Abstract
Thermal screening of coral source material is likely crucial to enhancing long-term restoration success under ocean warming. It is unclear, however, whether reef-based donor colonies retain their thermal tolerance in a nursery environment. Here, we used CBASS acute thermal assays to compare standardized thermal tolerance thresholds (ED50s) of donor colonies from Acropora cytherea and Acropora florida from two sites in Pulau Lang Tengah, Malaysia to their ‘nursery propagules’ reared in a common garden coral nursery over 365 days. CBASS assays of reef-based donors and their nursery counterparts were conducted in parallel and over two seasons to assess retention of thermal tolerance following nursery rearing. After 6 months, average ED50s of A. cytherea nursery corals were significantly lower compared to their reef-based donor colonies, but such difference disappeared after 365 days. By comparison, no such differences were measurable for A. florida and thermal tolerances were retained. Further, we did not observe trade-offs between growth and thermal thresholds for either species. Based on our findings, in situ thermal tolerance differences are likely adaptive and, consequently, either retained or recovered in longer-term restoration settings. Our findings further imply that thermal screening should be conducted prior to nursery propagation to avoid selection based on long-term acclimation artifacts.
Similar content being viewed by others
Introduction
Hard corals are the foundational architects and engines of tropical coral reef ecosystems and associated marine biodiversity1. Given their high ecological and economic value2, reef managers, scientists, and stakeholders have been developing and implementing active measures, such as in situ coral restoration3, to counteract past, present, and anticipated future losses of reef-building hard corals4,5,6. In situ coral restoration predominantly aims at increasing hard coral cover through the propagation of selected coral species7,8, a practice that has been expanding in scope and popularity, and which is now widely implemented across many of the worldʼs tropical reef regions9. However, concurrently with advances in coral restoration practice, ocean warming is resulting in longer, more widespread, and more frequent marine heatwaves10,11, resulting in severe mass coral bleaching and mortality events in all ocean basins12,13. Therefore, long-term coral restoration success is thought to increasingly depend on the preferential selection (screening) of heat tolerant corals14,15,16.
Thermal tolerance of hard corals greatly varies across and within species17,18,19,20, geographic reef regions21,22, reef environments23,24, and within single reefs 25,26,27. Differences in how individual coral colonies respond to heat stress have been associated with their algal symbiont assemblage28,29, acclimatory responses following prior exposure to heat stress30, genetic predisposition31,32, as well as bacterial associations of the coral host33,34. Despite the complex underpinnings of coral thermal tolerance, recent global advances have been achieved to develop standardized diagnostic tests to identify coral phenotypes with superior heat stress tolerance25,35,36,37. However, given that susceptibility to heat stress varies across time and space38,39, it remains largely unresolved whether heat tolerant donor colonies give rise to heat tolerant nursery corals or whether an acclimatization period to novel coral nursery and reef environments is required. Knowledge regarding this is critical to understand whether coral thermal tolerance is retained in various coral restoration settings, such as coral nurseries40,41 and following propagation to replenish denuded reef sites42.
Propagating naturally occurring heat resilient corals to build nurseries and inform restoration is a promising strategy43. Preferential selection based on experimentally derived standardized thermal thresholds has been shown to produce multi-species coral nurseries with enhanced ability to survive coral bleaching events43. In contrast, without pre-screening, the accidental selection of less resilient corals may impair the heat tolerance of nursery corals41. However, the current lack of repeated and parallel thermal stress testing over time of both, reef-based donors and nursery corals is limiting our ability to resolve retention or increase vs. loss and/or subsequent recovery of thermal tolerance in restoration practices41. Such testing would also provide clues as to whether the observed thermal tolerance variation is due to adaptive or acclimatory differences.
Although preferential selection of heat tolerant corals for restoration is considered crucial under warming oceans14,15, this may come with associated risks. For example, compromised growth and reduced survivorship of outplanted thermally tolerant corals have been noted44,45. Such trade-offs may stem from associations with thermally tolerant algal symbiont types46. However, contrasting evidence exists as well. Co-benefits (i.e., positive associations) between physiological performance traits such as fecundity, growth, and thermal tolerance were found in corals from the Indo-Pacific47, while cross-tolerance to multiple stressors has been identified for the common Indo-Pacific coral Acropora millepora48. Thus, trade-offs associated with thermal tolerance may not be universally present across species or universally manifest across environments24.
To support the optimization of coral restoration outcomes through thermal phenotype pre-screening, we conducted parallel and repeated heat stress testing of nursery and corresponding reef-based donor corals of two hard coral species from two environmentally disparate sites using the Coral Bleaching Automated Stress System (CBASS)25,35,37. Parallel testing over time was performed to resolve potential temporal patterns of coral thermal tolerance loss, retention, recovery, or increase. Moreover, putative trade-offs between coral thermal tolerance and coral growth44,49 during the initial growth-focused nursery phase were investigated.
Methods
Study location and coral sampling
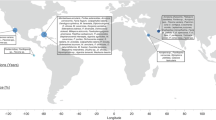
We conducted a year-long investigation to assess coral thermal tolerance dynamics at an ongoing restoration site around Pulau Lang Tengah (5°47′43.2"N, 102°53′39.7"E), in northeastern Peninsular Malaysia (Fig. 1). In March 2022, reef-based donor colonies of two fast-growing species used within local restoration efforts, Acropora florida and Acropora cytherea (Henry et al.50), were tagged, mapped, and photographed. A total of 40 colonies of A. florida and 42 coral colonies of A. cytherea from two distinctive reef environments (n = 20 and n = 21 colonies per site, respectively) were tagged, two replicate fragments from each colony were removed with gardening shears, placed in numbered zip-lock bags, and immediately transferred to a common garden in situ nursery site in a bucket filled with local seawater. Selected donor colonies were visually healthy, showed no signs of disease, bleaching, or partial mortality, and were sampled along a broad depth range between 4.4 and 15.2 m (±1 m tidal range) in a proportionate fashion (i.e., an equal number of colonies per depth zone). A minimum distance of 5 m between adjacent colonies was maintained to maximize phenotypic diversity and to avoid sampling of clonal genotypes51.
a Location of Pulau Lang Tengah (5°47′ 43.2"N, 102°53′39.7"E) along the northeastern coast of Peninsular Malaysia. The location pin in the zoomed-out figure shows the location of the nearest major city (Kuala Lumpur). Site 1 and Site 2 are reef locations from which coral source material was collected, with Site 1 being the location of the common garden nursery. Examples of donor and nursery coral species: (b, c) Acropora florida and (d, e) Acropora cytherea.
To assess the putative impact of the site of origin on coral thermal tolerance, coral source material was collected from two sites: one where the common garden nursery was established (Site 1) and a second site exhibiting an environmentally disparate reef setting (Site 2) (Fig. 1). Key habitat differences between these reef sites are differential exposure to wind forcing (sensu Szereday et al., 202427, Site 2 more exposed), difference in diel temperature profiles (Szereday et al., 202427, Site 1 exhibits greater diel temperature variability), and greater micro-habitat heterogeneity at Site 2 due to higher hard coral cover, density, and diversity compared to Site 152.
Common garden nursery
Four coral rebar frames were used as a coral nursery substrate53 (Fig. 1). The collected fragments from each donor colony were randomly dispersed across the rebar frames and secured with cable ties. To avoid overgrowth and shading within the nursery by adjacent corals, tabular A. cytherea were exclusively placed on the bottom half of the rebar frames. Site 1 was selected as the common garden site (Fig. 1) to allow comparison with previous studies that measured growth and survival of corals reared in adjacent rebar and coral tree nurseries50,54. The average depth of rebar frames was 9.0 m (i.e., middle point of the frame, top = 8.8 m, bottom 9.2 m) and was kept at the same depth level as the average depth of reef-based donor colonies (i.e., 9.2 m). Each coral fragment was tagged with a rubber tag to trace nursery fragment identity back to the respective reef-based donor colony. Coral nurseries were occasionally cleaned to remove algae and other biofouling organisms during the first 176 days of the study and once again after the northeast monsoon on day 360.
Coral growth monitoring
Following nursery stocking, coral fragment length, width, and height were measured along the rebar axis (Electronic Supplementary Material, Supplementary Fig. 1) to calculate initial fragment size, expressed as the geometric mean radius (GMR; cm, sensu Loya, 197655). The mean initial fragment size for A. florida was 3.39 ± 0.13 cm (SEM) and 2.77 ± 0.09 cm (SEM) for A. cytherea. Fragment size was re-measured on day 176 (i.e., before the CBASS assays), and the geometric mean radius was used to calculate the specific growth rate (SGR). Specific growth rate is calculated as50:
where t is the total time of growth (i.e., 176 days), with G1 and G2 being the GMR of the final (day 176) and initial size (day 0) in centimeters, respectively.
Standardized acute heat stress assays using the CBASS platform
To test whether coral thermal tolerance is retained, lost, or increased in coral nurseries, we used the previously established Coral Bleaching Automated Stress Systems (CBASS)25,35,37 for parallel heat stress testing of nursery corals and their corresponding reef-based donor colonies. Briefly, the CBASS platform consists of four replicate 10 L flow-through tanks with independent temperature profiles customized to prevailing local reef conditions. Here, we selected temperature profiles based on available NOAA climatology (Coral Reef Watch, version 3.1., https://coralreefwatch.noaa.gov/) binned to the nearest 5 km satellite pixel56. The control baseline temperature profile was set to 30.5 °C, based on the interpolation of NOAA’s historical maximum monthly mean (MMM) temperature of 29.94 °C with local in situ temperature data27. The three heat-hold treatment temperatures were then set to MMM + 4 °C (34.5 °C), MMM + 6 °C (36.5 °C), and MMM + 9 °C (39.5 °C) to induce mild, strong, and severe heat stress, respectively. Heat-hold temperatures were chosen to cover the spectrum of physiological responses to heat stress (from mild to extreme) for subsequent log-logistic modelling of photosynthetic efficiency loss over increasing temperatures, whereby the highest temperature is intended to induce severe bleaching and mortality36. Of note, we did not use replicated fragments (tanks) for each temperature treatment, since a prior study highlighted the absence of significant tank effects25 and to minimize biomass harvesting. Further, we assayed a high number of coral colonies (n = 24 for A. florida and n = 27 for A. cytherea) to capture biological variation, which also encompasses aspects of technical variance inherent to the experimental workflow.
After coral growth and survival monitoring on day 176 in October 2022, a subset of the total number of colonies was selected to achieve the maximum sample size of n = 14 per CBASS assay. Hereby, 24 nursery corals of A. florida (n = 12 from both sites) and 27 nursery corals of A. cytherea (n = 14 from Site 1, n = 13 from Site 2) were selected for acute heat stress testing together with their reef-based donor counterparts, representing 24 unique A. florida and 27 A. cytherea reef-based donor colonies. A total of 192 A. florida coral nubbins (i.e., 24 reef-based donors x 4 fragments per colony + 24 nursery corals x 4 fragments per colony) and 216 A. cytherea nubbins (27 reef-based donors x 4 fragments per colony + 27 nursery corals x 4 fragments per colony) were collected. The selection of the colony subsets was conducted haphazardly and included fragments of varying color intensity (i.e., from fully pigmented to slightly pale) of variable growth rates (i.e., 0.12–0.34% growth/day for A. florida, and 0.07–0.49% growth/day for A. cytherea). Of note, selected nursery corals for CBASS assays were on average larger than non-selected corals to ensure sufficient coral biomass for subsequent CBASS assays in March 2023. In further steps, the corresponding reef-based donor colonies were relocated, sampled, and acute heat stress tested to assess standardized thermal tolerances (i.e., ED50s) for both the nursery fragments and donor colonies in parallel (total n = 48 for A. florida and n = 54 for A. cytherea). Four replicate fragments of approximately 6.0–9.0 cm2 size57 were collected from each nursery coral and donor colony and randomly distributed across the four temperature treatment tanks, while their position within each tank was further randomized using a random number generator to determine each fragments position. Following the placement of all fragments so that each nursery and in situ coral was in each of the four temperature profiles, a standardized local diel temperature cycle, as per Voolstra et al. (2020)34, was applied to each experimental tank (Supplementary Fig. 2). The 7 h thermal cycles started at 1 pm and consisted of a 3 h heating phase to the desired heat stress temperature, a 3 h heat-hold phase of the maximum treatment temperature, and a 1 h cooling phase back to the baseline temperature. Temperatures in each treatment tank were controlled with low-cost temperature controllers (InkBird ITC-310T-B) that regulated thermo-electric chillers (IceProbe, NovaTec) and 200 W titanium heaters (Schego). Light was supplied with dimmable 165 W full spectrum LED aquarium lights, set at a 50–50 ratio of blue and white light, respectively, to match in situ reef conditions of approximately 35,000 lux intensity. Before each experiment, HOBO MX2202 Pendant MX temperature and light data loggers (Onset Computer Corporation, USA) were inter-calibrated and then used to continuously monitor temperature and light every minute in each tank throughout the experiment. Local seawater was collected on the morning of each experiment and adjusted to baseline temperature (i.e., 30.5 °C) in a 200 L insulated reservoir tank before the start of the CBASS assays. Once the water temperature was at baseline temperature, all treatment tanks were completely filled, and seawater was continuously supplied to treatment tanks with a submersible aquarium pump at a rate of 25-30 mL/min to achieve 100% turnover of treatment tank volume every six hours. During the 1-hour cooling phase (starting daily at dusk at 6 pm), the white LEDs were first switched off, then the blue LEDs (30 min later), so that all fragments were dark-acclimated. Dark-acclimated photosynthetic efficiency of PSII (Fv/Fm) of the coral algae was measured by pulse amplitude modulated (PAM) fluorometry (Diving-PAM underwater fluorometer; Heinz Walz GmbH, Effeltrich, Germany) to derive a physiological measure reflective of heat stress tolerance of corals58.
In March 2023, 365 days after the first nursery stocking, standardized heat stress assays were again conducted to assess the consistency of coral thermal tolerance. For this, previously tested nursery corals and reef-based donor colonies were resampled for acute heat stress testing, and new fragments from each previously tested nursery and reef-based donor were collected for subsequent CBASS assays. Minor sample size differences (A. florida n = 46 in March 2023 vs. n = 48 in October 2022; A. cytherea n = 47 in March 2023 vs. n = 54 in October 2022) due to detachment from the nursery, loss of coral tags, and some mortality of reef-based donors during the northeast monsoon are to be noted (Supplementary Table 1). The seasonal timing of acute heat stress testing was deliberate to account for the potential influences of seasonal temperature patterns. Locally, the warmest six-month period of the year is between April and September, and the coolest period occurs between November and February during the annual northeast monsoon, which increases rainfall, cloud cover, wind, and waves, resulting in lower temperatures. In situ temperatures at 1 and 15 m depths were recorded at the common garden nursery site with an Aqualink Smart buoy (Sofar Ocean Technologies Inc., USA). Additionally, in situ temperature data from Site 2 recorded at 8 m depth was partially available for the study period (Supplementary Fig. 3).
Standardized thermal tolerance threshold assessment – effective dose 50 (ED50)
Coral thermal tolerance thresholds (i.e., Effective Dose 50 - ED50s) are defined as the mean temperature at which 50% of the value measured (here Fv/Fm) at baseline temperature is retained36. For quantitative comparison of species-specific standardized thermal tolerance thresholds between nursery corals and reef-based donors, photochemical yields (Fv/Fm) measurements (see above) were used to fit log-logistic dose-response curves with the DRC package in R (Ritz et al., 2015) using the three-parameter model (LL.3) with model limits for the three parameters ‘hill’, ‘max’, and ‘ed50’ (upper limits = 100, 0.8, NA; lower limits = 10, 0.3, 30) to obtain effective dose 50 (ED50) values36,59. For ED50 determination, the measured mean temperatures of the 3 h heat-hold phase were used in lieu of the programmed target temperature (e.g., 31 °C instead of 30.5 °C for baseline, 35 °C instead of 34.5 °C, 37 °C instead of 36 °C, and 40 °C instead of 39.5 °C; measured temperatures of individual heat stress assays are provided in Supplementary Table 2–3). To calculate relative thermal tolerance thresholds, we subtracted the MMM from the determined ED50s to obtain relative thermal thresholds (ED50 – local MMM). This was done separately for each site and condition, i.e., Site 1 - Donor, Site 2 - Donor, Site 1 - Nursery, and Site 2 - Nursery.
Reproducibility of colony-specific ED50 values and rank/cohort assignments
To support the selection of individual coral colonies for restoration, the reproducibility of thermal tolerance thresholds (ED50s) by means of assignment into bottom and top performing colonies was investigated. The reproducibility of ED50 values on the colony level (i.e., for each genet60) was tested by comparing October 2022 ED50s to March 2023 ED50 values for each colony. Additionally, we assessed consistency of cohort assignment (top- vs. bottom-performing colonies) as a function of screening effort (i.e., number of colonies). Colonies were ranked by their ED50s and the mean overlap of the top 5 and bottom 5 colonies comparing donor and nursery corals in October 2022 and March 2023 were determined using random subsampling to assess accuracy as a function of screening effort (number of colonies assayed).
Statistics and Reproducibility
A linear mixed effect model (LME) was built to measure the contribution of individual response variables with ‘temperature’ (i.e., in situ measurements 31 °C, 35 °C, 37 °C, 40 °C) and ‘condition’ (i.e., nursery and donor) set as fixed factors as well as ‘site’ (i.e., Site 1, Site 2) and ‘time’ (i.e., October 2022 and March 2023) set as random factors. Since response to heat stress is inherently variable across species17, each species was tested independently using a separate model iteration. Model analysis was conducted in R Studio (www.r-project.org/) using the ‘lmerTest’ package61. Backward elimination was applied to simplify the model’s predictor variables (step function), referring to F-tests to compare complete and simplified models, following the removal of fixed effects. Shapiro-Wilk tests were used to test data for normality, and pairwise t-tests (two-tail) were used to compare relative thermal thresholds of individual corals across seasons (i.e., October 2022 and March 2023) within each site-condition group (four groups per species: Site 1-Donor, Site 2- Donor, Site 1-Nursery, and Site 2 -Nursery). Where the assumption of normality was violated, a signed non-parametric Wilcoxon test for paired samples was used instead of a pairwise t-test. Only complete pairs were considered for statistical comparison when sample size differences existed between CBASS assays across seasons. Site-specific differences in relative thermal tolerance thresholds for each species and site-condition group per season (as opposed to across seasons) were tested individually using a Tukey HSD for unequal sample size (two sites, two conditions, a total of four groups per season). Trade-offs between coral growth and relative thermal thresholds were investigated with Pearson’s rank correlation coefficient. Hereby, SGRs of nursery corals were measured on day 176 (see above), and the corresponding ED50 values were ranked per colony from highest to lowest, whereby a significantly negative correlation coefficient would substantiate trade-offs. Growth rate differences between coral species divided into site-condition groups were examined for significant differences with Tukey’s post hoc test for multiple comparisons. All data and custom codes detailed and required to reproduce this study are available online62 and are provided in the supplement (Supplement Data 1).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Species-specific retention vs. recovery of coral thermal tolerance
To assess whether nursery-maintained corals retain, lose, or increase their thermal tolerance in comparison to corresponding reef-based donors in their native habitat, we used the portable Coral Bleaching Automated Stress System (CBASS) and conducted parallel acute heat stress assays at the end of the annual warm season in October 2022 ( ~ 6 months, i.e., 176 days after nursery propagation) and at the end of the colder northeast monsoon season in March 2023 (1 year, i.e., 365 days after nursery propagation). We selected two Acropora species from two sites with varying diel temperature environments and disparate reef settings (Fig. 1) to collect coral source material for a common garden nursery at Site 1, established in March 2022. Thermal tolerance of reef-based donor colonies and nursery corals was investigated in parallel by recording dark-acclimated photosynthetic efficiency (Fv/Fm) across temperature treatment tanks after the heat-hold/ramp down-phase of the acute heat stress assay thermal cycling profiles for all donor colonies on day 176 (October 2022) and day 365 (March 2023), respectively.
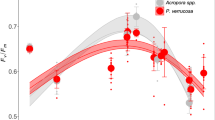
Results from the standardized acute heat stress assays showed species-specific recovery vs. retention of coral thermal tolerance following 365 days of nursery propagation (Fig. 2). Firstly, based on linear mixed effect models, an overall significant difference between nursery corals and the corresponding donor colonies existed for A. cytherea (n donor = 52, n nursery = 52, df = 400.02, t = −7.16, p < 0.0001, SE = 0.01) and A. florida (n donor = 46, n nursery = 48, df = 371.13, t = −2.65, p = 0.008, SE = 0.01) colonies, considering both reef sites and time points. To examine the overall significant differences in more detail, we conducted pairwise testing between reef-based donor colonies and nursery corals in consideration of the various spatial and temporal scales. This revealed the absence of significant differences between nursery corals and donor colonies for A. florida in October 2022 (i.e., day 176) and March 2023 (i.e., on day 365), as indicated by largely similar absolute (i.e., ED50) and relative thermal tolerance thresholds (i.e., ED50-MMM) (Figs. 2, 3, Supplementary Fig. 4), indicating retention of thermal tolerance in nursery settings over a 365-day long nursery period. In contrast, for A. cytherea relative thermal tolerance thresholds of nursery corals were significantly lower than donor colonies after 176 days in October 2022 (pairwise t-test p = 0.0006, relative ED50 nursery corals Site 1 = 5.02 ± 0.25 °C vs. relative ED50 donor colonies Site 1 = 7.70 ± 0.52 °C [mean ± SE]; pairwise t-test p = 0.02, ED50 nursery corals Site 2 = 5.66 ± 0.50 °C vs. donor colonies Site 2 = 7.27 ± 0.42 °C, Figs. 2–3). However, these intermediate thermal tolerance differences did not persist. Following the northeast monsoon season in March 2023 (i.e., after 365 days), thermal tolerance thresholds of nursery corals were similar to their reef-based donors (pairwise t-test p = 0.19, relative ED50 nursery corals Site 1 = 7.22 ± 0.46 °C vs. donor colonies Site 1 = 8.23 ± 0.59 °C; Wilcoxon pairwise test p = 0.10, relative ED50 nursery corals Site 2 = 7.09 ± 0.50 °C vs. donor colonies Site 2 = 7.55 ± 0.48 °C) (Fig. 3), suggesting intermediate loss of thermal tolerance followed by subsequent recovery to similar levels as the corresponding reef-based donor colonies.
a, b Three-parameter log-logistic regression of maximum photosynthetic efficiency of PSII (Fv/Fm) over temperature for Acropora florida as measured after the heat-hold/ramp down-phase of CBASS assays in (a) October 2023 ( ~ 6 months) and (b) March 2023 (1 year). c, d Three-parameter log-logistic regression of maximum photosynthetic efficiency of PSII (Fv/Fm) over temperature for Acropora cytherea as measured in (c) October 2023 ( ~ 6 months) and (d) March 2023 (1 year). The dotted lines represent 95% confidence intervals, and the standard error is indicated by the shaded area based on colony-based curve fits. Vertical lines represent the mean effective dose 50 values (ED50s in °C) of each site-condition group as a proxy for coral thermal tolerance, provided in the top right corner. Dots represent individual data points of the colony replicates.
Colony-specific and population-level thermal tolerance thresholds (mean ± S.E.M) relative to the maximum monthly mean temperature of the study site (relative ED50 = ED50 – MMM; MMM = 30.52 °C based on in situ interpolation of NOAA’s climatology data) are shown for each site-condition group (i.e., condition – nursery coral vs. reef-based donor, S1 – site 1, S2 – site 2) of a Acropora florida and b Acropora cytherea corals. Annotated letters highlight significant differences (at significance level p < 0.05) between groups following pairwise comparison (pairwise two-tail t-tests or signed non-parametric Wilcoxon test, see methods). Dots represent individual coral colony data points.
Through repeated and parallel CBASS testing, spatial and temporal patterns in coral thermal tolerance were investigated to determine the consistency of the thermal response. Although there was a difference in relative thermal thresholds between nursery and donor colonies at the end of the warm season after 176 days in October 2022 for A. cytherea, no significant differences between nursery corals across sites were found for both sampling time points, suggesting no effect due to site of origin (Tukey HSD p = 0.73, Site 1 October 2022, relative ED50 = 5.02 ± 0.25 °C vs. Site 2 October 2022, relative ED50 = 5.66 ± 0.50 °C and Tukey HSD p = 0.99, Site 1 March 2023, relative ED50 = 7.22 ± 0.46 °C vs. Site 2 March 2023, relative ED50 = 7.09 ± 0.50 °C) (Figs. 2, 3, Supplementary Fig. 4). Similarly, no significant differences in relative thermal thresholds of donor colonies across sites could be found (Tukey HSD p = 0.90, Site 1 October 2022, relative ED50 = 7.70 ± 0.52 °C vs. Site 2 October 2023, relative ED50 = 7.27 ± 0.42 °C and Tukey HSD p = 0.88, Site 1 March 2023, relative ED50 = 8.23 ± 0.59 °C vs. Site 2 March 2023, relative ED50 = 7.55 ± 0.48 °C (Figs. 2, 3, Supplementary Figs. 4). Finally, no significant differences were determined when comparing relative thermal thresholds of donor colonies across seasons (t-test p = 0.52, Site 1 October 2022, relative ED50 = 7.70 ± 0.52 °C vs. Site 1 March 2023, relative ED50 = 8.23 ± 0.59 °C; Wilcoxon pairwise test p = 0.58, Site 2 October 2022, relative ED50 = 7.27 ± 0.42 °C vs. Site 2 March 2023, relative ED50 = 7.55 ± 0.48 °C) (Figs. 2, 3). Taken together, the relative thermal thresholds of reef-based donor colonies of A. florida were consistent across time and space, while they were consistent across space for A. cytherea.
ED50 reproducibility and rank/cohort assignment consistency
Colony-level reproducibility of ED50 varied among species and conditions (i.e., Donor, Nursery). For A. florida, reproducibility was appreciably high as 57% of reef-based donor colonies (n = 21) and 62% of nursery corals (n = 21) re-tested at an ED50 difference < ±1 °C when considering ED50 across time points (i.e., October 2022 vs March 2023) (Supplementary Table 4). For A. cytherea, ED50 reproducibility was lower, as 50% of reef-based donor colonies (n = 24) and 37% of nursery corals (n = 19) re-tested at an ED50 difference < ±1 °C. This variation in ED50 reproducibility between species is further highlighted by the distribution of ED50 values for each site-condition group across time points (Supplementary Fig. 5). By comparison, cohort assignment into top vs. bottom performing colonies showed that ~1 colony was misassigned at a screening size of ≥15 colonies considering the top and bottom five colonies (Fig. 4).
a October 2022 and b March 2023. Colonies were ranked by their ED50s, subsampled to different sample sizes, and it was assessed whether the top and bottom five colonies were consistent between in situ and nursery corals. Mean overlap indicates the mean number of wrongly assigned colonies. SE = standard error.
Coral thermal tolerance and coral growth
To assess putative trade-offs between thermal tolerance and coral growth in a nursery setting following propagation (i.e., putative growth-focused nursery phase), we investigated correlations between ED50s and specific growth rates (SGR) of individual nursery fragments (Table 1, Supplementary Fig. 6). Species-specific coral growth rates after the 176-day nursery period were significantly higher for A. cytherea (Table 1). No differences in SGR were found within species across sites, denoting that relocation did not significantly affect growth rates for either species. A negative Pearson correlation of (r) > −0.30 was found for A. cytherea corals originating from Site 2. No significant correlations between coral thermal tolerance (i.e., ED50) and growth rates were found.
Discussion
The resilience of coral species to thermal stress is determined by long-term evolutionary processes63,64 and short-term acclimation65, which may both contribute equally to coral thermal tolerance31. However, and importantly, genotype predisposition to heat stress and coral bleaching remains unaltered when introduced to novel reef environments32. For instance, heat-resistant corals retained high metabolic functioning three months after their propagation to novel reef environments in Hawaii42. Similarly, in American Samoa and Australia, naturally occurring heat tolerant corals retained their thermal tolerance after relocation to a common garden nursery40,43. Taken together, these studies, in line with the results presented here, argue that coral thermal tolerance is adaptively fixed and ultimately retained in novel reef and nursery environments. However, an intermediate acclimation phase following relocation into the nursery may be observed, which can obscure thermal tolerance assessments. Therefore, it is critical to consider the thermal screening of reef-based coral colonies prior to coral nursery stocking, specifically since thermal tolerance is broadly distributed across species, sites, and conditions (Supplementary Fig. 5), suggesting wide thermal tolerance variability. Importantly, we found differences between different species within the same coral genus (i.e., Acropora), highlighting that retention vs. recovery of thermal tolerance in novel nursery environments is species-specific66, and caution should be applied when inferring on a genus basis. In our study, in the absence of repeated heat stress experiments over time, results would suggest a considerable loss in thermal tolerance of A. cytherea nursery corals in October 2022. Conversely, the initial period of thermal tolerance recovery between March 2022 and October 2022 would not have been noticed by conducting a single heat stress test on day 365 in March 2023, leading to potential implications for restoration practice (as discussed below). Ultimately, and irrespective of the above considerations, we are still missing a definitive understanding to what extent experimentally derived thermal tolerance thresholds map onto ecologically relevant bleaching outcomes67 regarding susceptibility and ability to recover68, critical for the implementation of resilience-based coral restoration69.
Drivers of coral thermal tolerance are complex and diverse. Whereas adaptive differences are fixed and maintained across changing environments31,42, short-term acclimatory responses may be due to gene expression changes70 or changes in coral holobiont composition, including Symbiodiniaceae29,71 and bacteria33. Such shifts can also occur following propagation to nursery environments72, possibly leading to temporary changes in coral thermal tolerance, as observed in this study. Although the specific reasons for the temporary difference in thermal tolerance between nursery corals and corresponding reef-based donors for A. cytherea remain undetermined, parallel heat stress testing across time shows that neither seasonality (i.e., site-wide differences in temperature, rainfall, and wind forcing between the warm season and the northeast monsoon season) nor trade-offs between growth and thermal tolerance are explanatory of the difference, as observed by previous studies47. Also, discernable differences due to site origin were not detected, but observed differences could be aligned to the species of origin. Thus, metabolomic signatures73 and protein markers74, among other variables, may be insightful to further investigate the differences and extent underlying coral thermal tolerance.
Our results suggest that thermal tolerance is adaptively fixed as both assayed species either retained or ultimately recovered their in situ (i.e., site of origin) thermal tolerance. However, species-specific differences in the form of temporary changes exist. Therefore, an intermediary adjustment period to nursery conditions may be required for some species, whereby coral restoration practitioners must consider the timing of thermal screening and nursery stocking. For example, during severe heat stress in 201927, three coral species (i.e., Acropora muricata, Acropora gemmifera, Montipora aequituberculata) in suspended rebar nurseries at this common garden site showed a higher average bleaching response than their corresponding reef-based donors, 90 days after coral nursery propagation54. However, no differences were found during a heat stress period in the subsequent year, suggesting and confirming recovery of initial (i.e., in situ) thermal tolerance following long-term rearing in the nursery40. Furthermore, while thermally more resilient phenotypes retained their tolerance in a common garden nursery in American Samoa, coral bleaching was induced at milder temperatures in the multi-species nursery compared to temperatures experienced by corresponding donor colonies in their native habitats43, possibly due to the short time frame elapsed since nursery stocking (i.e., approximately six months).
Taken together, corals can maintain and recover their stress response physiology75,76 but maximizing time in the nursery before potential periods of heat stress occur will be crucial to ensure that identified heat tolerant coral genotypes recover their original thermal tolerance (as measured for reef-based donor colonies). In addition, while the capacity of acute thermal screening (CBASS’ing) to reliably identify resilient phenotypes must be verified in situ, e.g., by following bleaching trajectories of diverse coral colonies in the field37,67,77, this study adds to previous research signifying the importance of preferential selection of thermally resilient coral phenotypes for restoration40,41,42,43,78. Pre-screening of coral phenotypes (i.e., acute heat stress testing to identify corals with superior thermal tolerance) should ideally occur before nursery stocking by testing a high number of reef-based donor colonies to safeguard coral nursery survival and restoration investment. Such efforts should further be accompanied by parallel genotyping of selected coral individuals to prevent genetic depression of nursery populations79 and to ensure cross-tolerance to other pervasive stressors44,69. Importantly, the reproducibility of colony-level ED50 is critical for restoration application to confidently select heat-tolerant corals. Although the ED50 reproducibility here was lower compared to Cunning et al., 2024 (i.e., 37–62% vs. 90% of corals re-tested at ED50 difference of < ±1 °C)60, the observed rates are still appreciably high, specifically when considering the thermal tolerance loss and recovery of A. cytherea nursery corals and known seasonal adjustments of ED50s of > 1 °C for Acropora species in the Red Sea80. For real-world application, consistency of cohort assignment (higher vs. lower thermal tolerance) may ultimately become more important than high reproducibility of ED50s. Our results show that top and bottom cohort assignment is highly consistent between reef-based donor and nursery corals for both time points, with the October 2022 time point showing higher consistency than the March 2023 time point. This may be attributable to differences in thermal tolerance threshold recovery, seasonal acclimation, etc. At large, our results suggest that with a reasonable screening effort of 20 colonies and subsequent selection of the top performing colonies, high accuracy can be achieved, emphasizing the suitability of CBASS assays to differentiate between heat tolerant and susceptible colonies across reef sites and species. However, at this conjecture, it is vitally important to resolve how ED50 differences map onto ecological in situ bleaching outcomes. This requires investigations of coral thermal tolerance and resilience to prevailing stressors, which must be conducted at a species and population level across large sample sizes and habitats. Importantly, discernable patterns of coral thermal retention and recovery are shown for two widely distributed Acropora species sourced from the same sites, reared in a common garden nursery, and subjected to equal heat loads during CBASS experiments. Thus, extending insights from one species to other species (even) of the same genus may undermine future research and restoration efforts.
Conclusion
Our study provides evidence that coral thermal tolerance is adaptive and retained in nursery settings, but discernible species-specific differences exist, whereby some species may require an adjustment period in common garden nurseries before original thermal tolerance is recovered. Due to these differences in two congeneric Acropora species, it is critical to conduct experiments at the species level without inferring results from one species to another, even if they are of the same genus. Results from this study imply that the identification of heat tolerant corals through heat stress screening of coral source material should ideally happen prior to the nursery phase by assaying reef-based donor colonies across diverse reef habitats. Similarly, coral nurseries should be stocked in foresight of heat stress periods to allow for sufficient time for corals to recover their original thermal tolerance. Future studies should determine changes in coral physiology and holobiont assemblage across coral restoration stages to identify drivers underlying temporary changes in thermal tolerance. Critically, in situ validation of experimentally derived thermal thresholds is required across reef scales at the species level to determine the capacity of acute thermal assays to reliably identify corals with increased thermal resilience under ecologically (i.e., real-world) relevant conditions for further application in coral restoration practice.
Data availability
All data required to perform the analyses and generate the results and figures in this manuscript are available in the Supplement Data 1 and are deposited open-access in the Zenodo repository: https://doi.org/10.5281/ZENODO.15880518.
Code availability
Statistical software codes and detailed data required to reproduce this study are available in the supplement as well as in the Zenodo repository: https://doi.org/10.5281/ZENODO.15880518.
References
Roberts, C. M. et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295, 1280–1284 (2002).
Spalding, M. et al. Mapping the global value and distribution of coral reef tourism. Mar. Policy 82, 104–113 (2017).
Suggett, D. J. et al. Restoration as a meaningful aid to ecological recovery of coral reefs. Npj Ocean Sustain. 3, 20–20 (2024).
Raquel, S. et al. The critical role of coral reef restoration in a changing world. Nat. Clim. Chang. 14, 1219–1222 (2024).
Carpenter, K. E. et al. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563 (2008).
Hughes, T. P. et al. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377 (2017).
Suggett, D. J. & van Oppen, M. J. H. Horizon scan of rapidly advancing coral restoration approaches for 21st century reef management. Emerg. Top. Life Sci. 6, 125–136 (2022).
Howlett, L. et al. Active coral propagation outcomes on coral communities at high-value Great Barrier Reef tourism sites. Biol. Conserv. 279, 109930 (2023).
Boström-Einarsson, L. et al. Coral restoration – A systematic review of current methods, successes, failures and future directions. PLoS ONE 15, e0226631 (2020).
Skirving, W. J. et al. The relentless march of mass coral bleaching: a global perspective of changing heat stress. Coral Reefs 38, 547–557 (2019).
Eakin, C. M., Sweatman, H. P. A. & Brainard, R. E. The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs 38, 539–545 (2019).
Hughes, T. P. et al. Global warming transforms coral reef assemblages. Nature 556, 492–496 (2018).
Reimer, J. D. et al. The Fourth Global Coral Bleaching Event: Where do we go from here?. Coral Reefs 43, 1121–1125 (2024).
Caruso, C., Hughes, K. & Drury, C. Selecting Heat-Tolerant Corals for Proactive Reef Restoration. Front. Mar. Sci. 8, (2021).
Voolstra, C. R. et al. Extending the natural adaptive capacity of coral holobionts. Nat. Rev. Earth Environ. 2, 747–762 (2021).
Hughes, T. P., Baird, A. H., Morrison, T. H. & Torda, G. Principles for coral reef restoration in the anthropocene. One Earth 6, 656–665 (2023).
Loya, Y. et al. Coral bleaching: The winners and the losers. Ecol. Lett. 4, 122–131 (2001).
Van Woesik, R., Sakai, K., Ganase, A. & Loya, Y. Revisiting the winners and the losers a decade after coral bleaching. Mar. Ecol. Prog. Ser. 434, 67–76 (2011).
Evensen, N. R. et al. Empirically derived thermal thresholds of four coral species along the Red Sea using a portable and standardized experimental approach. Coral Reefs 41, 239–252 (2022).
Naugle, M. S. et al. Heat tolerance varies considerably within a reef-building coral species on the Great Barrier Reef. Commun. Earth Environ. 5, 525–525 (2024).
Sully, S., Burkepile, D. E., Donovan, M. K., Hodgson, G. & Woesik, R. Van. A global analysis of coral bleaching over the past two decades. Nat. Commun. 10, (2019).
McClanahan, T. R. et al. Large geographic variability in the resistance of corals to thermal stress. Glob. Ecol. Biogeogr. 29, 2229–2247 (2020).
Oliver, T. A. & Palumbi, S. R. Do fluctuating temperature environments elevate coral thermal tolerance?. Coral Reefs 30, 429–440 (2011).
Rivera, H. E. et al. Palau’s warmest reefs harbor thermally tolerant corals that thrive across different habitats. Commun. Biol. 5, 1394–1394 (2022).
Voolstra, C. R. et al. Standardized short-term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Glob. Change Biol. 26, 4328–4343 (2020).
Pineda, J. et al. Two spatial scales in a bleaching event: Corals from the mildest and the most extreme thermal environments escape mortality. Limnol. Oceanogr. 58, 1531–1545 (2013).
Szereday, S., Voolstra, C. R. & Amri, A. Y. Back-to-back bleaching events in Peninsular Malaysia (2019–2020) selectively affect hard coral taxa across- and within-reef scales. Mar. Biol. 171, 183–183 (2024).
Baker, A. C., Starger, C. J., McClanahan, T. R. & Glynn, P. W. Baker et al. 2004. Nat. Commun. 430, 741–741 (2004).
Hume, B. C. C., Mejia-Restrepo, A., Voolstra, C. R. & Berumen, M. L. Fine-scale delineation of Symbiodiniaceae genotypes on a previously bleached central Red Sea reef system demonstrates a prevalence of coral host-specific associations. Coral Reefs 39, 583–601 (2020).
Thompson, D. M. & van Woesik, R. Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc. R. Soc. B Biol. Sci. 276, 2893–2901 (2009).
Palumbi, S. R., Barshis, D. J., Traylor-Knowles, N. & Bay, R. A. Mechanisms of reef coral resistance to future climate change. Science 344, 895–898 (2014).
Drury, C. & Lirman, D. Genotype by environment interactions in coral bleaching. Proc. R. Soc. B Biol. Sci. 288, 20210177 (2021).
Ziegler, M., Seneca, F. O., Yum, L. K., Palumbi, S. R. & Voolstra, C. R. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat. Commun. 8, 14213–14213 (2017).
Gardner, S. G. et al. Coral microbiome diversity reflects mass coral bleaching susceptibility during the 2016 El Niño heat wave. Ecol. Evol. 9, 938–956 (2019).
Voolstra, C. R. et al. Contrasting heat stress response patterns of coral holobionts across the Red Sea suggest distinct mechanisms of thermal tolerance. Mol. Ecol. 30, 4466–4480 (2021).
Voolstra, C. R. et al. Standardized methods to assess the impacts of thermal stress on coral reef marine life. Annu. Rev. Mar. Sci. 17, 193–226 (2025).
Evensen, N. R. et al. The Coral Bleaching Automated Stress System (CBASS): A low-cost, portable system for standardized empirical assessments of coral thermal limits. Limnol. Oceanogr. Methods 21, 421–434 (2023).
McClanahan, T. R., Baird, A. H., Marshall, P. A. & Toscano, M. A. Comparing bleaching and mortality responses of hard corals between southern Kenya and the Great Barrier Reef, Australia. Mar. Pollut. Bull. 48, 327–335 (2004).
Hughes, T. P. et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83 (2018).
Roper, C. D. et al. Coral Thermotolerance Retained Following Year-Long Exposure to a Novel Environment. Sci. Adv. 11, eadu3858 (2025).
Alderdice, R. et al. Loss of coral thermotolerance following year-long in situ nursery propagation with a consecutively high summer heat-load. Coral Reefs 43, 919–933 (2024).
Barott, K. L. et al. Coral bleaching response is unaltered following acclimatization to reefs with distinct environmental conditions. Proc. Natl Acad. Sci. USA 118, e2025435118 (2021).
Morikawa, M. K. & Palumbi, S. R. Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proc. Natl Acad. Sci. USA 116, 10586–10591 (2019).
Ladd, M. C., Shantz, A. A., Bartels, E. & Burkepile, D. E. Thermal stress reveals a genotype-specific tradeoff between growth and tissue loss in restored Acropora cervicornis. Mar. Ecol. Prog. Ser. 572, 129–139 (2017).
Ho’opai-Sylva, H. et al. Proactive Coral Reef Restoration Using Thermally Tolerant Corals in Hawai’i. bioRxiv 2025.03.28.646055-2025.03.28.646055, https://doi.org/10.1101/2025.03.28.646055 (2025).
Jones, A. & Berkelmans, R. Potential Costs of Acclimatization to a Warmer Climate: Growth of a Reef Coral with Heat Tolerant vs. Sensitive Symbiont Types. PLOS ONE 5, e10437 (2010).
Lachs, L. et al. No apparent trade-offs associated with heat tolerance in a reef-building coral. Commun. Biol. 6, 400–400 (2023).
Wright, R. M. et al. Positive genetic associations among fitness traits support evolvability of a reef-building coral under multiple stressors. Glob. Change Biol. 25, 3294–3304 (2019).
Roik, A. et al. Trade-offs in a reef-building coral after six years of thermal acclimation. Sci. Total Environ. 949, 174589 (2024).
Henry, J. A. et al. Using relative return-on-effort scoring to evaluate a novel coral nursery in Malaysia. Restor. Ecol. 31, e13767 (2023).
BAUMS, I. B. A restoration genetics guide for coral reef conservation. Mol. Ecol. 17, 2796–2811 (2008).
Bernard, G. G. R., Kellam, A. L. & Szereday, S. Differential size frequency distribution of hard coral colonies across physical reef health gradients in Northeast Peninsula Malaysia. Reg. Stud. Mar. Sci. 61, 102872 (2023).
Lange, I. D. et al. Coral restoration can drive rapid reef carbonate budget recovery. Curr. Biol. 34, 1341–1348.e3 (2024).
Henry, J. A., Szereday, S., Bernard, G. G. R. & Patterson, J. T. Improved coral nursery production through contingent heat stress events via depth manipulation. Aquaculture 605, 742558–742558 (2025).
LOYA, Y. The Red Sea coral Stylophora pistillata is an r strategist. Nature 259, 478–480 (1976).
Liu, G. et al. NOAA Coral Reef Watch’s 5km Satellite Coral Bleaching Heat Stress Monitoring Product Suite Version 3 and Four-Month Outlook Version 4. Reef Encount. N. J. Int. Soc. Reef. Stud. 32, 39–45 (2017).
Nielsen, J. J. V. et al. Experimental considerations of acute heat stress assays to quantify coral thermal tolerance. Sci. Rep. 12, 16831–16831 (2022).
Warner, M. E., Fitt, W. K. & Schmidt, G. W. The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: a novel approach. Plant Cell Environ. 19, 291–299 (1996).
Nicolas, R. et al. Remarkably high and consistent tolerance of a Red Sea coral to acute and chronic thermal stress exposures. Abstract Limnology and Oceanography 66 1718–1729 (2021).
Cunning, R. et al. On the use of rapid acute heat tolerance assays to resolve ecologically relevant differences among corals. Coral Reefs 43, 1793–1801 (2024).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 82, (2017).
Szereday, S., Chew, L. & Christian R. V. Coralku/commongarden: Data & Codes for Szereday et al. - Species-specific retention vs. recovery of coral thermal tolerance following nursery propagation. Zenodo https://doi.org/10.5281/ZENODO.15880518 (2025).
Logan, C. A., Dunne, J. P., Ryan, J. S., Baskett, M. L. & Donner, S. D. Quantifying global potential for coral evolutionary response to climate change. Nat. Clim. Change 11, 537–542 (2021).
Lachs, L. et al. Emergent increase in coral thermal tolerance reduces mass bleaching under climate change. Nat. Commun. 14, 4939 (2023).
Hughes, T. P. et al. Ecological memory modifies the cumulative impact of recurrent climate extremes. Nat. Clim. Change 9, 40–43 (2019).
Hsiao, W. V., Denis, V., Palmas, S. D. & Beger, M. Genus-scale taxonomic resolution is appropriate to study coral demographics: A case study with Pocillopora. Preprint at https://doi.org/10.22541/au.173770584.42663977/v1 (2025).
Voolstra, C. R. et al. Spatially restricted coral bleaching as an ecological manifestation of within-colony heterogeneity. Commun. Biol. 8, 740 (2025).
Caruso, C. et al. Short-term stress testing predicts subsequent natural bleaching variation. Coral Reefs 44, 399–409 (2025).
Shaver, E. C. et al. A roadmap to integrating resilience into the practice of coral reef restoration. Glob. Change Biol. 28, 4751–4764 (2022).
Savary, R. et al. Fast and pervasive transcriptomic resilience and acclimation of extremely heat-tolerant coral holobionts from the northern Red Sea. Proc. Natl. Acad. Sci. USA 118, e2023298118 (2021).
Silverstein, R. N., Cunning, R. & Baker, A. C. Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob. Change Biol. 21, 236–249 (2015).
Strudwick, P. et al. Impacts of nursery-based propagation and out-planting on coral-associated bacterial communities. Coral Reefs 41, 95–112 (2022).
Roach, T. N. F. et al. Metabolomic signatures of coral bleaching history. Nat. Ecol. Evol. 5, 495–503 (2021).
Nunn, B. L. et al. Protein signatures predict coral resilience and survival to thermal bleaching events. Commun. Earth Environ. 6, 191–191 (2025).
Wall, C. B. et al. Shifting baselines: Physiological legacies contribute to the response of reef corals to frequent heatwaves. Funct. Ecol. 35, 1366–1378 (2021).
Drury, C., Dilworth, J., Majerová, E., Caruso, C. & Greer, J. B. Expression plasticity regulates intraspecific variation in the acclimatization potential of a reef-building coral. Nat. Commun. 13, 4790 (2022).
Brown, K. T. et al. Divergent bleaching and recovery trajectories in reef-building corals following a decade of successive marine heatwaves. Proc. Natl Acad. Sci. USA 120, e2312104120 (2023).
Thomas, L. et al. Spatially varying selection between habitats drives physiological shifts and local adaptation in a broadcast spawning coral on a remote atoll in Western Australia. Sci. Adv. 8, eabl9185 (2023).
Baums, I. B. et al. Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecol. Appl. 29, e01978–e01978 (2019).
García, F. C. et al. Seasonal changes in coral thermal threshold suggest species-specific strategies for coping with temperature variations. Commun. Biol. 7, 1680–1680 (2024).
Acknowledgements
The research was conducted under permit number Prk. ML. 630-7Jld.12 (90), issued by the Department of Fisheries (DoF) Malaysia (Jabatan Perikanan Malaysia), and permit number EPU 40/200/19/3711 (13) issued by the Economic Planning Unit (Unit Perancang Ekonomi). We would like to thank research assistants Natasha Zulaikha, Mok Weng Dee, and Febrianne Sukiato for assisting with data collection and acute heat stress assays, and Affendi Yang Amri for providing us with the underwater pulse amplitude modulated (PAM) fluorometer to conduct the study. Special gratitude is extended to Lush Malaysia for funding the experimental systems by awarding a Lush Charity Pot grant to Coralku Solutions, and to Summer Bay Resort and the dive center team for supporting our fieldwork actively and in-kind.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Study design—S.S., C.R.V.; coral sampling and nursery stocking—S.S.; data collection—S.S., C.K.L.; formal analysis—S.S., C.R.V.; Original draft- S.S.; writing and revisions—S.S., C.R.V., C.K.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interest.
Peer review
Peer review information
Communications Biology thanks Saskia Jurriaans and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Michele Repetto. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szereday, S., Chew, K.L. & Voolstra, C.R. Species-specific retention vs. recovery of coral thermal tolerance following nursery propagation. Commun Biol 8, 1294 (2025). https://doi.org/10.1038/s42003-025-08657-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08657-w