Abstract
Lobopodians are an evolutionary grade of panarthropods characterized by their vermiform bodies and paired, unjointed lobopodous legs. A paraphyletic group, their study is of particular significance in understanding the evolution of extant panarthropods. Found exclusively in marine deposits from the Paleozoic, the great majority of species come from Cambrian Konservat-Lagerstätten, with only a few representatives known from the Ordovician, Silurian, and Carboniferous. Here we redescribe Palaeocampa anthrax from the Carboniferous Mazon Creek (USA) and Montceau-les-Mines (France) Lagerstätten as a lobopodian. First published in 1865, nearly fifty years before the discovery of the Burgess Shale, Palaeocampa is historically the first discovered lobopod, and its presence at the slightly younger Montceau-les-Mines (Gzhelian), makes this the youngest known fossil ‘xenusiid’ lobopodian species. We present the case that Palaeocampa most likely inhabited a freshwater environment, contesting the view that Paleozoic lobopodians were exclusively marine. Palaeocampa bears biomineralized dorso-lateral and lateral sclerite sets with a unique architecture unseen in other lobopodian sclerites, which may have been capable of secreting defensive chemicals at their tips. Palaeocampa anthrax represents a major evolutionary step in lobopodians, both in environmental adaptations and in defensive abilities.
Similar content being viewed by others
Introduction
Lobopodians are an informal group or evolutionary grade of vermiform panarthropods1 characterized by their elongate soft bodies and numerous paired lobopodous limbs, often equipped with sclerotized spines or plates. By its most liberal and useful definition, lobopodians can be said to include all panarthropods with the exception of arthropods and sometimes radiodonts2. This includes Onychophora (velvet worms) and Tardigrada (water bears) as extant representatives, but in the fossil record, the vast majority are known from marine paleozoic deposits3. Historically, the first known fossil lobopodian was Aysheaia pedunculata4, discovered in the Cambrian rocks of the Burgess Shale by Charles Walcott in 19105 and published in 1911. Walcott initially described it as a polychaete but quickly received letters from multiple entomologists (Prof. William Morton Wheeler, followed by Prof. Charles Schuchert in the same year) professing that Aysheaia likely represented a marine ancestor of Onychophorans. This was reinforced by further descriptions by Hutchinson6, Resser7, and later Whittington8. Hallucigenia sparsa4, another lobopodian also described in Walcott’s 1911 paper as a polychaete, took longer to be recognized as a lobopodian, famously interpreted upside down for many years9.
Lobopodians are notoriously difficult to classify, and the literature is full of similar misinterpretations and revisions. Xenusion auerswaldae10, a Cambrian species discovered shortly before the First World War, spent years unstudied in museum collections before undergoing similar debate11. Initially described as a possible onychophoran and once theorized as a late-surviving Ediacaran frond-organism12, it is now accepted as a lobopodian13. The species Acinocricus stichus14, initially described as a bizarre alga, is now known to be a heavily armoured collinsovermid lobopodian15,16. Facivermis yunnanicus17, first described as a polychaete and later considered to be a pentastomid worm18, is now understood to be a highly derived, tube-dwelling luolishaniid lobopodian19. A variety of small shelly fossils, hypothesized to belong to animals ranging from bradoriids to conodonts to enigmatic, conodont-like animals are now understood to be the spines of hallucigeniid lobopodians20. The misinterpretation of these fossils highlights the importance of revisiting and reevaluating fossil specimens with fresh perspectives and new technologies.
In this paper, we follow a similar vein of inquiry and revisit the obscure yet controversial Palaeocampa anthrax21,22,23,24 from the Carboniferous Mazon Creek (Moscovian; USA) and Montceau-les-Mines (Gzhelian; France) Lagerstätten. First published in 186521, nearly half a century before the discovery of the Burgess Shale, Palaeocampa anthrax was originally described from the coal measures of Illinois as a lepidopteran caterpillar (hence the name, although this theory can be discounted as the trunk lacks differentiated true legs and prolegs), then a worm25, a millipede26,27, and most recently as a polychaete fireworm24. Only once, in a paper informally detailing the Montceau-les-Mines fossil deposits28, has it been briefly allied with the onychophorans, but this was apparently done in error, confusing it with a co-occuring, then-undescribed velvet worm now called Antennipatus montceauensis29 (while the fossil is labeled an onychophoran, the illustration shows one without spines, alongside a polychaete representing Palaeocampa).
Palaeocampa has caused much confusion, not only over its own affinities, but also over the depositional environments in which it was found, and even the arrangements of palaeocontinents30. As fireworms are not known to occur in freshwater, its appearance at the apparently exclusively freshwater deposits of Montceau-les-Mines inspired various explanations, including an “Amazonian model”, where marine taxa over the course of a few million years were enclosed in a freshwater environment to which they eventually adapted31.
Here, we find that Palaeocampa anthrax does not belong to any of these groups, and instead represents the youngest known species of fossil lobopodian (as well as historically the first fossil lobopodian ever discovered) with a unique form of sclerite armature that likely included a toxic or noxious chemical defense mechanism (Fig. 1). Perhaps most significantly, Palaeocampa anthrax was a non-marine (likely freshwater) organism, unprecedented among fossil lobopodians other than tardigrades and onychophorans, which have previously been considered to be exclusively marine. This discovery reveals a novel frontier of panarthropod evolution, resolves centuries-old uncertainties, and changes our view of Paleozoic ecosystems.
a MCZ:IP:110208a, Overview of specimen. Faint red and blue grid lines from a wax pencil can still be seen across the specimen, made by the nineteenth century artist Katherine Pierson who illustrated pl. 26, Fig. 8 of27. b Detail of trunk, showing bases of some spine tufts and the sclerotized dermal papillae reinforcing the dorsum. c Detail of head, showing dorsal sclerite with septarian fractures115 and part of a small, secondary frontal appendage. d Longitudinal view of spines showing external ridges and other spines with interior exposed revealing the septa dividing inner segments of the spine. e Spines with jagged serrations projecting upward from the modified basement papillae. f Bases of spines emerging from modified basement papillae. g Crenellated, jagged terminus of one of the spines. crh, chemical residue halo; dss, dorsal sclerite spines; hs, head sclerite; lb, lobopod limb; lss, lateral sclerite spines; sha, secondary frontal appendage. Scale bars represent 5 mm in (a), 2 mm in (b) and (c), and 100 μm in d–g.
Systematic Paleontology
Systematic Paleontology
Panarthropoda Nielsen32,
Family Aysheaiidae Walcott4,
†Palaeocampa Meek & Worthen21,
Palaeocampa Meek & Worthen21: 52,22: 410, pl.32, Fig. 3,23: 565; Scudder,26: 161–170,27: 293; Pacaud et al. 33: 37,41; Rolfe et al. 34: 427;35: 390, pl. 1, Fig. 7; Heyler and Poplin,28; Fig. 1; Fitzhugh et al. 36: 80–81, Fig. 7A.29-7 A.30; Pleijel et al. 24, Fig. 1; Saleh et al. 37, Fig. 2C.
a External sculpture of sclerite spines, external and internal mould. b Internal sculpture of sclerite spines. Surface mostly smooth, with subtle, plate-like texture. Segments of spine divided by septa. c External sculpture of sclerite spines, with septa externally dividing segments of spine with thin, latitudinal line. d Hollow interior of sclerite spine, divided by septa. e Sclerotized dermal papillae from trunk dorsum. Details mostly lost, central pore empty. f Internal view of sclerite spine in cross section, showing thin, plate-like septa. g Crenellated terminus of sclerite spine with jagged, outwardly splaying peaks around the rim. h Modified basement papillae without occupying sclerite spines. Scale bars represent 100 μm in a, b, d, e, and g, and represent 50 μm in c and f.
Type species: Palaeocampa anthrax Meek & Worthen,21, by monotypy.
Emended Diagnosis- Lobopodian with 10 annulated lobopod pairs and corresponding biomineralized dorso-lateral and lateral sclerite sets. The lateral sclerites are composed of a bundle of radiating spines approximately equal in length to the width of the body, and the dorso-lateral sclerites are composed of a bundle of radiating spines approximately double the length of the width of the body. An additional set of long spines is situated on the posteriormost end radiating outwards. Each individual spine is straight, slightly tapering at the distal end, and externally has prominent longitudinal ridges separated by 2-3 smaller, less prominent ridges in between. Head bears a pair of stout, annulated antenniform appendages, a second pair of shorter and thinner appendages, and a smooth, slightly domed dorsal sclerite. Head and trunk covered in plicae with dermal papillae.
Remarks- This is a drastic revision to the previous diagnosis given by Pleijel et al. 24 which defined Palaeocampa as a member of the polychaete order Amphinomida. The presence of lobopodous walking legs and the spine structure of Palaeocampa (unlike the structure of any known extinct or extant polychaete chaetae) invalidates the assignment of Palaeocampa to any annelid taxon. We are in agreement with Pleijel et al. 24 that no marked difference exists between the geographically and temporally separate populations found in fossils from Mazon Creek and Montceau-les-Mines, and as such, we choose to keep Palaeocampa monospecific. An additional species of Palaeocampa, Palaeocampa(?) obscura, was doubtfully erected by Matthew38; however, based on the sole specimen figured, this species from New Brunswick, Canada, is more likely a genuine arthropod or polychaete, and decidedly not Palaeocampa. Additionally, Pleijel et al.24 following the suggestion of Pacaud et al.33 synonymized the monospecific fossil polychaete genus Rhaphidiophorus with Palaeocampa based on the perceived assumption that both genera have externally, longitudinally striated chaete. However, SEM analysis of Rhaphidiophorus hystrix39 chaete show no indication of external longitudinal striations nor do they have a similar internal structure (e.g., presence of septa) to that of Palaeocampa’s spines. Thompson39 who erected Rhaphidiophorus, noted that the outer sheath of the chaete “had no preserved surface morphology” (p.192). Thompson went on to suggest that any fine lateral ribbing which may be observed was internal longitudinal canals that are noted in the chaetae of some polychaetes. Furthermore, body shape, variable number of segments, and the distinct presence of parapodia from which the chaete emerge distinguish Rhaphidiophorus (annelid) from Palaeocampa (lobopod). As such, we reject the synonymization of Rhaphidiophorus and Palaeocampa and maintain them as two distinct genera (see Supplementary Fig. 2).
†Palaeocampa anthrax Meek & Worthen21,
Palaeocampa anthrax Meek & Worthen21: 52–53,22: 410–411, Fig. 3,23: 565; Scudder, [scudder1886]: 218,26: 161–170,27: 283–297, pl. 26, Figs. 1–9; Rolfe,35: 390, pl. 1, Fig. 7; Fitzhugh et al. 36: 80–81, Fig. 7A.29-7 A.30; Pleijel et al.24, Figs. 1–2; Saleh et al. 37, Fig. 2C.
a USNM PAL 38032a, specimen in dorsal perspective. Head region missing or incomplete. b USNM PAL 38032c, specimen in dorsal view. Head incomplete or missing. Some trunk lobopods are faintly preserved, but mostly hidden beneath spines. c USNM PAL 38032 d, specimen in dorsal view. Head and frontal appendages partially preserved, as well as some trunk lobopods, again mostly hidden by spines. d USNM PAL 38032b, specimen in lateral view. Dorsum of trunk reinforced with sclerotized dermal papillae, trunk lobopods preserved at various angles, and a highly elongate frontal appendage is seen at the far anterior. The middle portion of the appendage is missing, but continues around to overlap with the basal portion. Frontal appendage is evenly annulated, and lined with papillae. e Line drawing of USNM PAL 38032b. dsp dorsal sclerotized papillae, fa frontal appendage, tss terminal sclerite spines. Other abbreviations as in Fig. 1. Scale bars represent 5 mm.
a YPM IP 042834, specimen in dorsal view. Details of the head, if preserved, are obscure. b YPM IP 042833, specimen in dorsal view. Head missing. c YPM IP 042826, specimen in dorsal view. Possibly represents a molt. d YPM IP 036198, specimen in dorsal view. Head missing. The counterpart of this specimen was long previously coated in lead. Largest available specimen. e YPM IP 042827, specimen in dorsal view. Annuli and dermal papillae are preserved across the trunk dorsum. f YPM IP 042828, specimen in dorsal view. Possibly represents a molt. The anterior end of the trunk dorsum is strongly delineated, with a squared-off profile dorsally. g YPM IP 042831, specimen in lateral view. Trunk is strongly flexed, possibly in a defensive reflex during burial to better expose the spine tufts and protect the soft underbody. Trunk lobopods are preserved down the length of the trunk, with roughly circular cross sections occasionally exposed. Details of head obscure. Images courtesy of the Yale Peabody Museum (CC0). Abbreviations as in Figs. 1, 3. Scale bars represent 5 mm.
a MNHN SOT 3657, specimen in oblique lateral view. Five lobopodous trunk limbs are preserved anteriorly, roughly the same length as the diameter of the trunk, and lined with strong dermal papillae. Spine tufts are preserved on raised bases, the degree to which the spines were elevated from the trunk in life is ambiguous. b MNHN SOT 3657, detail of the anterior and trunk lobopods under low-angle lighting. c MHNN SOT 2148a, specimen preserved in dorsoventral view. Head region with expanded lateral regions and a circular sclerite plate, which bears a rim and concentric rings towards its center (which appears to be broken away). A pair of frontal appendages is incompletely preserved at the anterior of the head, evenly annulated, and lined with papillae. d Detail of frontal appendage from MHNN SOT 2148a. tn, trunk nodes. Other abbreviations as in Figs. 1, 3. Scale bars represent 5 mm in a and b, and represent 2 mm in c.
a MHNN SOT 2148b, dorsoventral, counterpart of MHNN SOT 2148a, from the Montceau-les-Mines Lagerstätte (Carboniferous, France). Some internal structure is exposed around the mid-trunk, potentially two distinct structures. A thin, central line is preserved extending beyond a much wider, pale, central structure. The thin structure is unlikely to represent the nervous system, as a paired nerve cord is strongly implied by phylogenetic bracketing. Conservatively, these structures may both, or either, represent the gut; although, the thinner, central structure could represent the remains of a heart or pericardinal sinus. b FMNH PE 12992a, specimen in dorsal, from the Mazon Creek Lagerstätte (Carboniferous, Illinois, USA). c FMNH PE 28735b, specimen in dorsal, from the Mazon Creek Lagerstätte (Carboniferous, Illinois, USA). d FMNH PE 33387a, specimen in lateral, from the Mazon Creek Lagerstätte (Carboniferous, Illinois, USA). Images b–d courtesy of the Field Museum of Natural History (CC BY-NC 4.0). cs, possible circulatory structure. Other abbreviations as in Figs. 1, 3, 5. Scale bars represent 5 mm.
a Scan images used to make masks that categorized each scanned area into fossil ‘Matrix’, ‘Spine’ or ‘Tip’ regions, using USNM 38032 d (Neotype MCZ:IP:110208 was also analyzed and incorporated into results). b Mean (thick lines) SNV-normalized spectra for each mask category, with 95% percentile ranges shown as confidence bands. c Principal component analysis of SNV pre-processed spectra for the three mask categories across both fossil specimens. d Spectral regions with statistically significant differences between each pair of categories. Upper and lower (Benjamini-Hochberg corrected) boundaries for significance indicated by dashed black horizontal lines with insignificant points grayed-out in between these lines. Differences between tip and spine categories (bottom, dark blue line), have shaded blue bands indicating regions where tip spectra show higher absorbance than spine spectra and have identified local maxima in the spectra corresponding to likely spectral peaks (black annotated numbers). e Spaghetti plot of variation across individual scanning runs between different categories. As in Supplementary Fig. 4, mean values for standard-normal-variate pre-processed, Savitzky-Golay smoothed spectra are presented for each category in each individual scanning run, overlaid to highlight systematic differences between categories in different spectral regions. Dotted lines with wavenumbers at the top denote peaks in the global average of the tip spectra, as identified by the ‘findpeaks’ function of the pracma package98, using minimum up and down values of 3 with minimum distance between peaks of 20.
a Palaeocampa anthrax (USNM PAL 30833) preserved alongside large, indeterminate plant fragments, suggesting an association with inland ecosystems, from the Mazon Creek Lagerstätte (Carboniferous, Illinois, USA). b Unnamed lobopodian (C1974a) with slab-like, branching trunk lobopods from the Soom Shale Lagerstätte (Ordovician, South Africa). Most likely an aysheaiid, given the combination of sclerotized claws on the trunk lobopods, and the large, complex frontal appendages. A possible secondary frontal appendage is preserved on the left side, which is reduced compared with the trunk lobopods and primary frontal appendages, and does not appear to occupy its own trunk somite. Image courtesy of Rowan Whittle. c Aysheaia pedunculata (USNM PAL 83942a), an aysheaiid lobopodian from the Burgess Shale Lagerstätte (Cambrian, BC, Canada). Image courtesy of The Smithsonian Museum Of Natural History (CC0). d Hadranax augustus (MGUH 24.527), a probable aysheaiid lobopodian from the Sirius Passet Lagerstätte (Cambrian, Greenland). An elongate, sparsely branching frontal appendage is preserved, crossing under the trunk, directed posteriorly. Claws are absent from the ends of the trunk lobopods. Image courtesy of the Natural History Museum of Denmark. e Thanahita distos (OUMNH C.29699), a basal, long-legged lobopodian from the Herefordshire Lagerstätte (Silurian, UK). Although the tufted papillae are superficially similar to the sclerite tufts of Palaeocampa anthrax, they are entirely soft, loosely arranged, and not exactly correlated with the limb insertions. cl, claw; dp, dorsal papillae; dtp, dorsal tufted papillae; lcf, lateral cuticular fringe; pl, plant material; te, tentacles; tl, tail; vlp, ventrolateral papillae. Other abbreviations as in Figs. 1, 3, 5. Scale bars represent 5 mm in a–c, and 10 mm in (d). Due to the array of perspectives shown, e is presented without a scale bar, however, the total preserved length of the specimen is 29.5 mm.
a Historical plate drawing (pl. 32, Fig. 322) of the original holotype of Palaeocampa anthrax, which was lost in a fire. b Phylogenetic position of Palaeocampa anthrax among lobopodians. Extinct taxa marked with a dagger (†), non-lobopodians in grey, non-marine inclusive species in red (Helenodora marked red pending further study), Palaeocampa anthrax bolded. Numbers below branch nodes represent the percentages of trees that recovered this node. Digital reconstruction of Palaeocampa anthrax, presented in (c) dorsal, (d) lateral, (e) oblique, trunk flexed, and f frontal perspectives. Colors are speculative, and based on extant onychophorans. Spines are shown with dramatic colors to advertise their chemical defenses. Frontal appendages are conservatively reconstructed without branches or endites.
Onychophore; Heyler and Poplin,28; Fig. 1.
Type- Holotype lost in fire, USNM 38032a previously designated as neotype, MCZ:IP:110208a,b (part and counterpart) hereby designated as neotype; YPM IP 042831, USNM 38032b, and MHNH SOT 2148 a, b are hereby designated as paratypes. Reposited in the Invertebrate Paleontology collection, Museum of Comparative Zoology, Harvard University, USA; Invertebrate Paleontology collection, Yale Peabody Museum, Yale University, USA; Department of Paleobiology, National Museum of Natural History, Smithsonian, USA; and the Sotty Collection, Muséum national d’Histoire naturelle, Paris (but held in Muséum d’Histoire naturelle, Autun), France; respectively.
Locality and horizon- Carbondale Formation, Francis Creek Shale Member, Mazon Creek, Moscovian (Mid-Late Carboniferous), Illinois, USA; Saint-Louis, Montceau-les-Mines, Late Gzhelian (Late Carboniferous), Bourgogne, France.
Other known specimens. Mazon Creek, Illinois, USA: USNM 38032a-d (4 specimens, parts and counterparts) and USNM 38033 (6 specimens, parts and counterparts), Invertebrate Paleontology Collection, Department of Paleobiology, National Museum of Natural History, Washington, D.C., USA; PE 57079 (part and counterpart), PE 88793 (part and counterpart), Fossil Invertebrate Collection, Field Museum, Chicago, Illinois, USA; 56540-493 (part only), Paleontological Collection, University of Illinois, Champaign, Illinois, USA; YPM IP 036198, 042823, 042825, 042826, 042827, 042828, 042830, 042831, 042833, 042834, Yale Peabody Museum, New Haven, Connecticut, USA. Montceau-les-Mines, France: MHNH SOT 2147, 2148, 2149, 3657, 3659, 5166, 7681, 95674 (8 specimens, parts and counterparts), Muséum national d’Histoire naturelle, Paris, France (but held in Muséum d’Histoire naturelle, Autun, France). See also Supplementary Table 1.
Emended Diagnosis-
As for the genus.
Description-
All specimens have been recovered within nodules, have biomineralized spines preserved three-dimensionally and varying degrees of soft tissue preservation (Fig. 1, Fig. 2, Fig. 3). The head emerges from under a protective hood of thickened dorsal cuticle with a squared-off anterior shape, the corners of which bore the anterior-most set of defensive spines (see Fig. 4f). Dorsally, the head bears a slightly-domed, circular, partially sclerotized structure, which due to its positioning in the holotype, we interpret as a dorsal head sclerite (Figs. 1a, 5c, 6a). No eyes or mouth are apparent on the anterior region of any of the specimens. A pair of annulated antenniform appendages come off the anterior region of the head. Only one specimen has a mostly complete frontal appendage preserved (Fig. 3d, 3e), while several others have the proximal areas of the appendage preserved but are missing distal features (Figs. 5c, 5d, 6a). The most completely preserved frontal appendage is curved in on itself towards the body, and the middle portion is missing. This appendage is roughly ~6.5 mm in length with a basal width of ~1.4 mm and a terminal width of ~.35 mm. This accounts for ~22% of the length of the rest of the body. A ventrolateral pair of shorter and thinner uniramous appendages sit posterior to the head shield (Figs. 1a, 5c, 5d, 6a). They appear to be relatively smooth and non-annulated. Because of the incomplete nature of the primary frontal appendage, branching structures or projections cannot be confidently identified, although small bumps in the most complete frontal appendage may represent the bases of such structures. The annuli of the frontal appendages are lined with small, regularly distributed papillae (Fig. 5d), but the annuli themselves are apparently undifferentiated, in contrast with onychophorans29.
Complete specimens range in body length from ~23 mm–40 mm not including spines or frontal appendages (see Figs. 4, 6). The body is elongate, vermiform, and maintains a relatively equal diameter throughout with a slight tapering towards the posterior. The cuticle is annulated with plicae and papillae (Figs. 1a, 3d, e, 5c, 4g, 6a). Papillae density is greatest on the dorsal surface (e.g., Figures 1a, 3d, e), where the individual papillae are enlarged and partially sclerotized; sclerotized papillae retain a terminal pore, similar to the modified spine-bearing papillae of the trunk nodes, although not bearing obvious spines themselves. No structures occupying these dermal papillae have yet been observed. Internal structures are preserved in some specimens, possibly the remains of the gut, and of a heart or pericardial sinus (see Fig. 6a). Complete specimens possess ten pairs of ventrolaterally-oriented lobopodous walking legs which are stout, approximately equal in length to the width of the trunk. Lobopods are annulated with rows of papillae (Fig. 5b). No terminal claws are apparent on the legs.
The trunk is covered by ten pairs of dorsolateral and lateral sclerite sets, corresponding to each walking leg pair, perhaps set on small mounds of soft tissue. Each sclerite set consists of alternating rows of modified basement papillae from which long straight spines emerge. The sum of the alternating rows of modified basement papillae forms a round to sub-round basal shape to each sclerite set. Dorsolateral sclerites are ~1.5x the length of trunk diameter and lateral sclerites are ~1.0 x length of the trunk diameter. The spines articulate independently, allowing for the splaying structure preserved three-dimensionally in some specimens. The spines remain in their set even when the rest of the body is disarticulated, suggesting that the modified basement papillae are firmly connected basally (see Supplementary Data Fig. 1). Spine growth is incremental, and growth lines are most prominent at the base of the spine. Externally, the spines have raised longitudinal ridges separated by 2-3 smaller, lesser ridges. The ridges are slightly serrated along their whole length, with serrations directed upward toward the distal end (more shingle-like than barbed). Septa are formed internally and at semi-regular intervals within the spine. A spongy, hollow structure, unattached to the spinal walls, is found between septa (Figs. 1d, 1f, 6d). The spines are round in cross-section and maintain their diameter throughout, only slightly tapering at the distal end. The terminus possesses jagged projections, likely as a means of mechanical defense. In several specimens, there are circular discolorations found at the tips of the spines, indicating the expulsion of fluid from the tips (probably induced taphonomically, or by stress at the time of burial), demonstrating that the porous inner material of the spines was fluid-filled in life. Such expulsion of fluid through the spines has not been observed in any other sclerite-bearing lobopodian.
Results and Discussion
Sclerite architecture and chemical defenses
Exudates preserved at the tips of the spines suggest that Palaeocampa had chemical defense abilities as well, either as venom or other noxious chemical deterrents. Palaeocampa’s spines are its most prominent feature and the defining characteristic that distinguishes it from other lobopods. Apart from the relative length and sheer number of spines per animal (n ≥ 1000), the basal growth of a fixed diameter (maintained from base to apex (Figs. 1d–f, Fig. 2) seen in Palaeocampa’s spines differs greatly from the accretionary ‘cone-in-cone’ growth seen in other lobopod sclerites (e.g., Fig. 2G in40). Additionally, the terminus of typical lobopod spines form a point or tip unlike the apex of Palaeocampa’s spines which maintains a relatively similar diameter throughout, yet does form sharp points along the circumference of the terminus giving it a ‘castle tower’ appearance (Figs. 1g, 2g). It is at this apex where some specimens of Palaeocampa possess circular discolorations suggestive of a chemical defense (Figs. 1, 3, 7a). To quantify the possible existence of a chemical defense (beyond the suggestive details of the spine’s inner hollow structure and consistent position of the circular tips at the distal end), two specimens (Fig. 3c) that possess well-defined circular discolorations at their spine’s tips were examined using Fourier-transform spectrometry analysis (FTIR) as a nondestructive method to identify biomarkers.
FTIR, As an analytical tool to identify inorganic and organic contents in fossils, is a growing method in paleontology with demonstrable success41,42, and refs. within] and in recent years has been used to resolve affinities of Rhynie Chert fossils43, identify Neoproterozoic fungal microfossils44, investigate bone diagenesis from Holocene fossils45, and distinguish soft tissues of vertebrates and invertebrates in the Carboniferous Mazon Creek Formation46. FTIR analysis of fossil material is successful because chemical and molecular signatures of organic molecules have been shown to be preserved as far back as the Proterozoic47,48 and experimentally shown to survive diagenesis49. Furthermore, recent studies using other techniques, such as full scan gas chromatography–mass spectrometry analysis, have successfully recovered terpenoid biomarkers (phytohopanoids and fernane/arborane derived aromatic products) from fossil ferns in Mazon Creek nodules50. The ability to preserve such biomarkers within Mazon Creek nodules is likely the result of both rapid entombment and cementation and the low thermal stress and diagenesis of the formation51.
FTIR was used to examine whether the material preserved at the tip of the spines was of different composition than the spines or the matrix. FTIR results showed statistically significant differences between the matrix, spines, and tips (Fig. 7) indicating that the circular discolorations at the spine tips are not taphonomic artifacts but possess a distinct organic signature. This signature is only detected at the apex of the spine, indicating expulsion from a fluid-filled interior as opposed to movement along the exterior using capillary action via the longitudinal ridges. Given the lack of a point at the apex and the absence of clearly defined internal canals, it is unlikely that the chemical would have been delivered as an injection but rather as a passive secretion stimulated as a stress response.
While not definitive, the spectra obtained on the tips of Palaeocampa’s spines are suggestive of the presence of aldehydes as shown in the FTIR spectrum by the aldehydic C-H stretch band at 2694–2873 cm−1 and the aldehydic C-H bend at 1394 cm-1 (Fig. 7e). Aldehydes typically have a strong C=O stretching band, usually the most intense peak in the spectrum52. A C=O stretching band is seen at 1770 cm−1 in the tip spectra, although its intensity is weak. Yet, the presence of several medium intensity bands between 1400 cm-1 and 1100 cm−1 (Fig. 7e), despite being a spectral range for many functional groups including siderite and other carbonate minerals of calcite structure, may be further support for the presence of aldehydes52,53 in which the bands are a result of organic vibrational modes arising from organic macromolecules. Most likely, the bands observed between 1400 cm−1 and 1100 cm−1 (Fig. 7e) are a combination of organic and mineralogic vibration modes. The presence of aldehydes would not be altogether surprising. Invertebrates commonly use aldehydes as a chemical defense that serves as deterrents against predators [e.g. ref. 54] and are typically possessed by slow-moving organisms that sit low on the food chain55,56. In addition to a chemical defense, aldehydes are also used in other roles such as communication and predation57, but these are secondary compared to their widespread use in defense. Without reference scans or knowledge of extant descendants of Palaeocampa for comparison, deciphering the full composition of the chemical is not yet possible. Nonetheless, identifying the presence of a spine-mediated chemical defense would mark the first of its kind among lobopods and an important evolutionary development for this group.
Extant fireworms (Amphinomidae) take their name from the intense burning pain caused by contact with their chaetae58. There has been intense debate over these chaetae, regarding their structure and capability for producing or delivering venom59. No studies to date have proven a method for producing or delivering venom, such as an apical pore or a toxin-producing gland, however, other studies have found genetic evidence for the production of venom among fireworm species60, and others maintain that venom may be stored within the chaetae59. Fireworms have both notochaetae and neurochaetae (per the notopodium and neuropodium), which may be further differentiated into many different kinds on the same animal (as well as into aciculae and capillary chaetae)61,62. Internally, they have a honeycomb structure of tubules, and a probably hollow internal cavity59. They may have a spur or bifurcate, or have serrations oriented away from the apex59. There is little morphological similarity with the spines of Palaeocampa, both internally or externally, which has a single spine morphology, only varying between the dorsal and lateral sets in total size. Lepidopteran larvae (caterpillars) and some spiders, particularly tarantulas, are also known for their urticating hairs, which are painful upon contact63. They are typically hollow, loosely attached or held within a socket in order to come loose and stay embedded in skin, assisted by barbs that also cause mechanical irritation64. In many of these, the barbs are directed towards the distal end65, similar to the serrations of Palaeocampa. By contrast, the spines of Palaeocampa are much larger (in width and height), have a more complex structure (Figs. 1, 2), and are firmly embedded in their set (Supplementary Material Fig. 1). Some caterpillars have larger, more complex spines, ending in a sealed point which is easily broken at its end, allowing it to inject venom upon contact63. These are perhaps less comparable morphologically, but may have served a more similar purpose to the spines of Palaeocampa, in being potentially adapted to break towards the apex and release the chemical defense, rather than the entire spine being ejected and stuck into the threat. In this case, the tip of the spine would be returned to its original state upon each molting. The crenellated spine apex, as well as the serrations, would have also served as a mechanical irritant upon contact.
Phylogeny
Several examples of macroscopic marine Lobopodians are known to have survived well past the Cambrian, with a handful from the early Ordovician66,67, Silurian68, and a few as late as the Carboniferous (Moscovian)69, after which they appear to disappear completely. The paucity of the lobopodian fossil record post-Cambrian mirrors the diminishing number of Lagerstätten over time70 and is therefore likely to be a taphonomic artifact, with their true abundance remaining stable throughout most of the Paleozoic, continuing to be cosmopolitan in distribution and yet locally scarce. There is no evidence to suggest they survived into the Permian; however, it is plausible that some persevered up until the end-Permian extinction event. Regardless, the rarity of post-Cambrian fossils provides a challenge in interpreting lobopod relationships, which can already be difficult among Cambrian species (Fig. 8). Post-Cambrian lobopodians suffer from a cryptic lineage and large temporo-spatial gaps between individual taxa which confuse attempts to place them in an evolutionary context. Palaeocampa anthrax possesses multiple features unprecedented among lobopodians, and is therefore particularly difficult to place.
Primarily using a modified version of the character matrix developed by Kihm et al. 71, (further details, including character list and references, can be found in the Supplementary Material), we tentatively resolve Palaeocampa anthrax as the sister taxon to Hadranax augustus72 within the larger Aysheaiidae (also including Aysheaia, and an unnamed species with branching trunk lobopods from the Ordovician Soom Shale of South Africa). Aysheaiidae as a whole is recovered as just basal Antennopoda, which includes Onychophora, Radiodonta, and Euarthropoda (Fig. 9). We also recover Luolishaniida as sister taxa to Tardigrada in agreement with71.
Hadranax augustus, known thus far from three described fossils (only the holotype mostly complete, see Fig. 8d)72 has long been a difficult species to place. It possessed highly elongate, unsclerotized frontal appendages, hardened node-like structures lining each segment, a lateral fringe of soft tissue, and apparently clawless walking legs72. Previous phylogenetic analyses have placed it alongside Xenusion auerswaldae (a short-legged, clawless, but also acute spine-bearing species) between the siberiids and more crownward total-group euarthropods73, or as a relative of kerygmachelids74. Palaeocampa provides a unique opportunity for comparison. Both taxa are stout-legged (walking appendage lengths approximately equal to body width), apparently clawless lobopodians, bearing elongate, unsclerotized frontal appendages, and segments lined with four evenly spaced “nodes”. These dorsal structures are large but rather poorly defined in Hadranax; however, they are comparable to the bases of the spinule bundles found in Palaeocampa. The lateral fringe seen in Hadranax is perhaps comparable to the thickened dorsum in Palaeocampa (see Fig. 4f). Together, they are both broadly similar to the stout-legged Aysheaia pedunculata from the Burgess Shale, although Aysheaia possesses claws, and lacks any obvious dorsal “nodes” (Fig. 8c).
Grossly, Palaeocampa resembles Aysheaia in its low number of body segments, non-terminal frontal appendages, and short limbs. The trunk of Aysheaia are lined with obvious papillae, which are enlarged dorsally (similar to the sclerotized dorsal papillae of Palaeocampa)8. The dorsal papillae in Aysheaia house small, possibly sensory setae8 (considering the role of the spines in Palaeocampa, a defensive, urticating role might not be ruled out), which may be analogous to the sclerotized papillae (with a central hole, probably housing similar setae, see Fig. 2e) and spinules of Palaeocampa, which themselves emerge from modified papillae along the dorsum (Figs. 1f, 2h). Still, considerable differences exist between the two – Palaeocampa lacks apparent claws, and bears a distinct cephalic sclerite, comparable to structures seen in some luolishaniids16 (the presence of such a sclerite remains a possibility with Hadranax, for which the head region is essentially unknown). It is possible that features such as claws and head sclerites have a higher degree of plasticity within the lobopodians as a whole, as the majority of morphological features still support Palaeocampa’s alignment with the aysheaiids. Another likely aysheaiid, an unnamed species from the Ordovician-age Soom Shale67 (Fig. 8b), lacks obvious trunk nodes and possesses claws, similar to Aysheaia – it does however have a pair of frontal appendages, with possibly a second smaller pair of frontal appendages behind them, comparable to Palaeocampa. This structure in the Soom Shale lobopodian awaits further investigation. Assignment of Palaeocampa to Aysheaiidae, then, seems the most reasonable interpretation, although we caution that more study of the group may need to be done to resolve the issues of claws and head sclerites.
Palaeocampa, in possessing nodal rows of four, is only superficially similar to Thanahita distos68 from the Silurian-age Herefordshire Biota, wherein the nodal rows of four are poorly organized (generally, one row above the legs, with three rows between leg pairs, the center-most row directed further laterally and composed of only two nodes, as well as two pairs of ventrolateral nodes between the legs, offset and smallest to the posterior), and the nodes are developed into unsclerotized papillae tufts (Fig. 8e). The walking legs point to a hallucigeniid affinity, with long thin lobopods ending in a small number of large claws (appendages six and beyond ending in a single claw, appendages five, four, and possibly three ending in one large and one small claw), with anterior-most appendages specialized into tentacular lobopods. Our analysis recovered Thanahita as basal within the ‘archonychophorans’75. Palaeocampa is also dissimilar from Carbotubulus waloszeki69, which co-occurs at Mazon Creek. Carbotubulus is a hallucigeniid, with differentiated posterior and anterior walking limb sets and a distinct neck region bearing tentacular limbs (although, the presence or absence of spines has not yet been satisfactorily investigated in this taxon).
Colonization of brackish and freshwater environments by lobopodians
Palaeocampa co-occurs in the Mazon Creek with the lobopodian Helenodora inopinata76, about which there has been some debate over whether it represents a marine or terrestrial organism. Originally described as a possible terrestrial onychophoran, the holotype and paratype of Helenodora are preserved in siderite nodules from Peabody Coal Company Pit 11 (apparently a marine deposit)76. The fossils have up to 20 pairs of very stout (roughly three times shorter than body width) lobopodous legs, and a more elongate, flexible pair of appendages at the far anterior, comparable to the antennae of modern onychophorans (possibly possessing also slime papillae, see ref. 69). The trunk is densely annulated, questionably with dermal papillae. Murdock et al.77 redescribed Helenodora, and concluded it was more likely a basal marine lobopodian (sister to Paucipodia inermis78), as no conclusive evidence for eyes, slime papillae, claws, or jaws was found, and where they had previously been identified were reinterpreted as taphonomic artifacts77. This reinterpretation was questioned by Oliveria et al.79, who considered the dark patches originally identified as claws as possibly genuine. Regarding the slime papillae, these they considered might be exceptionally difficult to recognize in a fossil like Helenodora. Based on its gross morphology, we consider an onychophoran identity much more likely than as a stem lobopod like Paucipodia. Another Carboniferous fossil worm, Antennipatus montceauensis from the Montceau-les-Mines Lagerstätte in France, previously described as “identical” to Helenodora34, demonstrated much more confidently the onychophoran characters that are ambiguous in Helenodora29. The annulated trunk was clearly ornamented with well developed dermal papillae, and a pair of well-differentiated slime papillae were present. Based on these, Antennipatus was probably a terrestrial animal, and represents either a stem or crown group onychophoran80.
The redescription of Palaeocampa anthrax, which co-occurs with both of these possible onychophoran genera, reignites discussion of the depositional environments and life habits of these animals. The Carboniferous Lagerstätte preserved at Montceau-les-Mines has been widely considered to preserve a freshwater-intermontane environment31,81,82,83,84, with the nearest open-marine environment being at least several hundred kilometers southwest of the locality84. It was tropical and equatorial, preserving a thriving coal forest element83. It is for this reason also that a marine habitat for Antennipatus was discounted29, citing the lack of any sedimentological, structural, or paleogeographic evidence for a marine influence, as well as the abundance of either freshwater (previously, the presence of arthropods like the xiphorusan Alanops magnificus81 and the euthycarcinoids31 were considered to be marine influence30, but these were more likely freshwater inhabitants85,86) or wholly terrestrial animals, as well as in-situ tree trunks indicating deposition close to land. Of the marine faunal influence present at Montceau-les-Mines cited by Schultze30, only Palaeocampa and the alleged myxinid (hagfish) Myxineidus gononorum87 remain problematic. Hagfish are highly intolerant of low salinity and high temperature water88, with all extant taxa living in deep-water marine environments. X-ray synchrotron microtomography of the fossil revealed details more strongly indicative of a lamprey identity89 (petromyzontiformes, also adapted to freshwater environments), or stem lamprey identity as found by phylogenetic analysis90, which would better agree with an equatorial, freshwater setting89. It is not inconceivable that a ‘xenusiid’ (lobopodians excluding onychophorans, tardigrades, and arthropods), which have to date only been found in marine deposits and are thus considered exclusively marine91, may have adapted to a non-marine environment. The ability for onychophorans and tardigrades to adapt to non-marine, even terrestrial environments, means that we should not rule out the possibility that other lobopodian groups may have had similar developments through the Paleozoic. For example, the Chengjiang Biota of China (Yu’anshan Member, Chiungchussu Formation, Cambrian Series 2) has recently been proposed to have inhabited an extremely shallow, deltaic front environment, where the influx of freshwater was a primary stressor92. Despite this, it is still host to a diverse lobopodian fauna3, perhaps even including a stem-onychophoran, Antennacanthopodia gracilis93, demonstrating at least a tolerance for brackish conditions as early as the Cambrian.
Mazon Creek specimens of Palaeocampa provide little indication of habitat beyond occasionally preserving alongside large plant fragments (Fig. 8a) typical of nodules preserving washout fauna/flora which originated from non-marine environments. Mazon Creek Palaeocampa specimens are also associated with the freshwater belinurine xiphosuran Euproops danae21 and various terrestrial arachnids23. However, Palaeocampa also occurs at Montceau-les-Mines, present in the same horizons as Alanops magnificus (a freshwater xiphosuran), at least two species of freshwater euthycarcinoids, various species of freshwater crustaceans, and a probably terrestrial94 pill millipede (Amynilyspes fatimae82)31. In agreement with Garwood et al.29, there remains no convincing evidence for a marine influence at Montceau-les-Mines, and we maintain that Palaeocampa itself does not discredit the widely accepted freshwater-terrestrial, intermontane setting of the Montceau-les-Mines Lagerstätte (Fig. 10). Palaeocampa anthrax, then, is best interpreted as a freshwater dwelling lobopodian, although we cannot rule out the possibility it was amphibious or terrestrial instead (subaerial adaptations such as spiracles were not observed, and the lobopods seem ill-equipped for sustained terrestriality). Lobopodians have thus colonized non-marine environments several times in their evolutionary history, including tardigrades95, onychophorans80, and now aysheaiids. This raises interesting questions about how prolific ‘xenusiid’ lobopodians were in freshwater environments in the late Paleozoic, and when exactly this colonization began. It remains to be seen if further non-marine lobopodians may be discovered in the future.
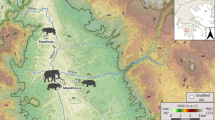
A large (40 mm) Palaeocampa anthrax is depicted at the edge of a shallow inland streambed, shadowed by the lush vegetation and mist of a coal forest, flanked by several euthycarcinoids, Sottyxerxes multiplex116 (max size ~35 mm), and a pair of freshwater xiphosurans, Alanops magnificus (max size ~25 mm). The nearest ocean environment is several hundred kilometers southwest.
Material and methods
Microscopy
Specimens were studied under a binocular microscope and Keyence digital microscope and photographed using a Nikon D7500 DSLR digital camera equipped with a Laowa 100 mm f/2.8 Ca-Dreamer Macro 2x lens under dry, wet, and cross-polarized light conditions. Photographs were then focus-stacked using Adobe Photoshop 2024 (v.25.4.0) to create high-resolution images. Images were further analyzed and measured using ImageJ (1.53t). Backscatter scanning electron microscopy and energy-dispersive spectroscopy-based elemental mapping were performed with a JEOL 7900 F SEM at the Center for Nanoscale Systems at Harvard University.
Fourier-transform Spectrometry, data filtering and pre-processing
A FTIR spectrometer with a microscope (Bruker, Vertex v70v and Hyperion 2000) was used to measure the spectral reflectivity over the fossil surface in the mid-infrared range (wavenumber = 600~4000 cm−1) where the vibrational energy transitions of the major chemical bonds correlated with organic molecules centered at. The spectral reflectivities qualitatively reveal the presence of molecular bonds and therefore differentiate the measured materials by their chemical compositions. Five FTIR raster-scans were conducted on different areas containing tip, spine and matrix areas of fossil specimen 38032, and four were conducted on specimen 42834, producing nine scans total. All scans used an aperture of 60 µm, and step sizes of these scans ranged from 20–50 µm, for a total of 12,175 individual spectra collected across both fossils. Spectra in which multiple category types overlapped were then excluded, resulting in 9114 individual spectra. Systematic differences between measured spectra were observed between scans for each fossil (Fig. 7), and so individual spectra were pre-processed using the Standard Normal Variate (SNV) method of the prospectr package96, which is a common pre-processing technique for analysis of FTIR spectra that removes the multiplicative interferences of scatter and particle size, accounting for overall differences in mean values and variance between individual spectra by mean centering each spectrum and dividing it by its own standard deviation97. For graphical analysis and subsequent statistical testing, SNV-normalized spectra were smoothed via quadratic Savitzky-Golay smoothing as implemented in the ‘savgol’ function of the pracma package98, using a window size of 25, based on the recommendations of99 for minimizing the likelihood of introducing overfitting of narrow peaks during smoothing. It should also be noted that the Carbondale Formation, which the Francis Creek Shale is a member of, has a thermal maturity of immature (407 °C) to barely mature (435 °C) based on Rock-Eval pyrolysis analysis100, which is reflected in the FTIR spectra with the presence of more aliphatic associated bands than aromatic ones.
Principal component analysis
Principal component analysis (PCA) was conducted on raw and SNV pre-processed spectra to examine the overall differences in spectral composition between different fossil specimens and categories. Input spectra were first scaled and then analyzed via PCA (Singular Vector Decomposition) using the StandardScaler and PCA functions of the sklearn package in Python. Analyses were conducted in Python using the pandas101, matplotlib102 and sklearn packages103.
Statistical analysis and reproducibility of spectral regions of interest
We identified spectral regions with statistically significant differences between each pair of categories, employing t-tests between the SNV-normalized values for each wavenumber for each pair of categories, followed by correction of all p-values via the method of Benjamini-Hochberg104 to account for multiple testing (Fig. 7d). We used local maxima in t-value plots as a means of identifying a subset of wavenumbers which were most statistically distinct between each pair of categories for further discussion and analysis. Statistical analyses and final plotting of SNV-normalized spectra and t-value plots were conducted in R (4.0.3), using the plyr105, dplyr106, prospectr107, tidyr108, and ggplot2108 packages.
Phylogenetic Analysis
A character matrix of 114 morphological characters and 74 taxa was assembled using Mesquite 3.70109, with an emphasis on lobopodians, and rooted on the Cambrian worm, Acosmia maotiania110. The matrix was analyzed using TNT 1.6111 using New Technology Search with Sectorial Search, Ratchet, Drift, and Tree Fusing enabled, all under standard settings, set to find the minimum length 500 times under equal weights, with the option to collapse trees after search enabled. The resultant topology was visualized using TreeGraph 2.15112, and the character matrix is included in the Supplementary Note 2 and Supplementary Note 3. The Nexus file used in the phylogenetic analysis is available at Zenodo (https://doi.org/10.5281/zenodo.15774949)113.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data required to reproduce the findings of the study are included in the manuscript and supplementary material. Source data from the FTIR analysis in Fig. 7 and from the Supplementary Figs. 3, 4 are available at Dryad (https://doi.org/10.5061/dryad.p2ngf1w0j)114. R and Python codes used for statistical analysis are available at Zenodo (https://doi.org/10.5281/zenodo.15774949)113. Character coding and references used for phylogenetic analysis is found in Supplementary Note 1-3 and as a Nexus file available at Zenodo (https://doi.org/10.5281/zenodo.15774949)113.
Code availability
R and Python codes used for statistical analysis are available at Zenodo (https://doi.org/10.5281/zenodo.15774949)113. Character coding and references used for phylogenetic analysis is found in Supplementary Note 1-3 and as a Nexus file available at Zenodo (https://doi.org/10.5281/zenodo.15774949)113.
References
Budd, G. E. A Cambrian gilled lobopod from Greenland. Nature 364, 709–711 (1993).
Wu, R., Pisani, D. & Donoghue, P. C. J. The unbearable uncertainty of panarthropod relationships. Biol. Lett. 19, 20220497 (2023).
Ou, Q. & Mayer, G. A Cambrian unarmoured lobopodian, †Lenisambulatrix humboldti gen. et sp. nov., compared with new material of †Diania cactiformis. Sci. Rep. 8, 13667 (2018).
Walcott, C. D. Cambrian Geology and Paleontology II: Middle Cambrian annelids. Smithson. Misc. Collect. 57, 109–144 (1911).
Yochelson, E. Discovery, collection, and description of the Middle Cambrian Burgess Shale biota by Charles Doolittle Walcott. Proc. Am. Philos. Soc. 469–545 (1996).
Hutchinson, G. E. Restudy of some Burgess Shale fossils. Proc. U.S. Natl. Mus. (1930).
Walcott, C. D. & Resser, C. E. Addenda to descriptions of Burgess shale fossils (with 23 plates). Smithsonian Miscellaneous Collections (1931).
Whittington, H. B. The Lobopod Animal Aysheaia Pedunculata Walcott, Middle Cambrian, Burgess Shale, British Columbia. Philos. Trans. R. Soc. Lond. B, Biol. Sci. 284, 165–197 (1978).
Conway Morris, S. A new metazoan from the Cambrian Burgess Shale of British Columbia. Palaeontology 20, 623–640 (1977).
Pompeckj, J. F. Ein neues Zeugnis uralten Lebens. Pal. Zeitschr. 9, 287–313 (1927).
Jaeger, H. & Martinsson, A. Remarks on the Problematic Fossil Xenusion Auerswaldae. GFF 88, 435–452 (1967).
McMenamin, M. A. S. The Garden of Ediacara. PALAIOS 1, 178–182 (1986).
Dzik, J. & Krumbiegel, G. The oldest ‘onychophoran’Xenusion: a link connecting phyla?. Lethaia 22, 169–181 (1989).
Conway Morris, S. & Robison, R. A. More soft-bodied animals and algae from the middle Cambrian of Utah and British Columbia. Univ. Kans. Paleontol. Contrib.122, 1–48 (1988).
Ramsköld, L. & Chen, J.-Y. Cambrian lobopodians: morphology and phylogeny. in Arthropod fossils and phylogeny (ed. Edgecombe, G.) 107–150 (Columbia University Press, 1998)
Caron, J.-B. & Aria, C. The Collins’ monster, a spinous suspension-feeding lobopodian from the Cambrian Burgess Shale of British Columbia. Palaeontology 63, 979–994 (2020).
Hou, X.-G. & Chen, J.-Y. Early Cambrian tentacled worm-like animals (Facivermis gen. nov.) from Chengjiang, Yunnan. Acta Palaeontol. Sin. 28, 32–42 (1989).
Cave, L. D., Insom, E. & Simonetta, A. M. Advances, diversions, possible relapses and additional problems in understanding the early evolution of the Articulata. Ital. J. Zool. 65, 19–38 (1998).
Howard, R. J. et al. A Tube-Dwelling Early Cambrian Lobopodian. Curr. Biol. 30, 1529–1536.e2 (2020).
Caron, J.-B., Smith, M. R. & Harvey, T. H. P. Beyond the Burgess Shale: Cambrian microfossils track the rise and fall of hallucigeniid lobopodians. Proc. R. Soc. B: Biol. Sci. 280, 20131613 (2013).
Meek, F. B. & Worthen, A. H. Notice of some new types of organic remains, from the Coal Measures of Illinois. Proc. Acad. Nat. Sci. Phila. 41–48 (1865).
Meek, F. B. & Worthen, A. H. Descriptions of invertebrates. Geol. Surv. Ill. 2, 410–411 (1866).
Meek, F. B. & Worthen, A. H. Palaeontology of Illinois. Geol. Surv. Ill. 3, 565 (1868).
Pleijel, F., Rouse, G. W. & Vannier, J. Carboniferous fireworms (Amphinomida: Annelida), with a discussion of species taxa in palaeontology. Invert. Syst. 18, 693–700 (2004).
Scudder, S. H. The fossil insects of North America. Geol. Mag. 5, 216–222 (1868).
Scudder, S. H. The affinities of Palaeocampa Meek and Worthen as evidence of the wide diversity of type in the earliest known myriapods. Am. J. Sci. 3, 161–170 (1882).
Scudder, S. H. Two new and diverse types of Carboniferous myriapods. Boston Soc. Nat. Hist., Mem. 3, 283–297 (1884).
Heyler, D. & Poplin, C. M. The Fossils of Montceau-les-Mines. Sci. Am. 259, 104–111 (1988).
Garwood, R. J. et al. Carboniferous Onychophora from Montceau-les-Mines, France, and onychophoran terrestrialization. Invertebr. Biol. 135, 179–190 (2016).
Schultze, H.-P. Interpretation of marine and freshwater paleoenvironments in Permo–Carboniferous deposits. Palaeogeogr. Palaeoclimatol. Palaeoecol. 281, 126–136 (2009).
Racheboeuf, P. R., Vannier, J., Schram, F. R., Chabard, D. & Sotty, D. The euthycarcinoid arthropods from Montceau-les-Mines, France: functional morphology and affinities. Earth Environ. Sci. Trans. R. Soc. Edinb. 99, 11–25 (2008).
Nielsen, C. Animal Evolution: Interrelationships of the Living Phyla. (OUP Oxford, 1995).
Pacaud, G., Rolfe, W. D. I., Schram, F. R., Secretan, S. & Sotty, D. Quelques invertébrés nouveaux du Stéphanien de Montceau-les-Mines. Bull. Soc.’Hist. Nat.’Autun 97, 37–43 (1981).
Rolfe, W. D. I., Schram, F. R., Pacaud, G., Sotty, D. & Secretan, S. A Remarkable Stephanian Biota from Montceau-les-Mines, France. J. Paleontol. 56, 426–428 (1982).
Rolfe, W. D. I. Catalogue of type specimens in the invertebrate paleontological collections of the Museum of Comparative Zoology Arthropoda (Trilobita, Arachnida and Insecta excluded). Museum of Comparative Zoology at Harvard College (1963).
Fitzhugh, K. Polychaete worms. in Richardson’s Guide to the Fossil Fauna of Mazon Creek (eds. Shabica, C. W. and Hay, A. A.) 64-68 (Northeastern Illinois University, 1997).
Saleh, F., Clements, T., Perrier, V., Daley, A. C. & Antcliffe, J. B. Variations in preservation of exceptional fossils within concretions. Swiss J. Palaeontol. 142, 20 (2023).
Matthew, G. F. On the organic remains of the Little R. Group, No. 4. Trans. Ott. Nat. Field Club 4, 108–109 (1894).
Thompson, I. Errant polychaetes (Annelida) from the Pennsylvanian Essex fauna of northern Illinois. Palaeontographica 163, 169–199 (1979).
Yang, J. et al. A superarmored lobopodian from the Cambrian of China and early disparity in the evolution of Onychophora. Proc. Natl. Acad. Sci. 112, 8678–8683 (2015).
Olcott Marshall, A. & Marshall, C. P. Vibrational spectroscopy of fossils. Palaeontology 58, 201–211 (2015).
Bobroff, V., Chen, H.-H., Javerzat, S. & Petibois, C. What can infrared spectroscopy do for characterizing organic remnant in fossils?. TrAC Trends Anal. Chem. 82, 443–456 (2016).
Loron, C. C. et al. Molecular fingerprints resolve affinities of Rhynie chert organic fossils. Nat. Commun. 14, 1387 (2023).
Bonneville, S. et al. Molecular identification of fungi microfossils in a Neoproterozoic shale rock. Sci. Adv. 6, eaax7599 (2020).
Del Valle, H. et al. ATR-FTIR to distinguish Holocene fumier facies. A perspective from bone diagenesis at El Mirador cave (Sierra de Atapuerca, Spain). J. Archaeol. Sci. 141, 105582 (2022).
McCoy, V. E. et al. Chemical signatures of soft tissues distinguish between vertebrates and invertebrates from the Carboniferous Mazon Creek Lagerstätte of Illinois. Geobiology 18, 560–565 (2020).
Marshall, C. P., Javaux, E. J., Knoll, A. H. & Walter, M. R. Combined micro-Fourier transform infrared (FTIR) spectroscopy and micro-Raman spectroscopy of Proterozoic acritarchs: A new approach to Palaeobiology. Precambrian Res. 138, 208–224 (2005).
Igisu, M. et al. Micro-FTIR spectroscopic signatures of Bacterial lipids in Proterozoic microfossils. Precambrian Res. 173, 19–26 (2009).
Alleon, J. et al. Organic molecular heterogeneities can withstand diagenesis. Sci. Rep. 7, 1508 (2017).
Tripp, M. et al. Rapid encapsulation of true ferns and arborane/fernane compounds fossilised in siderite concretions supports analytical distinction of plant fossils. Sci. Rep. 13, 19851 (2023).
Cotroneo, S. et al. A new model of the formation of Pennsylvanian iron carbonate concretions hosting exceptional soft-bodied fossils in Mazon Creek, Illinois. Geobiology 14, 543–555 (2016).
Smith, B. C. Infrared Spectral Interpretation: A Systematic Approach. (CRC Press, Boca Raton, 2018).
Alves, J. F., Edwards, H. G. M., Korsakov, A. & de Oliveira, L. F. C. Revisiting the Raman Spectra of Carbonate Minerals. Minerals 13, 1358 (2023).
Vesović, N. et al. The chemical composition of the secretions, their antibacterial activity, and the pygidial gland morphology of selected European Carabini ground beetles (Coleoptera: Carabidae). Front. Ecol. Evol. 11, (2023).
Berenbaum, M. R. The chemistry of defense: theory and practice. Proc. Natl. Acad. Sci. 92, 2–8 (1995).
Dettner, K. Chemical defense and toxins of lower terrestrial and freshwater animals. in Comprehensive Natural Products II (eds. Liu, H. W. & Mander, L.) 387–410 (Elsevier, Amsterdam, 2010).
Sainz-Borgo, C., Leal, B., Cabrera, A. & Hernández, J. V. Mandibular and postpharyngeal gland secretions of Acromyrmex landolti (Hymenoptera: Formicidae) as chemical cues for nestmate recognition. Rev. de. Biol.ía Trop. 61, 1261–1273 (2013).
Tilic, E., Pauli, B. & Bartolomaeus, T. Getting to the root of fireworms’ stinging chaetae—chaetal arrangement and ultrastructure of Eurythoe complanata (Pallas, 1766) (Amphinomida). J. Morphol. 278, 865–876 (2017).
Righi, S., Savioli, M., Prevedelli, D., Simonini, R. & Malferrari, D. Unravelling the ultrastructure and mineralogical composition of fireworm stinging bristles. Zoology 144, 125851 (2021).
Verdes, A., Simpson, D. & Holford, M. Are Fireworms Venomous? Evidence for the Convergent Evolution of Toxin Homologs in Three Species of Fireworms (Annelida, Amphinomidae). Genome Biol. Evol. 10, 249–268 (2018).
Barroso, R. & Paiva, P. C. A new deep-sea species of Chloeia (Polychaeta: Amphinomidae) from southern Brazil. J. Mar. Biol. Assoc. U. Kingd. 91, 419–423 (2011).
Müller, J. et al. “Brittleworms”: Ultrastructure and arrangement of the calcified chaetae of Euphrosine (Amphinomida, Annelida). Invertebr. Biol. 140, e12353 (2021).
Battisti, A., Holm, G., Fagrell, B. & Larsson, S. Urticating hairs in arthropods: their nature and medical significance. Annu Rev. Entomol. 56, 203–220 (2011).
Petrucco Toffolo, E. et al. Size and dispersion of urticating setae in three species of processionary moths. Integr. Zool. 9, 320–327 (2014).
Faucheux, M. J. The urticating apparatus in the larva of the Lappet Moth, Streblote panda Hübner, 1820 (Lepidoptera: Lasiocampidae). Bonn. Zool. Bull. 61, 129–134 (2012).
Van Roy, P. et al. Ordovician faunas of Burgess Shale type. Nature 465, 215–218 (2010).
Whittle, R. J., Gabbott, S. E., Aldridge, R. J. & Theron, J. An Ordovician Lobopodian from the Soom Shale Lagerstätte, South Africa. Palaeontology 52, 561–567 (2009).
Siveter, D. J., Briggs, D. E. G., Siveter, D. J., Sutton, M. D. & Legg, D. A three-dimensionally preserved lobopodian from the Herefordshire (Silurian) Lagerstätte, UK. R. Soc. Open Sci. 5, 172101 (2018).
Haug, J. T., Mayer, G., Haug, C. & Briggs, D. E. G. A Carboniferous Non-Onychophoran Lobopodian Reveals Long-Term Survival of a Cambrian Morphotype. Curr. Biol. 22, 1673–1675 (2012).
Schiffbauer, J. D. & Laflamme, M. Lagerstätten through time: a collection of exceptional preservational pathways from the terminal neoproterozoic through today. PALAIOS 27, 275–278 (2012).
Kihm, J.-H. et al. Cambrian lobopodians shed light on the origin of the tardigrade body plan. Proc. Natl. Acad. Sci. 120, e2211251120 (2023).
Budd, G. E. & Peel, J. S. A new xenusiid lobopod from the Early Cambrian Sirius Passet fauna of North Greenland. Palaentology 41, 1201–1213 (1998).
Caron, J.-B. & Aria, C. Cambrian suspension-feeding lobopodians and the early radiation of panarthropods. BMC Evolut. Biol. 17, 29 (2017).
Lerosey-Aubril, R. & Ortega-Hernández, J. A new lobopodian from the middle Cambrian of Utah: did swimming body flaps convergently evolve in stem-group arthropods?. Pap. Palaeontol. 8, e1450 (2022).
Ma, X., Hou, X. & Bergström, J. Morphology of Luolishania longicruris (Lower Cambrian, Chengjiang Lagerstätte, SW China) and the phylogenetic relationships within lobopodians. Arthropod Struct. Dev. 38, 271–291 (2009).
Thompson, I. & Jones, D. S. A Possible Onychophoran from the Middle Pennsylvanian Mazon Creek Beds of Northern Illinois. J. Paleontol. 54, 588–596 (1980).
Murdock, D. J. E., Gabbott, S. E. & Purnell, M. A. The impact of taphonomic data on phylogenetic resolution: Helenodora inopinata (Carboniferous, Mazon Creek Lagerstätte) and the onychophoran stem lineage. BMC Evolut. Biol. 16, 19 (2016).
Chen, J.-Y., Zhou, G.-Q. & Ramsköld, L. A new Early Cambrian onychophoran-like animal, Paucipodia gen. nov., from the Chengjiang fauna, China. Earth Environ. Sci. Trans. R. Soc. Edinb. 85, 275–282 (1994).
Oliveira, I. et al. Earliest Onychophoran in Amber Reveals Gondwanan Migration Patterns. Curr. Biol. 26, 2594–2601 (2016).
Oliveira, I. de S. An updated world checklist of velvet worms (Onychophora) with notes on nomenclature and status of names. Zookeys 1184, 133–260 (2023).
Racheboeuf, P. R., Vannier, J. & Anderson, L. I. A New Three-Dimensionally Preserved Xiphosuran Chelicerate from the Montceau-Les-Mines Lagerstätte (Carboniferous, France). Palaeontology 45, 125–147 (2002).
Racheboeuf, P. R., Hannibal, J. T. & Vannier, J. A new species of the diplopod Amynilyspes (Oniscomorpha) from the Stephanian Lagerstätte of Montceau-les-Mines, France. J. Paleontol. 78, 221–229 (2004).
Charbonnier, S. et al. Diversity and Paleoenvironment of the Flora From the Nodules of the Montceau-Les-Mines Biota (Late Carboniferous, France). PALAIOS 23, 210–222 (2008).
Perrier, V. & Charbonnier, S. The Montceau-les-Mines Lagerstätte (Late Carboniferous, France). Comptes Rendus Palevol 13, 353–367 (2014).
Lamsdell, J. C. Horseshoe crab phylogeny and independent colonizations of fresh water: ecological invasion as a driver for morphological innovation. Palaeontology 59, 181–194 (2016).
Ortega-Hernández, J., Legg, D. A., Tremewan, J. & Braddy, S. J. Euthycarcinoids. Geol. Today 26, 195–198 (2010).
Poplin, C., Sotty, D. & Janvier, P. Un Myxinoı̈de (Craniata, Hyperotreti) dans le Konservat-Lagerstätte Carbonifère supérieur de Montceau-les-Mines (Allier, France). Comptes Rendus Acad. Sci. Ser. IIA - Earth Planet. Sci. 332, 345–350 (2001).
Sardella, B. A., Baker, D. W. & Brauner, C. J. The effects of variable water salinity and ionic composition on the plasma status of the Pacific Hagfish (Eptatretus stoutii). J. Comp. Physiol. B 179, 721–728 (2009).
Germain, D., Sanchez, S., Janvier, P. & Tafforeau, P. The presumed hagfish Myxineidus gononorum from the Upper Carboniferous of Montceau-les-Mines (Saône-et-Loire, France): New data obtained by means of Propagation Phase Contrast X-ray Synchrotron Microtomography. Ann. Paléontol 100, 131–135 (2014).
Miyashita, T. et al. Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological–molecular conflict in early vertebrate phylogeny. Proc. Natl. Acad. Sci. 116, 2146–2151 (2019).
Hou, X.-G. & Bergström, J. Cambrian lobopodians-ancestors of extant onychophorans?. Zool. J. Linn. Soc. 114, 3–19 (1995).
Saleh, F. et al. The Chengjiang Biota inhabited a deltaic environment. Nat. Commun. 13, 1569 (2022).
Ou, Q. et al. A rare onychophoran-like lobopodian from the Lower Cambrian Chengjiang Lagerstätte, southwestern China, and its phylogenetic implications. J. Paleontol. 85, 587–594 (2011).
Lheritier, M. et al. Fossils from the Montceau-les-Mines Lagerstätte (305 Ma) shed light on the anatomy, ecology and phylogeny of Carboniferous millipedes. J. Syst. Palaeontol. 21, 2169891 (2023).
McInnes, S. J. Zoogeographic distribution of terrestrial/freshwater tardigrades from current literature. J. Nat. Hist. 28, 257–352 (1994).
Stevens, A. and Ramirez-Lopez, L. An introduction to the prospectr package. (2024).
Barnes, R. J., Dhanoa, M. S. & Lister, S. J. Standard Normal Variate Transformation and De-Trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 43, 772–777 (1989).
Borchers, H. W. and Borchers, M. H. W. Package ‘pracma’. Practical numerical math functions, version 2.4.4. (2023).
Zimmermann, B. & Kohler, A. Optimizing Savitzky–Golay parameters for improving spectral resolution and quantification in infrared spectroscopy. Appl. Spectrosc. 67, 892–902 (2013).
Chou, M.-I. M., Dickerson, D. R., Chou, S.-F. J. & Sargent, M. L. Hydrocarbon source potential and organic geochemical nature of source rocks and crude oils in the Illinois Basin. Illinois petroleum no. 136 (1991).
McKinney, W. Data structures for statistical computing in Python. SciPy 445, 51–56 (2010).
Hunter, J. D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Pedregosa, F. et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Benjamini, Y. & Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc.: Ser. B (Methodol.) 57, 289–300 (1995).
Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 40, 1–29 (2011).
Wickham, H., François, R., Henry, L. & Müller, K. dplyr: A Grammar of Data Manipulation. R package version 0.7.6.(2018).
Wickham, H. and Henry, L. tidyr: Tidy Messy Data. R package version 1.1. 2. CRAN. R-project. org/package= tidyr. (2020).
Wickham, H. Data Analysis. In ggplot2: Elegant Graphics for Data Analysis (ed. Wickham, H.) 189–201 (Springer International Publishing, Cham, 2016).
Maddison, W. P. and D. R. Maddison. Mesquite: a modular system for evolutionary analysis. Version 3.81. http://www.mesquiteproject.org (2023).
Chen, J.-Y. & Zhou, G.-Q. Biology of the Chengjiang Fauna. Collect. Res. 10, 11–105 (1997).
Goloboff, P. A. & Morales, M. E. TNT version 1.6, with a graphical interface for MacOS and Linux, including new routines in parallel. Cladistics 39, 144–153 (2023).
Stöver, B. C. & Müller, K. F. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinforma. 11, 7 (2010).
Knecht, R. J., McCall, C. R. A., Tsai, C.-C., Childers, R. A. R., Yu, N. Data Files for Palaeocampa anthrax, an armored freshwater lobopodian with chemical defenses from the Carboniferous, Knecht et al., 2025. Zenodo (https://doi.org/10.5281/zenodo.15774949) (2025).
Knecht, R. J., McCall, C. R. A., Tsai, C.-C., Childers, R. A. R., Yu, N. An armored, freshwater Lobopodian with chemical defenses from the Carboniferous. Dryad (https://doi.org/10.5061/dryad.p2ngf1w0j) (2025).
Woodland, B. G. & Stenstrom, R. C. The occurrence and origin of siderite concretions in the Francis Creek Shale (Pennsylvanian) of northeastern Illinois. In Mazon Creek Fossils (ed. Nitecki, M. H.) 69–103 (Academic Press, 1979).
Schram, F. R. & Rolfe, W. D. I. New Euthycarcinoid Arthropods from the Upper Pennsylvanian of France and Illinois. J. Paleontol. 56, 1434–1450 (1982).
Acknowledgements
The authors wish to thank the following people for their helpful conversations on the material and manuscript: Naomi Pierce, Gonzalo Giribet, Jacob Benner, and Mark Renczkowski. We also wish to thank the following institutions for their assistance in obtaining and viewing specimens: Museum of Comparative Zoology (Harvard University): Jessica Cundiff and Mark Renczkowski; Peabody Museum (Yale University): Susan Butts; Field Museum: Scott Lidgard, Paul Mayer; National Museum of Natural History (Smithsonian): Gene Hunt, Doug Erwin; Muséum d’Histoire Naturelle (Autun, France): Sylvain Charbonnier, Aude Medina; Prairie Institute, Illinois Center for Paleontology: Sam Heads; New Brunswick Museum: Matt Stimson. We thank Laura Cotton and Arden Bashforth (Natural History Museum of Denmark, Copenhagen, Denmark) for providing photographs of Hadranax. We thank Rowan Whittle (British Antarctic Survey) for providing photographs of the Soom Shale Lobopodian. We thank Rich Holm (Earth Science Club of Northern Illinois) for providing photographs of Palaeocampa from his private collection. We thank Tim Cavanaugh for his help in imaging specimens with the SEM. We thank Isabella Kirkland for the early artistic renditions of Palaeocampa. We thank four anonymous reviewers for their comments. RJK acknowledges funding support from the National Science Foundation (NSF) GRFP. Funds for open access were generously provided by the Wetmore Colles Fund (awarded to Richard Knecht and Naomi Pierce). CCT and NY acknowledges funding support from the Gordon and Betty Moore Foundation Experimental Physics Investigators (EPI) Initiative (grant No. 11561).
Author information
Authors and Affiliations
Contributions
R.J.K. devised and facilitated the study. R.J.K. located specimens and performed the photography. C.M. and R.J.K. devised the figures. C.-C.T. and N.Y. carried out FTIR measurement. C.M. performed the phylogenetic analyses and created the reconstructions. R.C. performed statistical analyses. R.J.K. and C.M. wrote and revised the paper. All authors discussed the data, participated in interpretation, and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Graham Budd, C Marshall and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Johannes Stortz. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
.Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Knecht, R.J., McCall, C.R.A., Tsai, CC. et al. Palaeocampa anthrax, an armored freshwater lobopodian with chemical defenses from the Carboniferous. Commun Biol 8, 1080 (2025). https://doi.org/10.1038/s42003-025-08483-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08483-0