Abstract
Brain tumors are commonly treated with radiotherapy, but the efficacy of the treatment is limited by its toxicity to the normal tissue including post-irradiation contrast enhanced lesions often linked to necrosis. The poorly understood mechanisms behind such brain lesions were studied using cerebral organoids. Here we show that irradiation of such organoids leads to dose-dependent growth retardation and formation of liquid-filled cavities but is not correlated with necrosis. Instead, the radiation-induced changes comprise of an enhancement of cortical hem markers, altered neuroepithelial stem cell differentiation, and an increase of ZO1+/AQP1+/CLDN3+-choroid plexus (CP)-like structures accompanied by an upregulation of IGF2 mRNA, known to be expressed in CP and cerebrospinal fluid. The altered differentiation is attributed to changes in the WNT/BMP signaling pathways. We conclude that aberrant CP formation can be involved in radiation-induced brain lesions providing additional strategies for possible countermeasures.

Similar content being viewed by others
Introduction
Primary brain tumors, which may arise in pediatric or adult patients are commonly treated with cranial irradiation. Historical studies have suggested improved disease control with dose-escalated radiation therapy employing either photons (X-rays) or charged particles (e.g. protons). However, dose escalation is hampered by the development of radiation necrosis (RN)1,2,3,4, a common side effect in many types of brain tumors including low-grade and high-grade glioma, meningioma, and metastatic brain tumors5,6,7,8,9. In pediatric patients, low-grade gliomas including pilocytic astrocytoma and ependymoma, or other tumor types such as medulloblastoma are more common than high-grade glioma, and patients are often treated with high-energy protons in order to spare normal brain tissues to a greater extent than X-rays do10. However, clinical studies raised concerns that pediatric brain tumor patients treated with protons experienced unexpectedly high rates of RN11,12, and a variety of other radiation-induced lesions13.
RN varies in severity and is often associated with contrast-enhancing lesions (CEL) in post-treatment magnetic resonance imaging (MRI) scans. RN is diagnosed in up to 25% of the patients treated with cranial radiation14. For symptomatic patients, bevacizumab has been shown to lower the rate of RN15 and is recommended in several guidelines. However, the diagnosis and treatment of RN remains extremely challenging as the observed CEL in brain tumor patients may represent a variety of pathophysiologies including RN, blood-brain barrier disruption (BBD), or tumor progression. Necrosis and progression occur commonly in recurrent glioblastoma, and brain metastases16, and often cannot be reproducibly diagnosed by pathologists17. Both BBD and RN are believed to stem from radiation-induced vascular damage leading to local ischemia and hypoxia that will eventually induce necrosis or angiogenesis and subsequent brain edema due to increased permeability of the newly formed blood vessels. While BBD occurs within the first six months of radiotherapy, RN usually occurs 6-18 months after radiation exposure but has also been observed in pediatric patients as early as 1.2 months (median time to onset18). Within the context of stereotactic radiotherapy, this sequela can present years or decades after radiotherapy19. BBD is generally believed to be transient but can transition into RN. Progressive, untreated RN, which is characterized by large edematous lesions and pronounced clinical symptoms, maybe both irreversible and lethal19.
Contemporary clinical studies do not offer clear insights into the onset and progression of RN and to the classification of CEL as necrosis. We elected to use cerebral organoids from human pluripotent stem cells as a model to study the impact that radiation has on stem and progenitor cells within the neuronal network. As these cells mainly govern the maintenance and regeneration of the normal neuronal tissue, their contribution to the radiation-induced CEL is of great importance to avoid or treat such potential debilitating side effects. While CELs often only represent late stage RN and biopsy material is scarce and only from severe cases, cerebral organoids offer the unique opportunity to study the etiology and progression of the normal tissue responses which lead to CEL. The cerebral organoid model has been used with great success to uncover fundamental mechanisms and alterations in normal brain tissue for various neurological diseases, serving as a bridge between knowledge gained from basic in vitro-, animal- and human epidemiological studies20. Since CEL are of particular concern for pediatric patients, who demonstrate high cure rates and subsequently expected long lifespans, we used high-energy protons, which are preferably used for the treatment of pediatric malignancies, in addition to high-energy X-rays, which are received by the majority of cancer patients, in our study.
Results
As CELs occur in various brain regions depending on the localization of the tumor and the radiation field, we used an unguided differentiation approach according to Lancaster et al. 21,22. (Fig. 1) to generate cerebral organoids in which neural progenitors are allowed to differentiate spontaneously with maximum diversity. As shown in Fig. 1, organoids were irradiated after 20 days (d) of maturation at which time they are mainly comprised of sex-determining region Y (SRY)-box 2 (SOX2)+/nestin (NES)+/paired box protein-6 (PAX6)+ neuroepithelial progenitor cells clustered in evolving ventricular zone-like rosettes (immature organoids, Supplementary Fig. 1a, b20,23,). Organoids were also irradiated at d80 when the neuronal network matured. In contrast to d20 organoids, d80 organoids expressed the neuronal marker microtubule-associated protein 2 (MAP2) adjacent to regions with PAX6+ neuronal progenitors (mature organoids, Supplementary Fig. 1a, b). Given that d20 organoids still differentiate substantially, changing their cellular composition accordingly, samples were taken approximately 40 days later (at d60) to allow for the observation of radiation impacts on cell differentiation. In contrast, the more mature organoids, where no dramatic changes in cell differentiation and thus cell composition occur, were irradiated at d80 and followed up to d140 even though most analyses were performed at d100.
Exposure to radiation results in growth retardation and reduced proliferation, but is not correlated to necrosis
Cerebral organoids, generated with current protocols, represent the prenatal embryonic/fetal or early postnatal brain, respectively. Prenatal radiation exposure leads to growth retardation and small head/brain size (reviewed in B. Yang et al. 24). Thus, we hypothesized the d20 organoids, representing a prenatal brain, to be very sensitive to irradiation responding with growth retardation reflected in a smaller organoid size while the d80 organoids were expected to be less radiation-sensitive. To study the impact of radiation, cerebral organoids were subjected to X-ray and proton irradiation (Fig. 1). X-rays were used as reference radiation and all samples were compared to sham-irradiated controls (0 Gy). Due to the above-mentioned fetal/neonatal radiosensitivity, originating from neuroepithelial stem cells25, for immature organoids (d20) doses of 1, 2, and 8 Gy were used. For the more mature organoids (d80), 3, 10, and 15 Gy were applied. In addition, d80 organoids were irradiated with high-energy protons (3, 10, and 15 Gy), both in the plateau (normal tissue) phase and in the Spread Out Bragg Peak (SOBP; tumor tissue), commonly used for radiotherapy of pediatric cancers26.
Growth was evaluated via commonly used microscopic bright field imaging (area measurement27, Fig. 2a, b) and evaluated in conjunction with cell proliferation (Ki67 staining, Fig. 2c, d), necrosis (LDH release assay, Fig. 2e, f) and apoptosis (Caspase 3-active and 3/7 assays, Supplementary Fig. S2). Measurements of the circular area (Fig. 2a, b) revealed that compared to the controls at the day of irradiation (dotted lines), all sham controls showed an increase in size (~84% increase for d60 organoids compared to the size at d20 and ~56% increase for d100 organoids compared to the size at d80). Organoids irradiated at d20 with X-rays (Fig. 2a) displayed a significant dose-dependent decrease in size at d60 when compared to the respective sham-controls (~31% decrease after 1 Gy, ~52% decrease after 2 Gy and ~92% decrease in size after 8 Gy). In the highest dose-cohort (8 Gy), this decrease resulted in an even smaller size than the d20 samples (analyzed 40 d prior) exhibited. The response of mature organoids irradiated on d80 (Fig. 2b) was less drastic. Here significant growth retardation was only observed in samples subjected to 10 Gy (~20% decrease in size) and 15 Gy X-rays (~28% decrease in size) or 10 Gy (~39% decrease in size) and 15 Gy proton irradiation in SOBP (~43% decrease in size), corresponding to the irradiation of the tumor bed. Irradiation in the entrance channel (plateau, corresponding to the surrounding normal tissue) was not significantly altered compared to the sham controls. This irradiation-related decrease in size corresponded to a decrease in proliferation as determined by Ki67 staining (Fig. 2c, d). While d20 organoids showed ample Ki67 staining throughout the neuronal rosettes/proliferation zones prior to irradiation, Ki67 was restricted to the inside of some, but not all neuronal rosettes in d60 sham irradiated controls (Fig. 2c). Irradiation with 1 Gy X-rays led to a decrease in proliferation, while Ki67 was not detectable anymore in the mostly deteriorated organoids subjected to 8 Gy X-rays. Due to their more mature character/progressed differentiation stage, d80 organoids and their d100 counterparts showed hardly any proliferation (Fig. 2d). However, irradiation with 3 and 15 Gy X-rays further diminished Ki67 expression.
a, b The organoid size measured as circular area [mm2] for organoids irradiated at d20 (immature) and at d80 (mature) measured 40 and 20 days post-irradiation (d60 and d100), respectively, using different radiation qualities and doses: X-rays: 1–8 Gy for d20 organoids and 3–15 Gy for d80 organoids; protons in SOBP or plateau, respectively: 3–15 Gy for d80 organoids in comparison to their respective sham-irradiated controls. Dashed lines delineate the size of the controls at the day of irradiation. Statistical analysis was done either by Brown-Forsythe and Welch ANOVA or one-way ANOVA with Dunnett´s post-test. Data are presented as mean ± SEM for three independent experiments (N = 3) and 25 to 36 organoids per experiment (n = 25–36) (X-rays, d20), N = 4 and n = 40-54 (X-rays, d80), N = 1 and n = 6–9 (protons in plateau, d80) and N = 1 and n = 9–10 (protons in SOBP, d80). **p = 0.0039; mean difference ± SE of difference: 3.704 ± 1.092; 95% CI of difference: 1.017 to 6.39 for 1 Gy at d20; ****p < 0.0001; mean difference ± SE of difference: 6.094 ± 0.9881; 95% CI of difference: 3.647 to 8.540 for 2 Gy at d20; ****p < 0.0001; mean difference ± SE of difference: 10.90 ± 0.8476; 95% CI of difference: 8.750 to 13.05 for 8 Gy at d20; *p = 0.0448; mean difference ± SE of difference: 10.35 ± 4.299; 95% CI of difference: 0.1884 to 20.52 for 10 Gy X-rays at d80; ***p = 0.0008; mean difference ± SE of difference: 14.78 ± 4; 95% CI of difference: 5.320 to 24.23 for 15 X-rays Gy at d80; *p = 0.0360; mean difference ± SE of difference: 16.75 ± 6.431; 95% CI of difference: 0.9284 to 32.57 for 10 Gy protons at d80; *p = 0.0209; mean difference ± SE of difference: 18.22 ± 6.431; 95% CI of difference: 2.396 to 34.04 for 15 Gy protons at d80. c,d Representative immunofluorescence staining of Ki67 (red) in d20 and d80 cerebral organoids, nuclei stained with DAPI (blue), scale bar: 200 µm, and 20 µm for magnification. e, f Lactate dehydrogenase (LDH) levels secreted by organoids irradiated with X-rays at d20 (0–8 Gy), measured 1, 20 and 40 days post-irradiation, and secreted by organoids irradiated at d80 (0–15 Gy), measured 1, 20 and 60 days post-irradiation. LDH level is directly proportional to absorbance values measured at 490 nm and subtracted from background values measured at 680 nm. Statistical analysis was done by two-way ANOVA with Tukey´s post-test. Data are presented as mean ± SD for three independent experiments (N = 3) and three organoids per experiment (n = 3). *p = 0.0263; predicted mean difference: −0.02798; 95% CI of difference: −0.05424 to −0.001730 for 1 day control vs. 20 days control; ****p < 0.0001; predicted mean difference: −0.06953; 95% CI of difference: −0.09659 to −0.04247 for 1-day control vs. 40 days control; **p = 0.0080; predicted mean difference: −0.03187; 95% CI of difference: −0.05893 to -0.004810 for 1 day vs. 40 days (2 Gy); *p = 0.0303; predicted mean difference: 0.02762; 95% CI of difference: 0.001363 to 0.05387 for control vs. 8 Gy (20 days); *p = 0.0443; predicted mean difference: −0.02740; 95% CI of difference: -0.05447 to -0.0003435 for 20 days vs. 40 days (2 Gy); **p = 0.0048; predicted mean difference: 0.03308; 95% CI of difference: 0.006016 to 0.06014 for control vs. 1 Gy (40 days); **p = 0.0017; predicted mean difference: 0.03646; 95% CI of difference: 0.008610 to 0.06430 for control vs 2 Gy (d40); ****p < 0.0001; predicted mean difference: 0.06822; 95% CI of difference: 0.04116 to 0.09528 for control vs 8 Gy (d40).
For the measurement of necrosis, the lactate dehydrogenase (LDH) assay was used, and LDH release was measured on d21, d40, and d60 for immature organoids (corresponding to 1 d, 20 d, and 40 d after irradiation), and on d81, d100, and d140 for mature organoids (corresponding to 1 d, 20 d, and 60 d after irradiation) (Fig. 2e, f). In contrast to the general hypothesis that irradiation leads to high levels of necrosis, a significant increase in extracellular LDH release was only observed in the sham controls from 1 d to 40 d post-irradiation and a slight but not significant increase in the samples exposed to 8 Gy X-rays 1 d after irradiation (Fig. 2e). At all other time points, LDH release was lower in irradiated organoids compared to the respective sham controls. The most significant decrease was observed in organoids 40 d post-irradiation while in samples irradiated at d80, no significant changes could be observed at any time point (Fig. 2e, f).
To elucidate any apoptotic events, caspase staining was performed (Supplementary Fig. S2). 1 d after X-ray irradiation, organoids revealed a dose-dependent increase of apoptotic cells that was more pronounced in d20 organoids compared to d80 organoids (Supplementary Fig. S2a, b). However, at later time points (d60 or d100, Supplementary Fig. S2c, d), this response was not observed.
Irradiation leads to the formation of liquid-filled cavities
Irradiation of cerebral organoids resulted in the formation of liquid-filled cavities with a typical diameter between 0.1–0.8 mm (Fig. 3). However, extreme cases of these cavities were observed with one becoming 18.7 mm in diameter with the corresponding organoid having a diameter of 2.6 mm (Fig. 3a). The occurrence of these cavities, depicted in Fig. 3b, c, was as dose-dependent as the growth retardation of organoids (Fig. 2a, b), and it was highest in organoids irradiated with 8 Gy X-rays at d20 (Fig. 3b). In organoids irradiated at d80 (Fig. 3c), protons in the SOBP were more effective in inducing cavity formation than X-rays or protons in the plateau region at the same dose. The slope of the fitted dose-response curves (Supplementary Fig. S3) and the relative biological effectiveness (RBE) of proton beams compared to X-rays are reported in Supplementary Table 1.
a Organoid, irradiated at d80 with 15 Gy protons (Plateau region), displays massive liquid filled cavities. b Percentage of organoids displaying cavities after irradiation with X-rays at d20 (0–8 Gy) and c with various radiation qualities: X-rays, protons in SOBP and in plateau, respectively at d80 (0–15 Gy). Quantitative analysis of the dose-response curve including a linear fit of the data and relative biological effectiveness are reported in Supplementary Table 1 and Supplementary Fig. S3. Statistical analysis was done by one-way ANOVA with Dunnett´s post- test for organoids at d60 and d100 where applicable. Data in b-c are presented as mean ± SD for two to three independent experiments (N = 2–3) or as single experiment (N = 1) and n = 10 organoids per variant/group. ***p = 0.0006; mean difference ± SE of difference: −30 ± 4.714; 95% CI of difference: −43.58 to −16.42 for 1 Gy at d20; ***p < 0.0001; mean difference ± SE of difference: −50 ± 4.714; 95% CI of difference: −63.58 to −36.42 for 2 Gy at d20; ***p < 0.0001; mean difference ± SE of difference: −73.33 ± 4.714; 95% CI of difference: −86.91 to −59.76 for 8 Gy at d20; *p = 0.0127; mean difference ± SE of difference: −19.67 ± 5.137; 95% CI of difference: −34.46 to −4.873 for 10 Gy at d80; ***p = 0.0007; mean difference ± SE of difference: −32 ± 5.137; 95% CI of difference: −46.79 to -17.21 for 15 Gy at d80.
Radiation-induced liquid-filled cavities represent choroid plexus
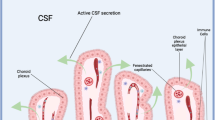
Liquid-filled cavities observed after irradiation of cerebral organoids morphologically resembled choroid plexus with cerebrospinal fluid (CSF)-filled cavities28,29. The choroid plexus (CP) is comprised of highly vascularized stroma with fenestrated capillaries and epithelial tissue, which forms the blood-CSF barrier and is the production site for CSF in the vertebrate brain30. The CP regulates the entry of compounds into the brain, its development, and function. The CP epithelium arises from multipotent pre-neurogenic neuroepithelial stem cells31, rather than later-stage neurogenic radial glia, and the CP in turn supports these stem cells30. In addition, CP formation is preceded by and depends on cortical hem (CH) signaling by BMPs and WNTs (reviewed in Moore and Iulianella32). Therefore, we examined the expression of neuroepithelial stem cell, CH and CP markers in organoids before and after irradiation as shown in Figs. 4 and 5 and Supplementary Figs. S4 and S6.
a Relative mRNA expression of SOX2, nestin (NES), PAX6, and MAP2 in d66 organoids subjected to 8 Gy X-rays at d20, mean ± SD for three independent experiments (N = 3) and n = 3 organoids per experiment. Values were normalized to 18S rRNA levels. Statistical analysis was done by multiple unpaired two-tailed t-test or Welch´s t-test, ****p = 0.000016 for; effect size ± SE of difference: 9.694 ± 0.2900 for NES. b Representative immunofluorescence staining of SOX2 (red) and NES (green) or c PAX6 (red) and MAP2 (green) at d66 (irradiation at d20). Nuclei stained with DAPI (blue), scale bar: 200 µm, and d20 µm for magnification. d Relative mRNA expression of SOX2, NES, PAX6, and MAP2 in d100 organoids subjected to 15 Gy X-rays at d80, mean ± SD for three independent experiments (N = 3) and n = 3 organoids per experiment. Values were normalized to 18S rRNA levels. Statistical analysis was done by multiple unpaired two-tailed t-test or Welch´s t-test. e Representative immunofluorescence staining of SOX2 (red) and NES (green) or f PAX6 (red) and MAP2 (green) at d100 (irradiation at d80). Nuclei stained with DAPI (blue), scale bar: 200 µm, 20 µm for magnification.
a–c Relative mRNA expression of the CH and CP lineage marker MSX1 and LMX1A, choroid epithelium marker OTX2, CP markers ZO1, AQP1, CLDN3, IGF2 and KIR7.1 in d66 organoids subjected to 8 Gy X-rays at d20, mean ± SD for at least four independent experiments (N = 4) and n = 3 organoids per experiment, and in d100 organoids subjected to either 15 Gy X-rays or 15 Gy protons at d80, mean ± SD for at least five independent experiments (N = 5) and n = 3 organoids for X-rays and for two to three independent experiments (N = 2–3) and n = 3 organoids for protons. Values were normalized to 18S rRNA levels and expressed relative to values for sham-irradiated control. Statistical analysis was done by two-tailed Welch´s t-test, *p = 0.0208; effect size ± SEM: 0.4363 ± 0.1454; 95% CI: 0.09006 to 0.7826 for MSX1. The lines indicate the levels in the sham-irradiated controls. d, e Representative immunofluorescence stainings of the CP markers AQP1 (red) and CLDN3 (red), and nuclei stained with DAPI (blue), scale bar = 100 µm, f Cerebral organoid after ink-based barrier test. g Cerebral organoids before and after collection of the fluid from organoid cavities.
qPCR analyses revealed that irradiation at d20 did not significantly change the expression of SOX2 mRNA in 60 days old organoids compared to the sham-irradiated control (Fig. 4a). A slight but not significant decrease in the expression of PAX6 and MAP2 mRNA was observed after irradiation with 1 Gy X-rays only, whereas irradiation of organoids with the dose of 8 Gy X-rays resulted in a significant increase of NES, and a slight but not significant increase in PAX6 and MAP2 mRNA level compared to the sham irradiated controls (Fig. 4a). Exemplary d60 IF analyses of sham-irradiated controls displayed neuronal rosettes whose cores were predominantly NES+/PAX6+ with scarce, scattered SOX2 expression surrounded by MAP2+ cells (Fig. 4b, c). Cells positive for homeodomain-only protein (HOPX), which is known as a marker of outer radial glial cells (oRGs)33, were particularly prominent at the rim, and GFAP+ cells were scarcely present (Supplementary Fig. 4a). In contrast, organoids subjected to 1 Gy X-rays (Fig. 4b, c, middle panel) showed NES+/PAX6+ rosette-like structures surrounded by MAP2+ cells as seen in the controls, but they also displayed highly SOX2+/PAX6- epithelial-like structures that were partially NES+ and bordered cavities. In general, SOX2 expression was more pronounced compared to the sham controls. HOPX and GFAP showed a slight decrease or no expression change in organoids subjected to 1 Gy X-rays compared to their respective sham controls (Supplementary Fig. 4a). Organoids irradiated with the highest dose at d20 showed deterioration, which made their evaluation more difficult (Fig. 4b, c, Supplementary Fig. S4a, b). Nevertheless, irradiation with 8 Gy X-rays led to areas with prominent nuclei condensation, identified by DAPI staining. MAP2 staining was restricted to these areas, whereas adjacent apparently vital cells stained for SOX2 and partially for PAX6 and NES. HOPX staining was generally less intense and observed throughout the entire organoid, while GFAP+ cells were mostly absent when compared to their respective controls (Supplementary Fig. S4a).
Irradiation at d80 did not significantly change the mRNA expression of SOX2 and MAP2 in organoids at d100 compared to the sham-irradiated control (Fig. 4d). A slight, but not significant increase in the expression of NES mRNA level was observed. PAX6 mRNA level was slightly but not significantly increased after 3 Gy X-rays, but slightly although not significantly decreased after 15 Gy X-rays compared to sham irradiated controls (Fig. 4d). Exemplary IF analyses show structural changes in irradiated compared to non-irradiated sham controls in Fig. 4e, f, for instance, sham controls at d100 were devoid of rosette-like structures. However, cells with low SOX2 expression were found throughout the organoid, while PAX6+ cells were predominantly detectable as a rim adjacent to areas with higher MAP2 expression or intermingled with cells displaying lower MAP2, SOX2, and NES expression (Fig. 4e, f). The rim of the organoid was enriched with HOPX+ cells, and less dominant astrocytes with an intense expression of GFAP (Supplementary Fig. 4b). Irradiation with 3 Gy X-rays (Fig. 4e, f, Supplementary Fig. S4b, middle panel) led to scant SOX2 expression. Even though NES expression was detected as well, co-staining of both markers was rarely detected. MAP2 was strongly expressed at the outer rim of structures that resembled late rosette-like features, but these were devoid of PAX6. HOPX and GFAP both showed a slight decrease in the expression compared to their respective sham controls. Irradiation with 15 Gy X-rays (Fig. 4e, f; two different organoids) led to a strong SOX2 and NES expression. In some areas, theses markers were co-expressed, but there were also areas with single expression. PAX6/MAP2 co-staining revealed the heterogeneity of organoids: Of the two organoids stained, one was exclusively PAX6+ while the other was almost exclusively MAP2+ (Fig. 4f). HOPX expression was overall less intense compared to the control but this change was not significant. The expression level of GFAP varied strongly between organoids of the irradiated group, but GFAP expression was similar to sham controls and remained restricted to the rim (Supplementary Fig. S4b).
As irradiated organoids apparently retained neuroepithelial stem cells in rosettes and the observed cavities showed similarities to CP structures, we probed for the CH marker MSX1, which instructs the differentiation of the CP. In addition, CH and CP lineage marker LMX1A34, choroid epithelium marker OTX235, and common CP markers zona occludens (ZO) 1, aquaporin (AQP) 1, and claudin (CLDN) 3 were analyzed. We also determined the mRNA level of insulin-like growth factor (IGF) 2 in the irradiated organoids. This marker was found to be highly enriched in CSF and is expressed during brain development36. IGF2 was shown to be restricted to a subtype of the CP epithelium, termed dark cells, which were uncovered using CP organoids37. The inwardly rectifying potassium channel KIR7.1, which is highly expressed in CP epithelium38, was chosen as it is a prerequisite in the proper generation of CSF39.
Compared to sham-irradiated controls, the level of MSX1 mRNA was significantly increased in samples subjected to 8 Gy X-rays at d20 and analyzed at d60. The levels of LMX1A, OTX2, ZO1, AQP1, CLDN3, and IGF2 mRNA, although not significantly upregulated, all showed a slight increase compared to the sham-irradiated control. Only KIR7.1 was expressed to a lesser extent in the irradiated versus the non-irradiated young organoids (Fig. 5a). A similar expression pattern for all markers could be seen in the d100 organoids that were X-ray-irradiated at d80 (Fig. 5b). Proton irradiation led to substantially higher mRNA expression of MSX1, LMX1A, OTX2, AQP1, CLDN3, IGF2, and KIR7.1 (Fig. 5c). However, the changes were not significant due to the lower sample size.
The existence of CP-like structures with their distinct morphology as a monolayer of epithelial cells in irradiated organoids was confirmed in young organoids subjected to X-rays at d20 and analyzed at d60 using H&E staining (Supplementary Fig. S5) and immunofluorescence staining against AQP1 and CLDN3 (Fig. 5d, e), whose co-expression is only found in CP. While AQP1 and CLDN3 staining in cells resembling early CP could be observed occasionally in sham controls, irradiated organoids showed multiple AQP1 and CLDN3 lined cavities of various sizes. The same was observed in organoids irradiated at d80 and analyzed at d140. Here, the effects were less pronounced compared to the d20 organoids reflecting the same trend seen in the qPCR analyses. The CP-like structures were also positive for the tight junction protein ZO1 and CLDN3. However, whereas the fluorescence signal for CLDN3 was restricted to CP-like structures, ZO1 was found in both, ventricular/neuroepithelial proliferation zones and CP-like structures (Supplementary Fig. S6). The barrier function of the cell layer lining the cavities was directly assessed by examining the entry of ink from the medium into the cavities. The ink was completely excluded from the cavities, proving intact barrier characteristics over the observed time of up to 5 min (Fig. 5f). The fluid contained within the cavities was actively produced by organoids as the contents were completely restored within a few days after its removal from the cavities (Fig. 5g).
Altered WNT/BMP signaling reflects organoid’s response to irradiation and CP formation
Irradiation to the brain can affect neurogenesis40,41 by inducing changes in genes that regulate proliferation, cell cycle, and differentiation42. Signaling pathways that govern neurogenesis and/or choroid plexus formation include neurogenic locus notch homolog protein (NOTCH)43, wingless-related integration site (WNT)44,45 and bone morphogenic protein (BMP) signaling46, NOTCH directly induces hairy and enhancer of split (HES) 1, 3, and 5, whose expression leads to the specification of CP epithelium at the expense of neurogenin (NGN) 2. Thus, we assessed the mRNA expression of NOTCH1 and 2, NGN2 as well as HES1 and 5 (Fig. 6). Compared to the sham controls, NOTCH1 and 2, and NGN2 showed similar expression, while HES5 showed a slight increase and HES1 was significantly increased in organoids irradiated at d20 (Fig. 6a). In organoids exposed to X-ray or proton irradiation (SOBP) at d80, the mRNA expression of NOTCH1 and 2, NGN2, HES1 and HES5 were slightly decreased compared to the respective sham controls (Fig. 6b, c).
a–c Relative mRNA expression of the markers NOTCH1, NOTCH2, NGN2, HES1, HES5, WNT3, WNT5a, LEF1 and BMP4 in d66 organoids subjected to 8 Gy X-rays at d20, mean ± SD for at least three independent experiments (N = 3) and n = 3 organoids per experiment, and in d100 organoids subjected to either 15 Gy X-rays, mean ± SD, for at least four independent experiments (N = 4) and n = 3 organoids per experiment or to 15 Gy protons (SOBP) at d80, for at least two independent experiments (N = 2) and n = 3 organoids per experiment. Values were normalized to 18S rRNA levels and expressed relative to values for sham-irradiated control. Statistical analysis was done by two-tailed Welch´s t-test, *p = 0.0122; effect size ± SEM: 61.16 ± 14.06; 95% CI: 22.11 to 100.2 for HES1; *p = 0.0145; effect size ± SEM: 0.05273 ± 0.01277; 95% CI: 0.01727 to 0.08819 for WNT5a, *p = 0.0233; effect size ± SEM: 0.006565 ± 0.001837; 95% CI: 0.001464 to 0.01167 for LEF1. The lines indicate the levels in the sham controls.
The WNT signaling pathway is known to also play a role in CP formation. WNT3a null mutants exhibit smaller CP areas, while treatment with WNT3 supports BMP4-mediated CP derivation from embryonic stem cells35. WNT5a promotes the formation of the tight epithelium in the developing CP and lack of WNT5a is associated with reduced CP size and complexity and loss of apicobasally polarized morphology44. As WNT signaling via b-catenin acts as a co-activator of lymphoid enhancer factor 1(LEF1)/TCF transcription factors47, we included LEF1 in the analyses. mRNA levels of WNT3, WNT5a, and LEF1 were elevated in both, organoids irradiated at d20 and d80 irrespective of the radiation quality (Fig. 6). These changes were significant for WNT5a and LEF1 in the d20 irradiated samples (Fig. 6a). BMP4, which is regulated by WNT3a (27) and is sufficient for CP epithelium induction46, was upregulated in organoids subjected to X-rays at d20 and after proton irradiation at d80 albeit with higher variability, while no increase was observed after d80 X-ray irradiation (Fig. 6b, c).
Discussion
Cerebral organoids have been used to mimic the onset and progression of several neurological diseases20 including the dysregulation of brain and CP cell types in patients with severe SARS-CoV-2 infections28,29. Here, we use cerebral organoids to elucidate the nature of CELs that are generally attributed to radiation necrosis as a result of damage to the vasculature of the brain and the blood-brain barrier. In contrast to the general opinion that radiation induces necrosis, irradiated cerebral organoids generated via unguided differentiation displayed lower levels of necrosis than sham-irradiated controls. This effect - most striking in d20 samples analyzed at the latest time point - stems from an intrinsic issue of the organoid model deprived of vasculature: as they enlarge, reaching diameters of several millimeters, they exceed the limit of passive diffusion of oxygen and nutrient supply, resulting in necrotic cells at the core48. Initial radiation-induced cell killing, particularly evident by the nuclei condensation and positive staining for an apoptotic marker after exposure of young organoids to 8 Gy X-rays, and decrease in cell proliferation led to the observed growth retardation (i.e. decrease in the size) in organoids, thus, resulting in an alleviated necrosis in the irradiated organoids compared to the controls.
However, we show that CEL-like appearance involves the blood-CSF barrier or choroid plexus, and the formation of liquid-filled cavities is clearly attributable to the radiation impact. Cerebral organoids generated in an unguided differentiation process according to the protocol by Lancaster et al. 21,22. are capable of developing CP. However, this process is rare and occurred in less than 10% of all control organoids in our study, compared to up to 50-70% in irradiated organoids. Indeed, to generate CP organoids, dorsalization has to be extrinsically induced using bone morphogenic protein (BMP) 437 that both in vitro and in vivo stimulates epithelial cell formation within the dorsal midline by repressing forkhead box (FOX) G1, which in turn supports neural stem/progenitor cell proliferation49. The formation of liquid-filled cavities in such induced conditions is observed after differentiation for more than 28 days and follows the appearance of characteristic cuboidal epithelium as has been shown in our study as well. Therefore, the radiation impact mimicked the guided differentiation of CP organoids as judged also by the expression of CP markers such as AQP1, the water channel essential for CSF production, and the tight junction proteins ZO1 and CLDN3, which ensure the integrity of the CP50. While ZO1, even though being a more general marker of tight junctions, is found in neuroepithelial proliferation zones and CP-like structures, the morphology of the latter is clearly distinguishable, rendering ZO1 a suitable marker for CP in addition to the others used in this study. Another characteristic marker is the potassium channel KIR7.1, although it was reported to some extent in Purkinje neurons and pyramidal neurons in the hippocampus51, in glia cells52, and retinal pigment epithelium53 as it is mainly expressed in secretory cells of the CP38,54. A slight increase in the mRNA expression of KIR7.1 in response of mature organoids to proton irradiation only, suggests induction of changes in the function of the CP depending on both the organoid age and quality of radiation. The existence of members of the NOTCH/HES signaling pathway and especially the induction of WNT/BMP signaling, which are both required for the regulation of CP formation in vivo30,35,44, together with unchanging or decreased neural NGN2 have been identified as being the cause for the altered differentiation process by the radiation impact. For the NOTCH signaling, this effect was more pronounced in the immature organoids than in the mature ones, while WNT signaling induction was observed in all organoids irrespective of their maturation status and the type of irradiation used. This agrees with the findings that using the WNT activator CHIR in combination with BMB4 is sufficient to induce CP in cerebral organoids37. The expression of WNT ligands implies the presence of hem-like cells expressing MSX1 that secrete these ligands, which precede the formation of CP (34, reviewed in ref. 50). A significant increase in the expression of MSX1 in d60 organoids after irradiation, and a tendency towards an increase in MSX1, lineage markers LMX1A and OTX2 regardless of the organoid age and irradiation quality, support the gradual differentiation into CP. The enrichment of CP cells in cerebral organoids irradiated at d14 was also observed in a recent study55.
These findings open an alternative interpretation of the CEL seen in brain tumor patients who received radiation therapy, which is substantiated by two clinical studies. Recently, Eulitz et al. showed an increased radiosensitivity in the periventricular region correlating with an increase in late radiation-induced brain injuries (RIBI) when evaluating consecutive MRI scans as a follow-up after proton therapy in patients with glioma56. The median distances of the RIBI volume centers to the cerebral ventricles, where the CP is located, and to the clinical target volume border were 2.1 mm and 1.3 mm, respectively. The same correlation of CEL formation and proximity to the periventricular region has been observed in a second study57. From our findings and the onset of RIBI/CEL in the ventricular region, involvement of the CP in the onset of such lesions is likely. Our study implies that radiation induces focal CP formation from neuroepithelial stem cells leading to the typical frond-like structures with increasing CSF production that have great resemblance to the CEL seen in MRI scans of patients who received cerebral radiotherapy. Even though in our short-time observations, CP barrier function was intact, arguing against a possible in vivo leakage of contrast-enhancing agents into the CP cavities, a comparison of 5 min and 4 h post-intravenous (IV) administration of a single dose of gadolinium-based contrast agents in a small cohort of patients with clinically suspected endolymphatic hydrops revealed significant leakage of contrast agents into the CSF spaces only at 4 h, but not at 5 min post-IV58. Thus, a leakage into the newly formed CP cavities is still possible in vitro and in vivo. In vivo, such leakage has been observed under multiple circumstances: in patients with stroke, in those with previous surgery, and those with high-grade gliomas59. The ability of organoids to actively secrete fluid, found in their cavities, resembles that of CP to secrete CSF. It has been shown that embryonic as well as adult CSF can support adult neural stem cells in culture via IGF236. IGF2 levels are attributed largely to CP secretion and peak during brain development. CP/CSF-IGF2 simulates cell divisions by binding to receptors on the surface of neural stem cells36. In our study, IGF2 was found to be upregulated in mature organoids indicating CSF production and secretion. Likewise, neurogenesis can occur from neural precursors within the developing choroid plexus60. Therefore, the formation of CP at the expense of newly formed neurons and outer radial glial cells, as evidenced by the decrease in the expression of MAP2 and HOPX, occurred as a dose-dependent response of neuroepithelial stem cells (SOX2+/NES+/PAX6+) to irradiation. The late production of CSF may thus explain the late onset, but progressive enlargement of CEL, and why pediatric patients are more severely affected than adult patients, as the pediatric brain still exhibits a higher plasticity than the adult brain61. In addition, the efficacy of the current approach to treat CEL with bevacizumab does not contradict the involvement of CP formation. Even though this humanized anti-VEGF monoclonal antibody is applied to inhibit vascular growth and to normalize the blood-brain barrier62, thus alleviating brain damage caused by radiation-induced vascular injury, it will also impact CP cells as VEGF is required for CP maintenance and VEGF receptors are found in adult CP63. Likewise, thalidomide has been found to restore the blood-brain barrier and cerebral perfusion in a mouse model of RIBI. This restored function was attributed to the induction of platelet-derived growth factor receptor β (PDGFRβ) expression and subsequent rescue of pericytes64. However, thalidomide also downregulates LEF1, the co-activator of the WNT/β-catenin signaling required for CP formation as well as IGF2, which is crucial for CP generation and CSF secretion (see above). Therefore, like bevacizumab, thalidomide has a potentially inhibiting effect on CP formation/progression and CSF production65. Assuming that focal/excessive CP/CSF formation is the cause of at least some of the CEL observed in brain tumor patients, additional drugs such as topiramate or the diuretics acetazolamide and furosemide are a treatment option as well, as they decrease secretion of CSF from the CP66.
Conclusion
The formation of CP and underlying alterations in the WNT/BMP4 signaling in response to radiation as a cellular stressor has not been previously described. It appears irrespective of the radiation quality but strongly depends on the age of the neuronal tissue that is exposed. CP formation offers an alternative interpretation of the initiation and progression of radiation-induced CEL seen in brain cancer patients and opens up possible new treatment regimes.
Materials and Methods
Human embryonic stem cell culture
The feeder-independent hES cell line WA09-FI (H9) was used for all studies presented as approved according to §4 and §6 of the German Stem Cell Act (registry numbers 3.04.02/0125 and 3.04.02/0125-E01). The line was originally generated by the group of Dr. James Thomson at the University of Wisconsin (52). H9 cells were obtained from the WiCell Research Institute, Wisconsin, USA, at passage 23 and were used for experiments in passages 43–53. Cells were routinely cultured on Laminin-521-coated culture dishes (BioLamina, #600962, 10 µg/ml) in mTeSR1 medium (STEMCELL Technologies) supplemented with 50 U/ml penicillin and 5 µg/ml streptomycin (Merck, #A2212) and passaged every 7 d using ReleSR (STEMCELL Technologies, #05872). Briefly, the medium was aspirated, and cells were rinsed first with 2 ml PBS and then with 200 µl ReleSR. After aspiration of the non-enzymatic passaging reagent, cells were incubated for 2 min at 37 °C to detach only pluripotent cells. Detachment was stopped by the addition of pre-warmed mTeSR1 medium and cells were seeded to about 1.0 × 105 cells per cm2.
Generation of cerebral organoids
Cerebral organoids were generated as previously described21,22. Briefly, for the generation of embryonic bodies (EBs), H9 cells were detached using ReLeSR (Stemcell Technologies) for 3 min at 37 °C. 18000 cells in Embryoid Body medium (EBM) with addition of 4 ng/ml basic fibroblast growth factor (bFGF) and 50 µM Rho-associated protein kinase (ROCK) inhibitor (Tocris Bioscience) were plated into each well of an 96-U-bottom suspension plate (Sarstedt) pre-coated with anti-adherence solution (Stemcell Technologies) for 5 min, 1000 rpm, at RT. The medium was changed to EBM without additional factors on day 3 of culture. EBs were transferred to neural induction (NI) medium in 6-cm dish (Sarstedt) to form neuroepithelial tissues on day 5 of culture. Fresh NI medium was added every second day after transfer. EBs were embedded into Matrigel (Corning) droplets on day 11 and cultured in NI medium until day 13 when it was changed to an improved differentiation medium without vitamin A (IDM-A) with the addition of 3 µM CHIR99021 (Biovision). Medium was then changed every second day until day 18. EBs were then cultured on an orbital shaker in T-25 flasks. The medium was switched to improved differentiation medium with vitamin A (IDM + A) on day 20 of culture, and then changed every 3–4 days. On day 40 of culture, 20 µl/ml Matrigel was added to IDM + A medium.
Detection of LDH release from necrotic cells
To detect extracellular lactate dehydrogenase (LDH) released from necrotic cells in the media, CyQUANT™ LDH Cytotoxicity Assay Kit (Invitrogen) was used according to the manufacturer’s instructions. Briefly, the culture medium was collected at certain time points (1 day, 20 days, and 40 or 60 days) after X-ray irradiation with different doses, i.e. 0 Gy, 1 Gy, 2 Gy, and 8 Gy for organoids irradiated on day 20 of culture or 0 Gy, 3 Gy, 10 Gy and 15 Gy for organoids irradiated on day 80 of culture. On the day of assay, 50 µl of each sample medium, previously stored at -80 °C, was added to a 96-well black clear-bottom plate in duplicate wells. Then, 50 µl of Reaction Mixture from the kit was added to each sample and incubated at room temperature for 30 minutes in the dark to enable conversion of lactate to pyruvate in a reaction catalyzed by LDH where NAD+ is reduced to NADH. The reaction was stopped by adding 50 µl Stop Solution from the kit. The absorbance of red formazan product generated by the reduction of a tetrazolium by diaphorase, which also oxidizes NADH, was measured spectrophotometrically at 490 nm. 680-nm absorbance value (background) measured using SpectraMax i3x plate reader with SoftMaxPro software (v.7.0, Molecular Devices) was subtracted from the 490-nm absorbance. Obtained absorbance values are directly proportional to the amount of LDH released into the media.
Irradiation of cerebral organoids
At d20 or d80 of differentiation, 5–10 cerebral organoids in T25 suspension flasks were subjected either to X-rays or protons in a dose range of 1–8 Gy (X-rays, d20) or 3–15 Gy (X-rays and protons). X-ray irradiation was performed using a MXR320/26 X-ray tube (250 kV, 16 mA). Exposure to protons took place at the Heidelberg Ion Beam Therapy Center. Samples were subjected either to ion beams in a 30 mm Spread Out Bragg Peak (SOBP, LET: 2.5–8.9 keV/µm) representing the irradiation field of the tumor plus margin or to irradiation conditions in the plateau phase (LET: 0.9–1.5 keV/µm) representing the surrounding normal tissue. Controls were sham-irradiated. The medium was exchanged immediately after irradiation or up to 1 h thereafter.
Size measurements and quantification of cavity formation
For the measurement of the organoid size, pictures of 10 organoids were taken at d20 and d80 (time of irradiation) and d60 and d100 (40 and 20 d post-irradiation, respectively) using a standard digital camera (Sony DSC-W220). The organoid area was measured using ImageJ. The number of organoids with visible cavities was counted and presented as a percentage of the overall number of organoids.
Analyses of cavity permeability
To examine the barrier function, an ink test was carried out. For this purpose, 6 drops of dark blue writing ink were carefully added to the cultivation medium under the microscope to observe the permeability of the cavity walls. Videos were recorded for 40 sec at a frame rate of 31.68 frames per second using the Eclipse Ts2 microscope (Nikon). Maximum observation time was ≤ 5 min.
Analysis of fluid production by CP-like cells in cerebral organoids
To examine whether organoids actively produce fluid leading to the observed cavities, organoids were washed once in PBS and the whole liquid content was collected from cavities using a 1 ml syringe with a 25 G needle and stored at −80 °C. Organoids were placed back into the culture medium in the incubator and monitored daily for the production of liquid in the cavities. Liquid collection from cavities was repeated with the same organoids once the cavities were filled again.
Immunofluorescence
For immunofluorescence staining, organoids were fixed in 3.7% paraformaldehyde (Carl Roth) at 4 °C overnight and then washed three times for 5 min with PBS. Organoids were dehydrated in sucrose (Sigma) gradient (7–60% sucrose in PBS) for 4 hours (for 7%, 10%, and 40% sucrose) or overnight (for 30% and 60% sucrose). Organoids were then embedded in 7.5% gelatin (Neolab)/10% sucrose using in-house 3D-printed PDMS embedding molds. Embedded organoids were frozen on dry ice and stored at −80 °C prior to being cryosectioned at 10 µm using CM1860 cryostat (Leica Biosystems). Cryosections were first blocked and permeabilized in 0.5% Triton X-100 (ThermoFisher Scientific)/1% BSA (Carl Roth) in PBS for 30 min, and then blocked with 1% BSA in PBS for another 30 min at room temperature (RT). Cryosections were incubated with primary antibodies in 1% BSA in PBS for 1 h at RT or at 4 °C overnight. After being washed three times for 5 min with PBS, cryosections were incubated with secondary antibodies in 1% BSA for 1 h at RT followed by incubation with 5 µg/ml DAPI for 4 min for nuclei staining. Then the cryosections were washed two times for 5 min in PBS followed by two wash steps for 5 min with Millipore water prior to mounting with fluorescence mounting medium (Dako). Images were captured with a fluorescence microscope (Zeiss Axio Imager.Z2) equipped with Metafer5 software (v. 4.3.12, Metasystems). Stainings were performed on samples from three independent preparations with at least three organoids per group. Images were processed with ImageJ (v1.53i, National Institute of Health (NIH)).
Antibodies used for immunofluorescence are listed in Supplementary Tables 2-4.
Real time RT-PCR analysis
For each condition to be analyzed 3-5 organoids were collected in QIAzol Lysis Reagent (Qiagen, #79306) and total RNA was isolated using the Qiagen RNeasy Mini Kit (#74106) according to the manufacturer´s instructions including a DNA removal step using the RNase-free DNase Set (Qiagen, #79254). 50 ng or 2 µg RNA were reverse-transcribed via the RevertAid RT Kit (Life Technologies, #K1691). Relative RNA expression was analyzed using the Hot FIREPol EvaGreen qPCR Mix Plus from Solis Biodyne (08-24-0000S) and the Quant Studio 3 Real-Time PCR System (Applied Biosystems) with Quant Studio Design & Analysis software (v. 1.5.3). Human fetal and adult brain mRNA served as controls, all target expression levels were normalized to 18S rRNA. Primer sequences can be found in the Supplement Table 5.
Statistics and reproducibility
Statistical comparisons were performed using GraphPad Prism (v 9.3.1) as stated in figure legends for a number of independent experiments (N) with a given number of organoids per experiment (n) and included an unpaired two-tailed test with or without Welch correction, Brown-Forsythe/Welch or mixed ANOVA model either with Dunnett´s or Tukey´s post-test. Samples of organoids were randomly assigned to different treatments. No statistical methods were used to pre-determine sample sizes. Because of the nature of the treatment (irradiation), data collection and analysis were not performed blind to the conditions of the experiments.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Raw data is uploaded on open access repository Zenodo (Version v1, https://doi.org/10.5281/zenodo.14750678)67. All other data, such as microscopy images, are available from the corresponding author on reasonable request.
References
Fitzek, M. M. et al. Dose-escalation with proton/photon irradiation for Daumas-Duport lower-grade glioma: results of an institutional phase I/II trial. Int J. Radiat. Oncol. Biol. Phys. 51, 131–137 (2001).
Matsuda, M. et al. Prognostic factors in glioblastoma multiforme patients receiving high-dose particle radiotherapy or conventional radiotherapy. Br. J. Radio. 84, S54–S60 (2011).
Mizumoto, M. et al. Phase I/II trial of hyperfractionated concomitant boost proton radiotherapy for supratentorial glioblastoma multiforme. Int J. Radiat. Oncol. Biol. Phys. 77, 98–105 (2010).
Tsien, C. I. et al. Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin. Cancer Res 18, 273–279 (2012).
Caccese, M. et al. The role of radiation therapy and systemic treatments in meningioma: The present and the future. Cancer Med 12, 16041–16053 (2023).
Chen, C., Lee, I., Tatsui, C., Elder, T. & Sloan, A. E. Laser interstitial thermotherapy (LITT) for the treatment of tumors of the brain and spine: a brief review. J. Neurooncol. 151, 429–442 (2021).
Corn, B. W. et al. White matter changes are correlated significantly with radiation dose. Observations from a randomized dose-escalation trial for malignant glioma (Radiation Therapy Oncology Group 83-02). Cancer 74, 2828–2835 (1994).
Kim, J. W. et al. Fractionated Stereotactic gamma knife radiosurgery for large brain metastases: a retrospective, single center study. PLoS One 11, e0163304 (2016).
Koffer, P. et al. Repeat stereotactic radiosurgery for locally recurrent brain metastases. World Neurosurg. 104, 589–593 (2017).
Durante, M., Orecchia, R. & Loeffler, J. S. Charged-particle therapy in cancer: clinical uses and future perspectives. Nat. Rev. Clin. Oncol. 14, 483–495 (2017).
Gunther, J. R. et al. Imaging changes in pediatric intracranial ependymoma patients treated with proton beam radiation therapy compared to intensity modulated radiation therapy. Int J. Radiat. Oncol. Biol. Phys. 93, 54–63 (2015).
Kralik, S. F. et al. Radiation necrosis in pediatric patients with brain tumors treated with proton radiotherapy. AJNR Am. J. Neuroradiol. 36, 1572–1578 (2015).
Bhattacharya, D. et al. Spectrum of neuroimaging findings post-proton beam therapy in a large pediatric cohort. Childs Nerv. Syst. 37, 435–446 (2021).
Gibson, E. & Monje, M. Effect of cancer therapy on neural stem cells: implications for cognitive function. Curr. Opin. Oncol. 24, 672–678 (2012).
Kulinich, D. P. et al. Radiotherapy versus combination radiotherapy-bevacizumab for the treatment of recurrent high-grade glioma: a systematic review. Acta Neurochir. 163, 1921–1934 (2021).
Mayo, Z. S., Billena, C., Suh, J. H., Lo, S. S. & Chao, S. T. The dilemma of radiation necrosis from diagnosis to treatment in the management of brain metastases. Neuro Oncol. 26, S56–S65 (2024).
Holdhoff, M. et al. The consistency of neuropathological diagnoses in patients undergoing surgery for suspected recurrence of glioblastoma. J. Neurooncol. 141, 347–354 (2019).
Plimpton, S. R. et al. Cerebral radiation necrosis in pediatric patients. Pediatr. Hematol. Oncol. 32, 78–83 (2015).
Bernhardt, D. et al. DEGRO practical guideline for central nervous system radiation necrosis part 1: classification and a multistep approach for diagnosis. Strahlenther. Onkol. 198, 873–883 (2022).
Eichmuller, O. L. & Knoblich, J. A. Human cerebral organoids - a new tool for clinical neurology research. Nat. Rev. Neurol. 18, 661–680 (2022).
Lancaster, M. A. et al. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 35, 659–666 (2017).
Lancaster, M. A. & Knoblich, J. A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 9, 2329–2340 (2014).
Nat, R. et al. Neurogenic neuroepithelial and radial glial cells generated from six human embryonic stem cell lines in serum-free suspension and adherent cultures. Glia 55, 385–399 (2007).
Yang, B., Ren, B. X. & Tang, F. R. Prenatal irradiation-induced brain neuropathology and cognitive impairment. Brain Dev. 39, 10–22 (2017).
Michaelidesova, A., Konirova, J., Bartunek, P. & Zikova, M. Effects of radiation therapy on neural stem cells. Genes 10, https://doi.org/10.3390/genes10090640 (2019).
Winter, S. F. et al. Mitigating radiotoxicity in the central nervous system: role of proton therapy. Curr. Treat. Options Oncol., https://doi.org/10.1007/s11864-023-01131-x (2023).
Schroter, J. et al. A large and diverse brain organoid dataset of 1,400 cross-laboratory images of 64 trackable brain organoids. Sci. Data 11, 514 (2024).
Yang, A. C. et al. Publisher Correction: Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 598, E4 (2021).
Yang, A. C. et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 595, 565–571 (2021).
Lun, M. P., Monuki, E. S. & Lehtinen, M. K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 16, 445–457 (2015).
Johansson, P. A. The choroid plexuses and their impact on developmental neurogenesis. Front Neurosci. 8, 340 (2014).
Moore, S. A. & Iulianella, A. Development of the mammalian cortical hem and its derivatives: the choroid plexus, Cajal-Retzius cells and hippocampus. Open Biol. 11, 210042 (2021).
Holst, C. B., Brochner, C. B., Vitting-Seerup, K. & Mollgard, K. The HOPX and BLBP landscape and gliogenic regions in developing human brain. J. Anat. 243, 23–38 (2023).
Shaker, M. R. et al. Choroid plexus defects in Down syndrome brain organoids enhance neurotropism of SARS-CoV-2. Sci. Adv. 10, eadj4735 (2024).
Parichha, A. et al. Constitutive activation of canonical Wnt signaling disrupts choroid plexus epithelial fate. Nat. Commun. 13, 633 (2022).
Lehtinen, M. K. et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69, 893–905 (2011).
Pellegrini, L. et al. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 369, https://doi.org/10.1126/science.aaz5626 (2020).
Doring, F. et al. The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties. J. Neurosci. 18, 8625–8636 (1998).
Millar, I. D., Bruce, J. & Brown, P. D. Ion channel diversity, channel expression and function in the choroid plexuses. Cerebrospinal Fluid Res. 4, 8 (2007).
Licursi, V. et al. X-ray irradiated cultures of mouse cortical neural stem/progenitor cells recover cell viability and proliferation with dose-dependent kinetics. Sci. Rep. 10, 6562 (2020).
Snyder, J. S., Kee, N. & Wojtowicz, J. M. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J. Neurophysiol. 85, 2423–2431 (2001).
Liang, D. et al. Radiotherapy side effects: comprehensive proteomic study unraveled neural stem cell degenerative differentiation upon ionizing radiation. Biomolecules 12, https://doi.org/10.3390/biom12121759 (2022).
Mase, S. et al. Notch1 and Notch2 collaboratively maintain radial glial cells in mouse neurogenesis. Neurosci. Res 170, 122–132 (2021).
Langford, M. B. et al. WNT5a regulates epithelial morphogenesis in the developing choroid Plexus. Cereb. Cortex 30, 3617–3631 (2020).
Lee, S. M., Tole, S., Grove, E. & McMahon, A. P. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development 127, 457–467 (2000).
Watanabe, M. et al. BMP4 sufficiency to induce choroid plexus epithelial fate from embryonic stem cell-derived neuroepithelial progenitors. J. Neurosci. 32, 15934–15945 (2012).
Bem, J. et al. Wnt/beta-catenin signaling in brain development and mental disorders: keeping TCF7L2 in mind. FEBS Lett. 593, 1654–1674 (2019).
Gong, J. et al. Three-dimensional in vitro tissue culture models of brain organoids. Exp. Neurol. 339, 113619 (2021).
Hou, P. S., hAilin, D. O., Vogel, T. & Hanashima, C. Transcription and Beyond: Delineating FOXG1 Function in Cortical Development and Disorders. Front Cell Neurosci. 14, 35 (2020).
Kompanikova, P. & Bryja, V. Regulation of choroid plexus development and its functions. Cell Mol. Life Sci. 79, 304 (2022).
Krapivinsky, G. et al. A novel inward rectifier K+ channel with unique pore properties. Neuron 20, 995–1005 (1998).
Papanikolaou, M., Lewis, A. & Butt, A. M. Store-operated calcium entry is essential for glial calcium signalling in CNS white matter. Brain Struct. Funct. 222, 2993–3005 (2017).
Carrington, S. J. et al. G protein-coupled receptors differentially regulate glycosylation and activity of the inwardly rectifying potassium channel Kir7.1. J. Biol. Chem. 293, 17739–17753 (2018).
Nakamura, N. et al. Inwardly rectifying K+ channel Kir7.1 is highly expressed in thyroid follicular cells, intestinal epithelial cells and choroid plexus epithelial cells: implication for a functional coupling with Na+,K+-ATPase. Biochem J. 342, 329–336 (1999).
Ribeiro, J. H. et al. A human-specific, concerted repression of microcephaly genes contributes to radiation-induced growth defects in cortical organoids. iScience 28, 111853 (2025).
Eulitz, J. et al. Increased relative biological effectiveness and periventricular radiosensitivity in proton therapy of glioma patients. Radiother. Oncol. 178, 109422 (2023).
Bahn, E. et al. Late contrast enhancing brain lesions in proton-treated patients with low-grade glioma: clinical evidence for increased periventricular sensitivity and variable RBE. Int J. Radiat. Oncol. Biol. Phys. 107, 571–578 (2020).
Ohashi, T., Naganawa, S., Iwata, S. & Kuno, K. Distribution of Gadolinium-based contrast agent after leaking into the cerebrospinal fluid: comparison between the cerebral cisterns and the lateral ventricles. Magn. Reson Med Sci. 20, 175–181 (2021).
Bozzao, A. et al. Cerebrospinal fluid changes after intravenous injection of gadolinium chelate: assessment by FLAIR MR imaging. Eur. Radio. 13, 592–597 (2003).
Prasongchean, W., Vernay, B., Asgarian, Z., Jannatul, N. & Ferretti, P. The neural milieu of the developing choroid plexus: neural stem cells, neurons and innervation. Front Neurosci. 9, 103 (2015).
Sorrells, S. F. et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381 (2018).
Yang, X., Ren, H. & Fu, J. Treatment of radiation-induced brain necrosis. Oxid. Med Cell Longev. 2021, 4793517 (2021).
Maharaj, A. S. et al. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J. Exp. Med. 205, 491–501 (2008).
Cheng, J. et al. A phase 2 study of thalidomide for the treatment of radiation-induced blood-brain barrier injury. Sci. Transl. Med. 15, eabm6543 (2023).
Meganathan, K. et al. Identification of thalidomide-specific transcriptomics and proteomics signatures during differentiation of human embryonic stem cells. PLoS One 7, e44228 (2012).
Scotton, W. J. et al. Topiramate is more effective than acetazolamide at lowering intracranial pressure. Cephalalgia 39, 209–218 (2019).
Bender, T. et al. Aberrant choroid plexus formation drives the development of treatment-related brain toxicity [data set] Zenodo, https://doi.org/10.5281/zenodo.14750678 (2025).
Acknowledgements
The authors thank Kim Sara Jung, Dr. Carola Hartel, Leonie Hartig, and Jennifer Persigehl for excellent technical assistance. We further thank Leon Kaysan and Dr. Timo Smit for microscopic analyses, Dr. Margot Mayer, Dr. M. Waleed Gaber, and Dr. Christine Beamish who shared their time and insights. We are grateful to Dr. Stephan Brons for his help with the planning and performance of proton irradiation experiments at Heidelberg Ion Beam Therapy Center (HIT). This work was funded by US NIH Grant 1RO1CA256848-01, and German Federal Ministry of Education and Research (BMBF) Grant 02 NUK 049A and 02NUK081A. This paper includes parts of results (Fig. 5f, Figure S2 (graphs), Figure S4) of the doctoral thesis “Effects of ionizing radiation on human cerebral organoids” submitted by Celine Schielke to the Johannes Gutenberg University Mainz in 2021 (https://doi.org/10.25358/openscience-6858).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: IS, MD, DRG. Methodology: IS, DRG, TB, ES, JD, MD. Investigation: TB, ES, CS, IS. Visualization: TB, ES, IS. Funding acquisition: IS, MD, DRG. Project administration: IS, MD, DRG. Supervision: IS, MD, DRG. Writing – original draft: IS, TB, ES. Writing – review & editing: MD, DRG, JD, IS
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Mohammed Shaker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Benjamin Bessieres. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bender, T., Schickel, E., Schielke, C. et al. Aberrant choroid plexus formation drives the development of treatment-related brain toxicity. Commun Biol 8, 276 (2025). https://doi.org/10.1038/s42003-025-07736-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-07736-2