis. Instead, Shh expression is limited to the caudal stomodeum and narrow domains on the anteromedial surface of the maxillary process (Fig. 2, Fig. S1). Downregulation of Hedgehog signaling does not affect proliferation of the neural crest-derived mesenchyme in the region where the frontonasal processes will form, but reduces cell proliferation in the maxillary and mandibular processes (Fig. S2-b, white arrows), with the effects less apparent at the base of each process (Fig. S2-b, red arrow). Furthermore, reduction in Hedgehog signaling does not lead to the complete loss of the premaxilla (Fig. 2G, H). Rather, reduction in Hedgehog signaling results in the loss of only the lateral processes of the premaxilla, reducing the premaxilla to a thin sliver of bone along the midline. This suggests that the premaxilla is derived from cells from both the FNP and maxillary process. A compound origin of the premaxilla is also supported by uCT skull scans of Stage 15 controls, where the midline septum and the two lateral portions of the premaxilla appear to form from independent ossification centers (Fig. S4). In addition to the absence of a clear midline Shh expression domain, Anolis also lacks the midline expression of Fgf8, thus a boundary between Shh and Fgf8 expression domains (an important piece of the FEZ model) does not exist within the lizard FNP. In contrast to chicks4,5, we observe limited expression of Fgf8 along the posterior maxillary prominence and around the nasal pits (Fig. 3). Furthermore, inhibition of FGF signaling leads to a dramatic reduction in the size of the maxilla. Inhibition of this signaling results in conspicuous reduction of proliferation in the area between maxillary and mandibular process (Fig. S2-d, red arrow) consistent with expression of Fgf8 at Stage 3 (Figs. 3 and 4). Together, our observations and experiments indicate that FGF signaling plays little role in the development of midline facial structures.
Mice have been described as having an FEZ that functionally parallels what is observed in birds5,35. However, like Anolis, mice also have a facial skeleton primarily composed of the maxillary and nasal bones. Recent debates about skeletal morphology have also questioned whether mammals possess a true premaxilla17,18. To date, few people have addressed the function of Shh and Fgf8 in the mammalian maxillary prominence, although their expression is readily visible in published in situ images2,5,15,35,36,37,38.
From embryonic Day 9.5–10.5, mouse embryos show epithelial Shh expression in the medial nasal and anteromedial maxillary prominences. Conditional inhibition in murine Hedgehog signaling in cranial neural crest-derived cells show phenotypes consistent with what we observe in Anolis: a shortened, downturned face and the formation of hypoplastic premaxilla, maxilla, and internarial septum15. Although inhibition of Hedgehog signaling reduces the overall length of the snout in Anolis and mice, this is likely a consequence of changes in growth within the internarial septum39. At embryonic day 9.5–10.5 in the mouse, there is diffuse Fgf8 expression across the FNP, strong Fgf8 expression around the margin of the nasal pits, and strong expression in the posterior mandibular process (Fig. 4)5,15,35,40. This diffuse pattern does not abut the paired Shh expression domains of the medial nasal prominences. The strong nasal Fgf8 expression domains provide a morphogenetic signaling center for olfactory neurogenesis and nasal cavity development35,41. Our data from Anolis shows the importance of distinguishing between Fgf8 expression in the facial midline (as in the avian FEZ), the nasal pits (as conserved in amniotes)42, and the telencephalon (also conserved among amniotes)42. We recommend a critical reassessment of Fgf8 expression and function in a diversity of amniote species in light of our results.
We show that the close spatial relationship between Shh and Fgf8 expression domains seen in birds is not conserved in the upper jaw of Anolis, although we observe a conserved FEZ-like expression domain in the mandibular process12,36. In contrast to the diversity of the facial skeleton, widespread conservation of expression domains of Shh and Fgf8 in the amniote mandible might reflect overall similarities in anatomy, structure, and growth of the mandibular ramus. In other words, we might predict better conservation of molecular signaling in parts of the body with strong conservation of morphology. The vertebrate limb, for example, maintains a “one bone, two bone, many bone” morphology throughout tetrapod evolution43 and limb development consistently relies on reciprocal signaling between apical ectodermal ridge and zone of polarizing activity for outgrowth44. But instead of morphological conservation, the amniote upper jaw shows widespread diversity among the major morphological clades (Fig. 1). Examining facial development in additional species with more ancestral features (e.g., geckos) and highly derived facial skeletons (e.g., turtles and snakes), could be fruitful in understanding how Hedgehog and FGF signaling contribute to broad patterns of cranial diversity. Likewise, comparison of Hedgehog and FGF signaling in eutherian and metatherian craniofacial development might clarify mechanisms responsible for stronger integration in the metatherian face in comparison with eutherians45.
A close spatial relationship between Shh and Fgf8 in the FEZ has been hypothesized to be a consequence of feedback between these two pathways, synergistically facilitating cell migration and proliferation in the facial prominences9,35,46. The evolutionary origin of the FEZ during the evolution of the beak may reflect the need for this structure to develop rapidly and relatively early in craniofacial development. The apparent proximity of weak Fgf8 expression with the Shh expression domains in the murine medial nasal processes may be coincidental rather than ancestral to all amniotes. This difference in the expression pattern of Fgf8 and Shh may also explain our observation that CNC mesenchyme migrates into the midline face relatively late in both Anolis and mouse.
Our finding that Shh expression in the maxillary prominence shapes premaxilla morphology in Anolis bears on recent debates on the homology of the mammalian premaxilla. We show that inhibition of Hedgehog signaling in the maxillary prominence of Anolis is sufficient to eliminate much of the premaxillary bone, particularly the maxillary process and tooth row (Fig. 2). Because this signaling pathway is not active in the frontonasal prominence of Anolis, and because other bones of frontonasal origin (e.g., the nasal bones) are not affected in this treatment, this implies a composite (i.e., both frontonasal and maxillary prominence) origin of the premaxilla in reptiles. Higashiyama and colleagues17,18 recently proposed that the mammalian premaxilla is distinct from all other amniote premaxillae in being formed from cellular contributions from the maxillary prominence. According to these authors (and the FEZ model), the amniote premaxilla is largely, if not entirely, derived of cells from frontonasal prominence and the true mammalian premaxilla was lost as mammals evolved a fleshy nose. Our data suggest a more widespread pattern across amniotes; rather than a mammalian evolutionary novelty, a combined frontonasal/maxillary prominence origin of the premaxilla may be the ancestral amniote state. The ancestral contribution of the maxillary prominence to the avian upper jaw may have been lost during the evolution of the highly enlarged premaxillary beak that is primarily derived from the FNP.
Amniote vertebrates exhibit a vast range of facial morphologies. Our description of Shh and Fgf8 expression and function in Anolis demonstrates the importance of looking beyond the mouse and chicken models to understand larger patterns and principles of amniote craniofacial development. Our finding that lizards lack a true FEZ, and that modulating Hedgehog and FGF signaling in reptiles does not replicate patterns of craniofacial diversity identified in birds, reiterates a fact that comparative morphologists have long understood: modern mammals and modern birds have highly-derived facial skeletons produced by highly-derived developmental programs which do not generalize across amniotes. Continued investigation of developmental programs, particularly in additional species with highly derived facial skeletons like snakes and turtles, will test what features of craniofacial patterning and outgrowth are evolutionary labile and which are deeply entrenched in the history of amniotes.
Methods
Husbandry and embryo collection
Gravid females of Anolis sagrei were collected from Coral Gables, Florida, USA, and housed at Loyola University Chicago under the Institutional Animal Care and Use Committee (IACUC) protocol #1992. Eggs were collected daily between 8.30 a.m. and 10 a.m. and incubated at 27 °C until used. Embryos were dissected, collected and fixed in 4% paraformaldehyde. After fixation embryos were washed and stored at different conditions depending on the downstream experiment. Embryos used for section in situ hybridization were embedded in Tissue-Tek O.C.T. Compound (Sakura) and stored at −80 °C; embryos for whole mount in situ hybridization were dehydrated using subsequential gradient of methanol and stored at −20 in 100% methanol. Embryos used for cell proliferation assay or phenotyping were dehydrated using subsequential gradients of ethanol and stored at 4 °C in 70% ethanol.
Shh and Fgf8 expression pattern
To characterize the expression pattern of Shh and Fgf8, we performed fluorescent hybridization chain reaction (HCR) in situ hybridization28,29 on whole mount embryos stored in 100% methanol and 8 µm sagittal sections obtained by embryos embedded in Tissue-Tek O.C.T. Compound (Sakura) and cut using cryostat CM1860 UV (Leica). We used HCR from Molecular Instruments following manufactures protocol. See Supplementary data for sequences submitted to Molecular Instruments for probe design. The sequences for Shh and Fgf8 were downloaded from the A. sagrei genome47. Before imaging, samples were stained with DAPI (Sigma Aldrich) for 15 min. To reduce autofluorescence, wholemount embryos and tissue sections were treated with TrueBlack (Biotium) following manufacturer’s protocol. We acquired 15 images for each embryo using AXIO Zoom.V16 Zeiss and visualized using the extended focus function in Zen Black software (Zeiss).
Inhibition of Shh and Fgf8 using pharmaceutical assay
To test the function of the Hedgehog or FGF pathways, we immersed eggs in 100 µM Cyclopamine (Toronto Research Chemicals) or 100 µM Infigratinib (BGJ398, Selleckchem) for 30 min on the day of oviposition. We immersed control embryos in PBS with the same amount of DMSO carrier added for the chemical treatments. After immersion, eggs were returned to the incubator at 27 °C degrees until tissue collection, 24 days after treatment. Dissection of the embryos for cell proliferation assay was performed 24 h after treatments. We acquired 15 images for each embryo using AXIO Zoom.V16 Zeiss and visualized using the extended focus function in Zen Black software (Zeiss).
Cell proliferation assay
To identify proliferating cells, 10 µl of 20 mg/ml 5-ethynyl-2’-deoxyuridine (EdU) in water was injected into eggs 24 h after treatment with DMSO, 100 µM Cyclopamine, or Infigratinib as described above. Eggs were then incubated at 27 °C for 1 h, then dissected and stored in 70% ethanol at 4 °C. Samples were dehydrated and cell proliferation assay was conducted using Click-iT EdU Alexa Fluor 546 Imaging kit (Invitrogen). We followed manufacturer’s protocol for EdU detection but incubated the reaction cocktail overnight instead of 30 min as protocol suggested. Embryos were then incubated with DAPI for 10 min before imaging. Images were taken using AXIO Zoom.V16 Zeiss and processed using Zen Black software. Because subtle variation in fluorescent intensity may be introduced between trials, we for standardized brightness between samples using the eye and brain as a reference.
MicroCT scanning and Segmentation
We acquired high-resolution µCT scans of embryonic skull bone development using the Perkin Elmer Quantum GX2 µCT scanner. Each embryo was scanned at high-resolution (512 slices) using 0.05 mm Aluminum filter at 50 kilovolts (kV), 160 microamperes (µA), 18 µm voxel size. To visualize and segment the embryo skulls, we imported the scans into VGStudio Max software (Zeiss) and adjusted levels to reduce background noise. We segmented the nasal, maxillary, and premaxilla bones.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The reconstructed CT data is publicly available at Morphosource, project 000691395, under the Standard Morphosource use agreement. (https://www.morphosource.org/projects/000691395?locale=en). All other data are available from the authors on reasonable request.
References
Young, N. M. et al. Embryonic bauplans and the developmental origins of facial diversity and constraint. Development 141, 1059–1063 (2014).
Tapadia, M. D., Cordero, D. R. & Helms, J. A. It’s all in your head: new insights into craniofacial development and deformation. J. Anat. 207, 461–477 (2005).
Selleri, L. & Rijli, F. M. Shaping faces: genetic and epigenetic control of craniofacial morphogenesis. Nat. Rev. Genet. 24, 610–626 (2023).
Hu, D., Marcucio, R. S. & Helms, J. A. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development 130, 1749–1758 (2003).
Hu, D. & Marcucio, R. S. Unique organization of the frontonasal ectodermal zone in birds and mammals. Dev. Biol. 325, 200–210 (2009).
Marcucio, R. S., Young, N. M., Hu, D. & Hallgrimsson, B. Mechanisms that underlie co-variation of the brain and face. Genesis 49, 177–189 (2011).
Chong, H. J. et al. Signaling by SHH rescues facial defects following blockade in the brain. Dev. Dyn. 241, 247–256 (2012).
Adameyko, I. & Fried, K. The nervous system orchestrates and integrates craniofacial development: a review. Front. Physiol. 7, 49–49 (2016).
Abzhanov, A. & Tabin, C. J. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev. Biol. 273, 134–148 (2004).
Inman, K. E., Purcell, P., Kume, T. & Trainor, P. A. Interaction between Foxc1 and Fgf8 during mammalian jaw patterning and in the pathogenesis of syngnathia. PLoS Genet 9, e1003949 (2013).
Billmyre, K. K. & Klingensmith, J. Sonic hedgehog from pharyngeal arch 1 epithelium is necessary for early mandibular arch cell survival and later cartilage condensation differentiation. Dev. Dyn. 244, 564–576 (2015).
Xu, J. et al. Hedgehog signaling patterns the oral-aboral axis of the mandibular arch. eLife 8, e40315 (2019).
Marcucio, R. S., Cordero, D. R., Hu, D. & Helms, J. A. Molecular interactions coordinating the development of the forebrain and face. Dev. Biol. 284, 48–61 (2005).
Marchini, M. et al. Wnt signaling drives correlated changes in facial morphology and brain shape. Front. Cell Dev. Biol. 9, 644099 (2021).
Jeong, J., Mao, J., Tenzen, T., Kottmann, A. H. & McMahon, A. P. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 18, 937–951 (2004).
Wada, N. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development 132, 3977–3988 (2005).
Higashiyama, H., Koyabu, D. & Kurihara, H. Evolution of the therian face through complete loss of the premaxilla. Evol. Dev. 25, 103–118 (2023).
Higashiyama, H. et al. Mammalian face as an evolutionary novelty. Proc. Natl. Acad. Sci. USA 118, e2111876118 (2021).
Iyyanar, P. P. R. et al. Developmental origin of the mammalian premaxilla. Dev. Biol. 503, 1–9 (2023).
Pincheira-Donoso, D., Bauer, A. M., Meiri, S. & Uetz, P. Global taxonomic diversity of living reptiles. PLoS ONE 8, e59741 (2013).
Carroll, R. L. The earliest reptiles. Zool. J. Linn. Soc. 45, 61–83 (1964).
Carroll, R. L. Vertebrate Paleontology and Evolution. (Freeman, 1988).
Hermyt, M., Janiszewska, K. & Rupik, W. Squamate egg tooth development revisited using three‐dimensional reconstructions of brown anole (Anolis sagrei, Squamata, Dactyloidae) dentition. J. Anat. 236, 1004–1020 (2020).
Tschopp, P. et al. A relative shift in cloacal location repositions external genitalia in amniote evolution. Nature 516, 391–394 (2014).
Sanger, T. J. Integrative developmental biology in the age of anthropogenic change. Evol. Dev. 23, 320–332 (2021).
Sanger, T. J. et al. The oestrogen pathway underlies the evolution of exaggerated male cranial shapes in Anolis lizards. Proc. R. Soc. B 281, 20140329 (2014).
Sanger, T. J., Losos, J. B. & Gibson-Brown, J. J. A developmental staging series for the lizard genus Anolis: a new system for the integration of evolution, development, and ecology. J. Morph. 269, 129–137 (2008).
Choi, H. M. T. et al. Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development 145, dev165753 (2018).
Choi, H. M. T. et al. Mapping a multiplexed zoo of mRNA expression. Development 143, 3632–3637 (2016).
Chen, J. K., Taipale, J., Cooper, M. K. & Beachy, P. A. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev 16, 2743–2748 (2002).
Flomerfelt, F. A. & Gress, R. E. Analysis of cell proliferation and homeostasis using EdU labeling. in T-Cell Development, Vol. 1323 (eds Bosselut, R. & S. Vacchio, M.) 211–220 (Springer, 2016).
Thisse, B. & Thisse, C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev. Biol. 287, 390–402 (2005).
Hao, Y., Tang, S., Yuan, Y., Liu, R. & Chen, Q. Roles of FGF8 subfamily in embryogenesis and oral‑maxillofacial diseases. Int J Oncol 54, 797–806 (2019).
Hu, D. et al. Signals from the brain induce variation in avian facial shape. Dev. Dyn. 244, 1133–1143 (2015).
Griffin, J. N. et al. Fgf8 dosage determines midfacial integration and polarity within the nasal and optic capsules. Dev. Biol. 374, 185–197 (2013).
Abzhanov, A., Cordero, D. R., Sen, J., Tabin, C. J. & Helms, J. A. Cross–regulatory interactions between Fgf8 and Shh in the avian frontonasal prominence. Congenit. Anon. 47, 136–148 (2007).
Fuchs, A. et al. Regulation of Tbx22 during facial and palatal development. Dev. Dyn. 239, 2860–2874 (2010).
Creuzet, S., Schuler, B., Couly, G. & Le Douarin, N. M. Reciprocal relationships between Fgf8 and neural crest cells in facial and forebrain development. Proc. Natl. Acad. Sci. USA 101, 4843–4847 (2004).
Kaucka, M. et al. Signals from the brain and olfactory epithelium control shaping of the mammalian nasal capsule cartilage. Elife 7, e34465 (2018).
Bachler, M. & Neubüser, A. Expression of members of the Fgf family and their receptors during midfacial development. Mech. Dev. 100, 313–316 (2001).
Kawauchi, S. et al. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development 132, 5211–5223 (2005).
Bhullar, B.-A. S. et al. A molecular mechanism for the origin of a key evolutionary innovation, the bird beak and palate, revealed by an integrative approach to major transitions in vertebrate history. Evolution 69, 1665–1677 (2015).
Shubin, N. Your Inner Fish: A Journey into the 3.5-Billion-Year History of the Human Body (Vintage Books, 2009).
Barresi, M. J. F. & Gilbert, S. F. Developmental Biology. (Oxford University Press, 2024).
Newton, A. H., Weisbecker, V., Pask, A. J. & Hipsley, C. A. Ontogenetic origins of cranial convergence between the extinct marsupial thylacine and placental gray wolf. Commun Biol 4, 51 (2021).
Minoux, M. & Rijli, F. M. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 137, 2605–2621 (2010).
Geneva, A. J. et al. Chromosome-scale genome assembly of the brown anole (Anolis sagrei), an emerging model species. Commun. Biol. 5, 1126 (2022).
Acknowledgements
We thank B. Czesny, S. Leanos, M. Brar, and A. Edwards for technical assistance and animal care. J. Pardo provided valuable feedback on early drafts of this manuscript. We thank V. Weisbecker and two anonymous reviewers for their helpful comments. This work was supported by the funds and resources from the Loyola University Chicago Department of Biology, Office of the Provost; the Undergraduate Research Opportunities Program; and the National Science Foundation [#1942250].
Author information
Authors and Affiliations
Contributions
M.M. and T.J.S. designed the study, analyzed data, and carried out experiments. G.K., N.K., R.S., and A.S.G. carried out experiments, analyzed the experimental data. M.M., R.S., A.A., and K.B.S. performed CT scanning and analysis. All authors contributed to writing the manuscript and preparing figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Vera Weisbecker and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editors: Borja Figueirido and Michele Repetto. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Marchini, M., Keller, G., Khan, N. et al. Sonic hedgehog and fibroblast growth factor 8 regulate the evolution of amniote facial proportions. Commun Biol 8, 84 (2025). https://doi.org/10.1038/s42003-025-07522-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-07522-0
This article is cited by
-
Positional programs in early murine facial development and their role in human facial shape variability
Nature Communications (2025)