Abstract
Chromogranin A (CgA), a neuroendocrine pro-hormone, is proteolytically cleaved into bioactive peptides, most notably catestatin (CST) and pancreastatin (PST), which exert opposing effects on metabolic and inflammatory processes. Using CgA- and CST knockout (KO) mice, we investigated the roles of these peptides in pancreatic endocrine function, morphology, neurotransmitter dynamics, and systemic glucose homeostasis. CST-KO mice displayed impaired insulin secretion in vivo (but not ex vivo), disrupted islet architecture, and elevated catecholamine levels. In contrast, CgA-KO mice, which lack both CST and PST, exhibited reduced beta-cell mass but improved insulin sensitivity due to absence of PST. Peptide supplementation experiments in CgA-KO mice revealed that CST suppressed gluconeogenesis and enhanced glucagon regulation, whereas PST promoted insulin resistance and glucose production. Spatial mass spectrometry further demonstrated altered neurotransmitter and polyamine profiles in KO islets, implicating disrupted nerve-immune-islet crosstalk as a contributor to the observed metabolic phenotypes. Collectively, these findings identify CgA-derived peptides as critical regulators of islet function and highlight CST as a promising therapeutic candidate for diabetes and metabolic-inflammatory disorders.
Similar content being viewed by others
Introduction
Chromogranin A (CgA), a 49 kDa pro-hormone, has been extensively studied in the initiation and regulation of dense-core secretory granule biogenesis as well as in the context of various metabolic and inflammatory diseases. Increased circulating CgA levels are linked to various diseases, including neuroendocrine tumors1, hypertension2,3, congestive heart failure4, renal failure5, inflammatory bowel disease6, rheumatoid arthritis7, sepsis8, and Alzheimer’s disease9,10. CgA is secreted by endocrine cells, such as chromaffin cells in the adrenal medulla, enterochromaffin cells in the gut, pancreatic islet cells, neurons, and immune cells, such as neutrophils, monocytes, and macrophages11. In the pancreatic islet, CgA is present in the alpha and beta cells12 and is important for maintaining islet volume, cellular composition, and function13. CgA mostly exerts its effects following proteolytic cleavage, which is why most studies have focused on the bioactive cleavage products. So far, we know that CgA gives rise to vasostatin I/II (VS-I, VS-II), serpinin, WE-14, prochromacin, pancreastatin (PST), and catestatin (CST)14,15. Of these, the roles of PST and CST have received the most scientific attention since they exert opposing effects16. For example, PST has anti-insulin and pro-inflammatory properties17, while CST has pro-insulin and anti-inflammatory properties18,19.
Here, we mainly focus on the neuropeptide CST, which exerts a wide variety of immune- and neuro-regulatory functions11. The 21-amino-acid-long CST acts as an anti-inflammatory19,20,21, anti-diabetic19, and cardioprotective peptide22. Decreased CST blood levels have been found in diet-induced obese (DIO) mice19 and type 2 diabetes (T2D) patients23, while elevated CST blood levels have been seen in inflammatory bowel disease6, rheumatoid arthritis24, COVID-19 infection25 and various cardiovascular diseases such as acute heart failure, arrhythmia and hypertension26,27,28,29,30. Recent studies implicated that higher CST plasma levels, in combination with low plasma catecholamines, were associated with a worse disease prognosis for heart failure (five-fold increase in mortality for high CST plasma compared to normal CST levels)26,27,28,29,30. At the same time, low plasma CST and high catecholamine levels were associated with insulin resistance19, T2D23 and other cardiovascular diseases, including hypertension3,23. In line with this, the supplementation of CST in rodents21,31,32,33 and humans34 with hypertension normalized blood pressure. CST supplementation also reduced chronic gut inflammation in murine colitis models35,36 and improved inflammation and insulin sensitivity in mice with diet-induced obesity19,37.
Based on the link between CgA and its cleavage products to various endocrine diseases and their presence in pancreatic islets, we aimed to investigate how CgA, CST, and PST may influence pancreatic islet endocrine function, islet morphology and the islet microenvironment. For this purpose, we used CgA full knockout mice (CgA-KO)31 and mice with selective deletion of the CST-coding region in the Chga gene (CST-KO)21. CgA-KO mice have been characterized with hypertension, elevated sympathoneuronal activity and affected endocrine cells in the adrenal gland and pancreas13,31. CST-KO mice have been found to be hypertensive, obese, and exhibit insulin resistance. Additionally, they displayed low grade organ inflammation, elevated amounts of norepinephrine (NE) and epinephrine (EPI) in the plasma, lower bacterial gut diversity, and impaired epithelial barrier function6,19,21. CgA-KO mice maintained on a normal chow diet (NCD) exhibited enhanced insulin sensitivity38, which has been attributed to the absence of the CgA cleavage product PST, a known promoter of hepatic gluconeogenesis38. Notably, insulin sensitivity in CgA-KO mice persisted even after 4 months of high-fat diet17, further reinforcing PST as a key contributor to insulin resistance. In contrast, NCD-fed CST-KO mice displayed insulin resistance, suggesting that CST plays an insulin-sensitizing role. Both CgA-KO and CST-KO mice developed hypertension due to the absence of CST, as evidenced by the normalization of blood pressure upon CST supplementation21,31.
To our knowledge, this is the first study to comprehensively assess the pancreatic microenvironment, including endocrine cell density, composition and hormone content, pancreatic endocrine function by glucose-stimulated pancreatic hormone secretion and gluconeogenesis, inflammatory cytokine levels, innervation, and spatial mass spectrometry for identifying neurotransmitter and metabolite levels. We show that islets lacking CgA and CST display aberrant ratios of alpha- and beta cells along with glucagon and insulin contents and consequent differences in blood glucose homeostasis. Various neurotransmitters involved in crucial processes, such as hormone regulation, were also affected by CgA or CST deletion. In addition, we found that PST promoted gluconeogenesis, which was inhibited by supplementing CgA-KO mice with CST or CST in combination with PST. Upon CST supplementation, gluconeogenesis was restored to the WT level. Thus, our findings contribute understanding the role of CgA and its cleavage products CST and PST in the pancreatic microenvironment with possible implications for the pathology of both T1D and T2D.
Results
CgA and CST in the pancreatic islet in homeostasis and in T1D
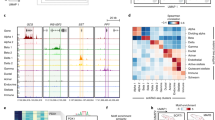
Since CST levels have been reported to be decreased3,21,23 or increased in various inflammatory diseases19,24,25,26,27,28,29,30, we measured plasma CST levels in healthy individuals and patients with T1D. In line with previous studies in patients with IBD6 and RA7, the CST levels were significantly increased in T1D individuals when compared to age-matched healthy controls (Fig. 1A). To visualize CST in the pancreas during autoimmune diabetes onset, we performed immunostainings in the pancreas of 4, 8 and 15 weeks-old female nonobese diabetic (NOD) mice to cover the disease onset process. Quantification of the insulin and CST staining showed that the ratio of double-positive cells (insulin and CST) remained constant in the NOD mice islets, indicating maintained CST production throughout disease progression (Fig. 1B, C). At the moment, the exact CgA cleavage products present in the pancreas, including the ones leading to CST are unknown (Fig. 1D). To compare the presence of CgA cleavage products in wild-type and 8-weeks NOD male and female mice, we performed immunoblotting of pancreatic islets stained for CST (Fig. 1E and Supplementary Fig. 1). To investigate tissue-specific processing of CgA into CST, we performed immunoblotting for CgA and CST in the adrenal medulla, pancreatic islets, and macrophages in WT mice (Fig. 1F, G and Supplementary Fig. 2). Consistent with inhibition of proteolytic processing by catecholamines in the adrenal gland39, we detected full-length CgA (75 kDa) in the adrenal medulla as compared to the pancreatic islets, which exhibited various CgA cleavage products, indicating rapid proteolytic processing of CgA (Fig. 1D, F). For both macrophages and pancreatic islets, CgA is effectively processed to CST as immunoblots with CST antibody showed bands comparable in size with the loaded synthetic CST peptide, as shown previously in heart tissue40 (Fig. 1F, G). Nevertheless, the presence of intermediate CgA cleavage products in the islets implies a potential role for CgA peptides in maintaining islet homeostasis.
A CST plasma levels in healthy (n = 13) and T1D participants (n = 23) graphs show mean ± SEM. B, C Quantification and representative images of immunofluorescent staining for catestatin (CST, magenta), insulin (green), CD3 (cyan) and nuclei (blue) in 4, 8, and 15-week-old NOD mice pancreas. n = 1 mouse pancreas per group, graphs show mean ± SEM. D CgA cleavage products towards CST. E Western blot showing CST staining in NOD mice pancreas. n = 2 mice per group. F Western blot showing CgA staining in the adrenal medulla, pancreatic islets and macrophages (mac). Arrows indicate full CgA and possible cleavage products of CgA. G Similar membrane stained for CST with synthetic CST peptide in adrenal medulla, pancreatic islets, and mac.
Islet morphology and endocrine islet composition is altered upon CST or CgA deletion
Staining of frozen pancreas sections from WT, CgA-KO, and CST-KO mice revealed CST presence in pancreatic islets of WT mice. The complete absence of CST staining in pancreata of CgA-KO or CST-KO knockout mice confirmed their KO phenotype (Supplementary Fig. 3). We also observed CST staining outside the pancreatic islets in WT tissue, likely attributing to CST content in immune cells and/or nerve fibers. To get an overview of the pancreas of WT, CgA-KO, and CST-KO sections, we made full-section scans of haematoxylin and eosin (H&E)-stained slides for male (Fig. 2A) and female mice (Supplementary Fig. 4A). These images seemed to show changes in islet morphology, which could indicate aberrations in islet function41,42. To investigate pancreatic islet morphology and endocrine cell composition in CgA-KO or CST-KO mice in more detail, we stained frozen pancreatic sections of KO mice for insulin, glucagon and somatostatin (Fig. 2B and Supplementary Fig. 4B). First, we quantified the islet density and islet area. Although the individual islet areas were unchanged, the islet density was significantly decreased in the CgA-KO pancreas when compared to WT and CST-KO pancreas (Fig. 2C, D), which is in line with previous findings for CgA-KO mice13. Next, islet shape was assessed, showing decreased islet circularity in CST-KO compared to WT islets (Fig. 2E). To double-check if the CST or CgA domain was absent in the pancreas, we ran a Western blot with CST or CgA antibody, which showed no CST band for CST-KO mice and no CgA band in CgA-KO mice, confirming CST and CgA KO (Fig. 2F and Supplementary Fig. 4–6). To assess endocrine cell composition, we quantified alpha, beta, and delta cell ratios per islet for WT, CgA-KO, and CST-KO mice (Fig. 2G; Supplementary Fig. 4C and Supplementary Method Fig. 1). In line with previous findings13, the quantification showed that CgA-KO mice displayed a significant increase in alpha cells and a decrease in beta cells per islet when compared to WT islets. The CST-KO mice only showed a decrease in beta cells when compared to the WT islets. In line with these findings, the numbers of hormone-negative cells in CST and CgA-KO islets were significantly increased. This means that CgA-KO and CST-KO mice have fewer endocrine cells per islet, which in combination with the decreased islet density in CgA-KO mice, may lead to altered endocrine function. Thus, the absence of CgA or CST in mice affects the endocrine cell composition and potentially also the function of the pancreatic islet.
A H&E staining of WT, CgA-KO, and CST-KO pancreatic slices, including annotations for islets (black dotted lines) and blood vessels (B). B Representative images of immunofluorescent stainings of glucagon (cyan), insulin (green), somatostatin (magenta), and Hoechst (blue) on WT, CgA-KO, and CST-KO pancreatic sections. C Islet area n = 5 mice per group. D Islet density n = 5 mice per group. E Islet circularity n = 5 mice per group. F Immunoblotting showing CST staining in pancreatic islets of WT and CST-KO mice with synthetic CST peptide (CST) as a positive control. n = 2 mice per group. G Quantification of alpha/beta/delta/hormone negative cells per islet. Quantification is based on the staining represented in (B). n = 5 mice per group. All graphs show mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Pancreatic islet function of mice lacking CgA or CST
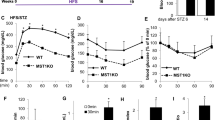
To determine the functional consequences of CgA or CST deficiency on glucose homeostasis and islet endocrine response, we performed in vivo glucose tolerance tests (GTT) in male mice. CgA-KO mice exhibited a significant reduction (~17% lower area under the curve (AUC)) in blood glucose levels during GTT compared to WT controls (Fig. 3A). This is consistent with the enhanced peripheral insulin sensitivity, likely due to suppression of hepatic gluconeogenesis, as reported previously38. These physiological findings support earlier reports of reduced islet numbers, decreased β-cell mass, and lower plasma insulin levels in CgA-KO mice relative to WT13. Conversely, CST-KO mice displayed significant elevation (~18% higher AUC) of blood glucose levels during GTT compared to WT mice, indicative of impaired β-cell function and/or insulin resistance, as described previously19.
A Graphs displaying combined and single data of glucose tolerance test (GTT; 2 g/kg dextrose intraperitoneal) results for WT (black), CgA-KO (pink) and CST-KO (cyan) over time (min) n = 8 mice per group (statistical power = 95%), including a graph displaying the calculated area under the curve (AUC in mg/dL*min). B Graphs displaying combined and single data of pyruvate tolerance test (PTT: i.p. 2 g/kg bw sodium pyruvate) blood glucose results (mg/dL) for WT (black), CgA-KO (pink) and CST-KO (cyan) over time (min) n = 15 mice per group (statistical power >95%) and a graph displaying the calculated AUC. C Graphs displaying combined and single data of PTT blood glucose results (mg/dL) over time (min) of CgA-KO mice supplemented with saline (blue), CST (purple), PST (pink) or CST + PST (green). n = 10 mice per group (statistical power = 95%) and a graph displaying the calculated AUC. Data were analyzed by one- or two-way ANOVA followed by Sidak’s multiple comparison test. All graphs show mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Given the central role of gluconeogenesis in glucose homeostasis and its suppression by insulin, we next conducted pyruvate tolerance tests (PTT) in WT, CgA-KO, and CST-KO mice to assess gluconeogenic capacity. We recorded body weight (Supplementary Fig. 7B, D), pancreatic weight (Supplementary Fig. 7C, E) and food intake (Supplementary Fig. 8A–D) before starting PTT in male and female WT, CgA-KO, and CST-KO mice, which showed increased body weight for CgA-KO and CST-KO male and female mice compared to WT mice. The CST-KO mice did not only increase in weight, but also lost the normal difference in weight between male and female mice. Consistent with a previous study38, CgA-KO mice exhibited marked reduction (~11 and ~8% lower AUC in male and female mice, respectively) in glucose levels during PTT (Fig. 3B; Supplementary Figs. 9 and 10), indicating inhibition of hepatic gluconeogenesis. In contrast, CST-KO mice showed increased (~14 and ~13% higher AUC in male and female mice, respectively) glucose levels (Fig. 3B and Supplementary Fig. 10A–D), suggesting enhanced gluconeogenesis in the absence of CST. In summary, deletion of CgA or CST results in affected glucose metabolism, where deletion of CgA results in increased insulin sensitivity, while deletion of only the CST domain results in decreased insulin sensitivity.
To delineate the contribution of specific CgA-derived peptides to the regulation of gluconeogenesis, we supplemented CgA-KO mice intraperitoneally with PST (1.4 µg/g body weight), CST (1 µg/g body weight), or an equimolar concentration (215 µM) of both and performed PTT after four weeks of treatment. We recorded body weight throughout the treatment, and pancreas weight was recorded after sacrificing mice for tissue harvesting. Male and female CgA-KO mice supplemented with saline, CST and CST + PST showed a similar pattern as for the normal CgA-KO mice in these aspects (Supplementary Figs. 7–10). CST-treated CgA-KO mice showed decreased glucose production (~7% and ~11% decrease in AUC in male and female mice, respectively) (Fig. 3C and Supplementary Fig. 10E), indicating inhibition of gluconeogenesis by CST specifically in female mice. In contrast, PST-treated mice exhibited increased glucose levels (~19% and ~22% increase in AUC in male and female mice, respectively) (Fig. 3C and Supplementary Fig. 10E), implicating PST as a pro-gluconeogenic peptide. Notably, co-administration of CST and PST prevented the PST-induced rise in glucose levels during PTT (Fig. 3C and Supplementary Fig. 10E), indicating that CST functionally antagonizes PST’s effects and exerts dominant control over gluconeogenesis. Thus, it seems that PST and CST have opposite effects in gluconeogenesis, where PST promotes and CST suppresses gluconeogenesis. The above findings are consistent with the observed increase in insulin sensitivity in CgA-KO mice38 in absence of the effects of PST. Similarly, it is also consistent with the increased insulin resistance in CST-KO mice19 in the absence of CST functions.
Glucose-stimulated insulin secretion from isolated CgA-KO and CST-KO pancreatic islets
To assess islet functionality further, we performed glucose-stimulated insulin secretion (GSIS) assays on islets isolated from WT, CgA-KO, and CST-KO mice. The islet yields per animal tended to be slightly higher for CST-KO mice when compared to WT and CgA-KO (Fig. 4A). The total insulin content per 10 islets was comparable across all genotypes, but insulin levels were slightly, but not significantly, increased in the CST-KO islets (Fig. 4B). Insulin secretion following stimulation with high glucose concentration was comparable between WT and CgA-KO mice (~12-fold versus ~13-fold) as compared to low glucose (Fig. 4C). In contrast, in CST-KO mice, the high glucose concentration caused a ~29-fold increase in insulin secretion (>2-fold higher than CgA-KO islets Fig. 4C). The fold-changes in insulin secretion in response to high glucose concentration were as follows: ~10-fold in WT islets; ~13-fold in CgA-KO islets and ~33-fold in CST-KO islets (Fig. 4D). Insulin secretion indices were as follows: ~10-fold for WT islets; ~14-fold for CgA-KO islets and ~36-fold for CST-KO islets (Fig. 4E). These data show a major increase in the ex vivo response to glucose from CST-KO islets compared to the in vivo GTT data.
A Islet yield per animal following isolation from WT, CgA-KO, and CST-KO pancreata. n = 4 mice per group. B Total insulin content (ng/mL) per 10 islets before (pre) and after (post) glucose stimulation for WT, CgA-KO, and CST-KO mice n = 6 mice per group. C GSIS from isolated islets from WT, CgA-KO, and CST-KO mice. Showing insulin content under basal glucose conditions (2.8 mM) before glucose stimulation (pre-GSIS) (left) and after high glucose stimulation (16.7 mM) (post-GSIS) (right). n = 6 mice per group. D Insulin secretion during GSIS on isolated islets from WT, CgA-KO, and CST-KO mice, expressed as percent of insulin content before and after GSIS. n = 6 mice per group. E Insulin secretion index = fold change of high vs. low GSIS (/content). n = 6 mice per group (Statistical Power = 80%). Data were analyzed by one- or two-way ANOVA followed by Sidak’s multiple comparison test. All graphs show mean ± SEM *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In vivo insulin and glucagon secretion after supplementation of CgA-KO mice with CST, PST or CST + PST
To investigate the role of CgA in islet endocrine function, we first assessed fasting (8 h) plasma insulin, glucagon, and C-peptide levels in male WT, CgA-KO, and CST-KO mice. Compared to WT mice, fasting plasma insulin levels were significantly reduced in CgA-KO (~28%) and CST-KO (~54%) mice (Fig. 5A). Decreased plasma insulin in CST-KO mice coincides with decreased C-peptide levels (Supplementary Fig. 7A). Paradoxically, fasting plasma glucagon levels were significantly decreased only in CST-KO mice (~50%), but not in CgA-KO mice (Fig. 5B). To further elucidate islet secretory dynamics, we conducted GTTs. Glucose administration in fasting (8 h) mice caused ~1.3- and ~1.6-fold increases in insulin secretion in WT and CgA-KO mice, respectively as compared to only ~1.1-fold in CST-KO mice (Fig. 5C), in line with the increased glucose levels during GTT (Fig. 3A, B). Suppression of glucagon secretion by glucose was observed only in fasting WT mice (~40%), as this response was abolished in both CgA-KO and CST-KO mice (Fig. 5D), implicating CST as a key regulator of islet glucagon dynamics.
A Basal fasting plasma insulin and B glucagon levels (ng/mL) from WT, CgA-KO, and CST-KO mice. n = 8 mice per group. C Graph showing plasma insulin and D glucagon concentrations under basal glucose conditions (fasting) and under high glucose conditions (15 min after i.p. injection of 2 g/kg dextrose) for WT, CgA-KO, and CST-KO mice, n = 8 mice per group. E Basal fasting plasma insulin and F glucagon levels (ng/mL) in CgA-KO mice treated for 7 days with saline, CST, PST, or CST + PST. n = 8 mice per group. G Graph showing fasting plasma insulin or (H) glucagon levels under basal glucose conditions and under high glucose conditions (15 min after i.p injection of 2 g/kg dextrose) injected for 4 weeks with saline (blue), CST (1 µg/g bw, i.p.) (purple), PST (1.4 µg/g bw, i.p.) (pink) or CST + PST (green). Data were analyzed by one- or two-way ANOVA followed by Sidak’s multiple comparison test. n = 8 mice per group (statistical power = 85%). All graphs show mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
To dissect the contributions of CgA-derived peptides to peripheral insulin resistance, we supplemented CgA-KO mice with intraperitoneal PST, CST, or a combination of both peptides at equimolar concentrations for one month. Basal plasma insulin levels were not affected significantly after treatments with CST or PST (Fig. 5E). However, co-administration of CST and PST significantly elevated (by ~39%) fasting insulin levels (Fig. 5E), suggesting that CST counteracted the effects of PST and enhanced basal insulin secretion. With respect to glucagon, CST monotherapy significantly reduced (by ~31%) fasting plasma glucagon levels (Fig. 5F), while PST had no effect. Notably, combined CST and PST treatment resulted in further suppression of glucagon (by ~46%; Fig. 5F), highlighting CST’s dominant role in regulating glucagon secretion.
GTT experiments in peptide-treated CgA-KO mice revealed: (i) enhanced glucose-induced insulin secretion (~60%) in saline-treated controls; (ii) moderate insulin secretion (~40%) with CST treatment; (iii) inhibition of insulin secretion by PST; and (iv) restoration of GSIS (~33%) in the presence of both CST and PST (Fig. 5G), indicating that CST is able to override PST-mediated inhibition. Finally, while glucose failed to suppress glucagon secretion in saline-treated CgA-KO mice, CST or CST + PST treatment restored this physiological suppression (~36%; Fig. 5H). In contrast, PST treatment alone led to paradoxical stimulation of glucagon secretion in response to glucose (Fig. 5H).
Neurotransmitter and metabolite levels are changed in CgA-KO and CST-KO pancreas
Neuronal input to islet endocrine cells affects their hormonal secretion. In order to explore potential differences in pancreatic neuronal activity, we employed mass spectrometry imaging of pancreatic sections from WT, CgA-KO, and CST-KO. We detected 12 pancreatic neurotransmitters and metabolites that met validation criteria (Supplementary Table 2). Values were extracted for WT, CgA-KO, and CST-KO total pancreas and differential expressions were visualized in a bar chart (Fig. 6A; Supplementary Figs. 11 and 12). These data showed that hypoxanthine, tyrosine, spermidine, histidine, cysteine, and spermine levels differ between CST-KO and WT. For most neurotransmitters, the expression seemed to be lowest in the CgA-KO pancreas overall when compared to WT and CST-KO. To be able to distinguish neurotransmitter levels between pancreatic islets and exocrine pancreatic tissue, we identified islets on consecutive H&E-stained sections (Supplementary Fig. 13). After scanning the pancreatic sections in a microscope, we matched the exported islet regions to the intensity distribution of the serotonin signal (as a marker for islets) and annotated islets and comparatively sized areas of exocrine tissue (serotonin negative) (Fig. 6B, Supplementary Method Figs. 2 and 3). Here, we visualized and quantified the content of serotonin, gamma-aminobutyric acid (GABA), histamine, cysteine, taurine, creatine, spermidine, histidine, NE, tyrosine, spermine, and hypoxanthine in the exocrine and endocrine parts of the pancreas (Fig. 6C). Altogether, this resulted in a comprehensive view of the neurotransmitters in the WT, CgA-KO, and CST-KO pancreas.
A Comparative analysis of analytes in pancreatic tissue. This chart displays the log2-fold change in expression of the analytes in total pancreatic tissue of WT (gray), CgA-KO (pink) and CST-KO (cyan). n = 3 mice per group. B Serotonin heatmap showing examples of WT (green annotations), CgA-KO (red annotations), and CST-KO (yellow annotations) pancreatic sections, including annotations for islets (colored) and exocrine tissue (white). C Comparative analysis of analytes in pancreatic tissues. This figure highlights the differential expression of neurotransmitters between pancreatic islets (blue) and adjacent exocrine tissue (red), based on the average intensity of root mean square (RMS) normalized regions. n = 3 mice per group.
Metabolic and neurotransmitter synthesis pathways are affected in CgA-KO and CST-KO pancreatic islets
In addition to the combined analysis, we took a closer look at the individual analytes in pancreatic islets and exocrine pancreas to identify which parts of the pathways were affected in islets and exocrine tissue of CgA-KO and CST-KO pancreata. For all three genotypes, serotonin and taurine levels were significantly higher in pancreatic islets when compared to exocrine tissue (Fig. 7A, B). Additionally, GABA and histamine levels were higher in islets when compared to exocrine tissue for WT and CST-KO (Fig. 7C, D). We also noted that CgA-KO had lower GABA levels in islets, and for both GABA and histamine, no differences between islet and exocrine tissue were detected. For CgA-KO islets and exocrine tissue, we also observed a decrease in amounts of cysteine compared to WT (Fig. 7E). Cysteine is a highly conserved amino acid involved in regulating catalysis, protein structure, redox sensitivity, and metal-ion transport43. Decreased cysteine levels might indicate disturbances within the homocysteine pathway.
A Peak area intensities for islets and exocrine tissue of WT, CgA-KO, and CST-KO pancreata of serotonin, B taurine, C GABA, D histamine, E cysteine, F spermidine, and spermine. Spermidine can be converted into spermine as illustrated in the schematic drawing. G Peak area intensities of creatine for islets and exocrine tissue of WT, CgA-KO, and CST-KO pancreata (H) hypoxantine (I) norepinephrine. n = 3 mice per group. J Plasma norepinephrine (pg/mL) and K epinephrine (pg/mL) of WT, CgA-K,O and CST-KO mice, n = 3 mice per group, where 28–30 islets and matching exocrine pancreas areas were selected per group (statistical power = >95%). Data were analyzed by one-way ANOVA followed by Sidak’s multiple comparison test. All graphs show mean ± SEM *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Polyamines (spermidine, spermine, and thermospine) play important roles in cell growth, proliferation, and differentiation. In the pancreas, polyamines seem to modulate beta cell function by affecting proinsulin biosynthesis and insulin secretion44. Spermidine is synthesized from putrescine by spermidine synthase. No differences in this process were seen among the genotypes as similar spermidine values were found for WT, CST-KO, and CgA-KO pancreata (Fig. 7F). Afterwards, spermidine is normally converted into spermine by spermine synthase. However, this process seems disturbed in both KO models as spermine levels were drastically decreased for the CgA-KO and CST-KO islets and exocrine tissue when compared to WT pancreas (Fig. 7F). This suggests that the conversion by spermine synthase is less efficient in the absence of CgA or CST.
The creatine pathway seems unaffected since creatine levels appeared equal for all three genotypes (Fig. 7G). Hypoxanthine levels were only significantly increased in CST-KO mice (Fig. 7H). Here, both exocrine tissue and islets showed high levels of hypoxanthine, which could indicate pancreatic necrosis45.
NE and dopamine are catecholamines that can regulate insulin secretion by islet beta cells44,46. Thereby, the synthesis and degradation of NE and dopamine are essential for normal islet endocrine function. In the past, increased adrenal and plasma catecholamine levels have been detected in the plasma and adrenal glands of CgA-KO and CST-KO mice21. In contrast, our data shows that NE levels in the pancreas are not significantly different compared to WT for CgA-KO and CST-KO mice since we observed high islet NE levels and lower NE exocrine levels for all three genotypes (Fig. 7I). These data suggest that catecholamine levels in the pancreas were normal. In contrast, plasma catecholamine levels (NE and EPI) were increased in CgA-KO and CST-KO mice (Fig. 7J, K), indicating that there might be a disturbance in local catecholamine degradation or an influence from circulating catecholamines.
Pancreatic sympathetic innervation and inflammatory signature in mice lacking CgA and CST
There is increasing evidence that pancreatic islet innervation is disordered in T1D and T2D, which may lead to changes in regulation of islet hormone release and metabolic regulation47. To investigate whether the observed changes in endocrine composition and/or function in CgA-KO and CST-KO mice may be related to islet innervation, we stained pancreatic sections for the NE transporter to visualize sympathetic neurons (Fig. 8A and Supplementary Fig. 14). Although the nerve network qualitatively seemed more disorganized in the CgA-KO and CST-KO pancreas, the quantification did not reveal major measurable differences regarding islet innervation between WT, CgA-KO and CST-KO pancreata (Fig. 8B). Comparing innervation in and around the pancreatic islet, the nerve density for WT islets was significantly lower when compared to the exocrine nerve network (Fig. 8C). However, this difference between in- and outside the islet was lost for the CgA-KO and CST-KO mice. This might indicate changes in the overall nerve network organization upon CgA or CST deletion. CST acts as a neuropeptide and has been shown to be produced by and have effect on nerves and macrophages, which may result in suppression of neuronal and neuroendocrine activity in an inflammation-dependent manner11. To investigate its role in the pancreas, we quantified nerve-macrophage interactions in pancreatic islets of CgA-KO and CST-KO mice (Fig. 8D–F). Although no differences were found in the numbers of macrophages per islet, we observed a trend for less macrophage-nerve interactions in the islet upon CgA or CST deletion when compared to WT (Fig. 8E, F). To gain a better insight into the role of inflammation in mice lacking CgA or CST, we measured plasma TNF-α, interleukin-6 (IL-6), IL-1β, C-X-C motif chemokine ligand 1 (CXCL-1), chemokine (C-C motif) ligand 2 (CCL2), and IL-10. In line with previous studies6,19,21, the lack of CST resulted in reduced plasma levels of the anti-inflammatory marker IL-10 and increased inflammatory cytokines (IL-6, IL-1β) and chemokine levels (CXCL-1, CCL-2), reinforcing CST as an anti-inflammatory peptide (Fig. 8G).
A Representative images of nerve staining norepinephrine transporter (NET, magenta), islets (insulin, green), and nuclei (Hoechst) in WT, CgA-KO and CST-KO pancreatic sections. B Quantification of the nerve density ratio between islet and exocrine tissue for WT, CgA-KO and CST-KO based on the stainings represented in A. n = 3 mice per group. C Quantification of nerve volume in the islet and the exocrine pancreas for WT, CgA-KO, and CST-KO. n = 3 mice per group. D Macrophage count per islet (every dot in the chart represents one islet). E Number of macrophages interacting with nerves per islet for WT, CgA-KO, and CST-KO. n = 3 mice per group. F Representative images of nerve staining NET (cyan), macrophages (IBA1, magenta) and nuclei (gray) in WT, CgA-KO, and CST-KO pancreatic slices. G Plasma IL-10, TNF-α, IL-β, IL-6, CXCL-1, and CCL-2 levels of WT, CgA-KO, and CST-KO mice. Data were analyzed by one-way ANOVA followed by Sidak’s multiple comparison test. n = 8 mice per group (statistical power = 85%). All graphs show mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Conclusion and discussion
This comprehensive longitudinal study underscores the pivotal role of CgA and CST in maintaining pancreatic homeostasis, including both morphological and functional components. CgA is a unique pro-hormone containing counter-regulatory motifs. Among these, PST (hCgA250–301)48 has been reported to exert pro-diabetic, pro-inflammatory, and obesogenic effects17, whereas CST (hCgA352−372)49 exhibits anti-diabetic19,37, anti-inflammatory19,37, and anti-obesogenic18 properties. Consequently, the simultaneous absence of both PST and CST in CgA-KO mice results in heightened insulin sensitivity, as previously documented in both NCD38 and DIO mice17. Supplementation of CgA-KO mice with PST led to increased glucose excursion during GTT and elevated glucose production during PTT, confirming PST as a pro-gluconeogenic, insulin-resistant peptide. Conversely, CST supplementation reduced glucose excursion in GTT and inhibited glucose production during PTT, highlighting CST as an insulin-sensitizing peptide as per our previous report in DIO mice19. Co-supplementation of CgA-KO mice with equimolar concentrations of PST and CST revealed that CST overrides PST’s gluconeogenic effect, suggesting CST’s dominance in regulating hepatic glucose output. Moreover, we have shown that CgA-KO mice maintain enhanced insulin sensitivity even after four months on a high-fat diet (60% calories from fat)17. CST’s beneficial effects on insulin sensitivity in DIO mice are mediated via suppression of monocyte-derived macrophage infiltration19, attenuation of gluconeogenesis19, and reduction of endoplasmic reticulum stress37.
Our morphometric studies demonstrated decreased beta cell numbers in CgA-KO and CST-KO mice, which align with biochemical findings of reduced insulin levels in these models. In contrast, increased alpha cell numbers in CgA-KO and CST-KO mice do not fully correlate with glucagon levels; no change in CgA-KO mice and decreased glucagon levels in CST-KO mice compared to WT mice. Intriguingly, despite elevated catecholamines, increased ketone bodies, and muscle insulin resistance, CgA-KO mice maintain euglycemia due to enhanced hepatic insulin sensitivity, as evidenced by data from GTT, insulin tolerance tests, PTT, and hyperinsulinemic euglycemic clamp studies38. The absence of the pro-gluconeogenic PST in CgA-KO mice likely contributes to this increased hepatic insulin sensitivity. Conversely, in CST-KO mice, PST was allowed to exert its gluconeogenic effect without intervention by CST. This made CST-KO mice, which still express PST, develop insulin resistance due to unopposed PST action. Our current data reinforce this concept, as CST supplementation effectively dominated PST in regulating glucagon dynamics and gluconeogenesis.
We also observed discrepancies between in vivo GTT (15 min exposure) in intact animals and ex vivo GSIS (60 min exposure). Notably, glucose injections failed to stimulate in vivo insulin secretion in CST-KO mice but induced ~29-fold insulin release ex vivo. This in vivo failure to respond to glucose with insulin secretion is likely attributable to elevated plasma catecholamines: NE (~1.6-fold increase) and EPI (~1.8-fold increase) in CST-KO mice21. Catecholamines are known to inhibit insulin secretion by activating α₂-adrenergic receptors on beta cells50,51. The α2A-adrenoceptor, the predominant subtype in mouse and human islets, reduces beta-cell excitability and insulin release by lowering cAMP levels and desensitizing of late exocytotic step(s) to cytosolic Ca2+50, leading to glucose intolerance in vivo. However, isolated islets studied ex vivo are free from catecholaminergic inhibition, enabling robust insulin secretion at high glucose concentration. Thus, CST deficiency results in elevated catecholamines that potently inhibit insulin secretion, a constraint removed under ex vivo conditions.
GABA is linked to T1D through the autoantigen GAD65 and is thought to increase beta cell content in the islet through beta cell proliferation52. Lower GABA levels might be one reason for the detected decrease in insulin-producing cells in the CgA-KO mouse islets. However, the differences seen in CST-KO islets are probably caused by other mechanisms affecting insulin content or beta cell numbers, since the GABA and histamine values in CST-KO islets were similar to WT islets. Beta cells contain high concentrations of polyamines (putrescine, spermidine, and spermine), where they regulate proinsulin biosynthesis and secretion of insulin53,54. The depletion of polyamines in isolated mouse islets has been associated with impaired GSIS, insulin content, insulin transcription, and DNA replication55,56. Polyamine levels were reported to be diminished in aging and obese mice57, which are resistant to insulin. CST-KO mice have also been reported to be insulin resistant19. The markedly decreased spermine levels in the CST-KO pancreas may be one reason for the observed increased insulin plasma levels and/or resistance to insulin in CST-KO mice. Also, the observed lower spermine levels in the CgA-KO and CST-KO mice can result in disturbed insulin secretion44 and less uptake of Ca2+ by the beta cells58. In obese mice the spermine to spermidine ratio is even similarly disturbed in the pancreatic islets as in the KO mice in the current study57.
Altogether, more research is necessary to identify the pathway(s) resulting in the affected islet composition in the CgA-KO and CST-KO mice. With our unique methodology, we were able to spatially investigate the pancreatic microenvironment—a crucial step towards identifying and understanding the pathways affecting islet composition in KO mice. However, since our current methodology is insufficient to detect enzyme expression and activity, future research should focus on studying the enzymes regulating catecholamine degradation and spermine pathways to clarify the underlying mechanisms of the pancreatic microenvironment further.
The CgA cleavage product CST is increasingly implicated in metabolic regulation, including insulin sensitivity, glucose homeostasis, and inflammation. The enhanced beta cell responsiveness in isolated islets of CST-deficient mice suggests a physiological role for CST in restraining insulin hypersecretion, possibly to prevent beta cell exhaustion in metabolic stress conditions. The discrepancy between in vivo GTT and in vitro GSIS, where isolated islets from CST-KO mice were highly responsive to glucose in vitro, but not in vivo, may be partially explained by the hyperadrenergic state of these mice. CST normally acts as a physiological brake on catecholamine release59,60, and the increased NE and EPI levels may thus limit insulin secretion from islet beta cells. Thus, modulating CST activity could potentially offer a novel therapeutic approach for metabolic disorders characterized by insulin resistance, such as T2D and obesity. Specifically, CST antagonists may enhance endogenous insulin output in the context of beta cell insufficiency, while CST mimetics/analogs could be beneficial where insulin hypersecretion contributes to disease progression. This is an area that requires further translational research.
Results of this study should also be interpreted with caution since the use of full body (systemic) KO models for CgA and CST has limitations. Whole-body KO models can introduce confounding effects from other tissues (e.g., liver, fat, muscle) or can be the result of developmental issues, which may also influence the observed metabolic phenotype. The development of a conditional CgA- and CST-KO model that can be turned on and off in target tissues such as the pancreas would be very useful for future research. Human and mouse islets are slightly different in morphology and endocrine composition61, therefore, the spatial MS, GSIS and fluorescent staining of this study should be repeated on CgA- or CST-stimulated human spheroids or islets to confirm the effects in human.
Future research in this matter should also address the long-term effects of CgA- or CST deletion, as the full effects of metabolic disturbances may appear later in life. Targets for CgA cleavage products, both in respect to receptors involved and tissues/cells affected should be carefully dissected, potentially through the use of mutated/truncated versions of these peptides. Additionally, future research should focus on further profiling of macrophages and other immune cell populations to clarify their role in neuro-immunomodulation in the context of CgA and CST absence. Surprisingly, we didn´t observe significant differences of catecholamine levels in the pancreas, while catecholamine plasma levels of the KO mice are increased. However, we didn´t look into NE or EPI degradation products, future research should confirm if the catecholamine degradation is indeed affected upon CgA- or CST-KO.
Recent studies have highlighted the crucial connection between the local immune system in the pancreas and the peripheral nervous system in the development of autoimmune diseases62,63,64. Interfering with pancreatic nerve signals through surgery, chemical blockage, or electric stimulation has been shown to prevent the onset of T1D in mice62,64. Additionally, depleting macrophages during the onset of autoimmune diabetes in mice can halt T1D onset63. These findings indicate that the connection between the local immune system in the pancreas and the peripheral nervous system plays an important role in the development of autoimmune disease. This connection may involve NE signaling, where NE is produced by neurons but locally regulated by nerve-associated macrophages, potentially through β2 adrenergic signaling, as observed in hypertension65. This mechanism may explain how CgA and CST influence autoimmune disease development since (1) NE/EPI levels are high in CgA-KO and CST-KO mice, (2) both KO mice are characterized with hypertension and (3) CgA and CST levels are elevated in autoimmune diseases. Clarification of this link between CgA/CST and autoimmune disease development through catecholamine signaling among nerves, immune cells, and endocrine cells could provide novel insights into therapeutic strategies for autoimmune diseases in the future.
Altogether, our findings delineate the opposing roles of CgA cleavage products PST and CST in orchestrating pancreatic and peripheral glucose homeostasis, highlighting a neuro-immune-endocrine regulatory axis (Fig. 9). Our PTT data establish CST’s dominance over PST in suppressing gluconeogenesis, underscoring the therapeutic potential of modulating CgA-derived peptides. By targeting the neuro-immune-endocrine (structural and functional components) axis, through CST-based therapy or PST inhibition could represent a novel strategy to improve insulin sensitivity and potentially ameliorate diabetes disease and related metabolic disorders. This study provides insights into how a potential dysregulation in the CgA system in various diseases can affect metabolic functions and underscores this as a factor of relevance in elucidating disease networks. In the extension, this study also provides foundation for further research into precision peptide therapeutics aimed at complex metabolic and autoimmune diseases.
A Summarized effects of chromogranin A cleavage products catestatin and pancreastatin (created in BioRender71). B Proposed working mechanism of how catestatin (CST, green) and pancreastatin (PST, purple) levels maintain euglycemia by affecting the α-2a-adrenergic signaling via catecholamines (red dots), resulting in release or inhibition of insulin (black dots) secretion from the beta (β) cell. Levels of hormones/peptides are indicated by arrow direction, high (facing up) and low (facing down) (created in BioRender72). C Proposed working mechanism of how CST (green) and PST (purple) maintain euglycemia by affecting the α-2a-adrenergic receptor via catecholamines (red dots) resulting in release or inhibition of glucagon (black dots) secretion from the alpha (α) cell (created in BioRender73).
Materials and methods
Human blood samples for CST ELISA
The collection of blood samples was approved by the Regional Research Ethical Committee in Uppsala (Dnr 2014/485) and was conducted in consistency with the Declaration of Helsinki. All participants gave their written informed consent prior to inclusion in the study. CST levels in plasma were determined using ELISA (CUSABIO CSB-E17355h). Patient characteristics are listed in Supplemntary Table 1.
Mice
Male and female wild-type (WT), CgA-KO mice and CST-KO mice (5–8 months old) on C57BL/6 background were kept in a 12 h dark/light cycle on NCD (13.5% calorie from fat; LabDiet 5001, Lab Supply, Fort Worth, TX). These mouse studies were approved by the UCSD and Veteran Affairs San Diego Institutional Animal Care and Use Committees and conform to relevant National Institutes of Health guidelines. Organs were harvested after deeply anesthetizing the mice with isoflurane, followed by cervical dislocation. For the immunoblotting and immunofluorescent experiments, organs (adrenal gland, pancreas, bone marrow) were harvested from male C57BL/6J mice (Taconic, Denmark) or NOD mice. These studies were approved by the Regional Animal Ethics committee in Uppsala, Sweden (permit no: 5.8.18-01462/2023, 5.8.18-01540/2024). All animal experiments were conducted according to local standard operating procedures regarding humane endpoints.
Tissue preparation for immunohistochemistry
Pancreata were harvested from WT, CST-KO, and CgA-KO mice. Pancreata were cut in two parts and snap frozen. For immunostainings, the pancreata were embedded in O.C.T., sectioned in 10 µm sections at −20 °C and captured on microscope glass slides (Epredia; J1830AMNZ). For mass spectrometry imaging, the frozen pancreas tissues were sectioned at a thickness of 12 μm using a Leica CM3050S cryostat set at −20 °C, thaw-mounted on stainless steel MALDI-target plates and subsequently stored at −80 °C until further analysis.
Fluorescent staining of the pancreatic islets
Pancreatic sections were fixed for 10 min in 4% paraformaldehyde followed by washes in phosphate-buffered saline with 0.1% Tween (PBST) (Medicago; 274713; Sigma-Aldrich; SZBA3190V). Afterwards, slides were treated for 10 min with 0.2% Triton X100, followed by 30 min in blocking solution (PBS supplemented with FBS 2%, saponin 0.2%, NaAz 0.1%, 1:1000 FC blocker (BD Bioscience; 553142)). To prevent antibody cross-reactivity, the insulin staining was performed first. To do so, slides were incubated overnight (o.n.) at 4 ˚°C with guinea pig insulin primary antibody in blocking buffer (1:1000, Fitzgerald; 20-IP35). The next day, slides were washed three times with PBST for 5 min followed by staining for 30 min at r.t. with the secondary antibody anti-guinea pig-488 (1:500, Invitrogen; A-11073). After washing three times, slides were incubated for 1 h at r.t. with either mouse anti-glucagon 1:200 (1:200, Proteintech; 67286-1-Ig) and rabbit anti-somatostatin (1:500, Abcam; ab111912) or rabbit anti-norephrine transporter (1:200, Abcam; ab254361) and rat anti-IBA1 (1:1000, Synaptic Systems; 234017) or rabbit anti-Tyrosine Hydroxylase (Abcam; ab137869) or 488-anti-mouse CD3 (1:300, Biolegend; 100210) and rabbit anti-CST (1:1000, Proteintech; 289-MM-0288). Slides were washed three times with PBST for 5 min followed by staining for 30 min at r.t. with anti-mouse 555 (1:1000, Invitrogen; A21127) and anti-rabbit-647 (1:2000, Invitrogen; A31573) or anti-rabbit 555 (1:1000, Invitrogen; A31572) and anti-rat-647 (1:1000, Invitrogen; A48272). After washing three times with PBST, the slides were stained for 10 min with Hoechst (1:10.000, ThermoFisher; 33342) to visualize the nuclei. Afterwards, slides were washed three times with PBS. After the final wash the cover glass (24 × 50 mm, VWR; 631-0147) was applied using ProLong™ Gold Antifade Mountant (ThermoFisher; P36934). For imaging, the slide scanner Zeiss Axio Scan Z1 with a 20× objective was used.
Analysis of insulin, glucagon and somatostatin cells in the pancreatic islet
Slide scanner pictures were analysed using QuPath66 (Supplementary Method Fig. 1). Islets were annotated based on staining in all channels visualizing the complete islet. The machine-learning software was trained on WT pancreas images to detect cell number per islet using the Hoechst nuclei staining channel and the following nucleus parameters: background radius: 15 px; median filter radius: 0 px; sigma: 3 px; minimum area: 10 px2; maximum area: 1000 px2; intensity parameters: threshold 100; cell parameters: cell expansion 5 px. The obtained cell classifier was used to determine islet shape features (area, length, circularity, solidity, maximum diameter, minimum diameter, and nucleus/cell area ratio) and to calculate the islet density (islet density = islet area/pancreatic section area). This was followed by obtaining the numbers of insulin, somatostatin, and glucagon-producing cells per islet. Afterwards, insulin, somatostatin, and glucagon cell ratios were calculated: Positive cells % per islet = (100/total cell number) × target cell number).
In vivo glucose tolerance test (GTT)
Mice were fasted o.n. for 8 h before starting the GTT. Blood glucose was measured from tail tip blood drops using a glucose strip (True Metrix, total diabetes, Whitestown, IN) and a glucometer (total diabetes, Whitestown, IN) after 15, 30, 60, 90 and 120 min of administration of intraperitoneal dextrose (2 g/kg). Data are presented as blood glucose concentration (mg/dL) over time and as are under the curve above baseline.
Pancreatic islet isolation and islet glucose-stimulated insulin secretion (GSIS)
Pancreatic islets were isolated from mice by injecting collagenase P solution (1.4 mg/mL; #11213873001, Roche) into the pancreas via the common bile duct. The pancreas was subsequently incubated at 37 °C with intermittent vortexing (10 s at medium speed) until fully digested. Enzymatic activity was quenched by adding ice-cold RPMI medium supplemented with 10% fetal bovine serum (FBS), followed by gentle agitation. The digested tissue was centrifuged at 300 × g for 3 min at 4 °C. The supernatant was discarded, and the pellet was washed twice with cold complete medium, then passed through a 500 µm filter (#U-CMN-500-B, component supply). The filtered suspension was centrifuged again and resuspended in a smaller volume to facilitate loading onto a 70 µm cell strainer (#352350, Corning). The strainer was then inverted into a 60 × 15 mm tissue culture dish (#83.3901.500, SARSTEDT) containing 5 mL of complete RPMI medium. Islets retained on the membrane were collected under a stereomicroscope and cultured in RPMI containing 11.1 mM glucose, 10% FBS, 100 U/mL penicillin–streptomycin, and 2 mM GlutaMAX. Following isolation, the following parameters were assessed: the number of islets per mouse, insulin content per 10 islets, GSIS, GSIS normalized to insulin content, and the insulin secretion index.
In vivo insulin and glucagon measurements
All mice were fasted for eight hours before starting the glucose injections. Blood (50 µl) from WT, CgA-KO, and CST-KO mice was collected at 0 min from the tail tip using a capillary tube and kept on ice. Mice were injected with dextrose (2 g/kg intraperitoneally) and blood (50 µl) was collected again after 15 min of dextrose administration. Blood samples were centrifuged at 12,500 RPM for 10 min at 4 °C to separate the plasma. Plasma was stored at −80 °C until analysis of insulin and glucagon levels. Twenty-five microliter plasma was used for insulin and glucagon assay using the MSD U-PLEX ELISA kit (Meso Scale Diagnostics, Rockville, MD).
Pyruvate tolerance test (PTT)
All mice were fasted for 8 h before starting the PTT. Blood glucose was measured on a blood drop collected from the tail tip using a glucose strip and a glucometer at 0 min. Sodium pyruvate (2 g/kg intraperitoneally) was injected, followed by blood glucose measurements from tail tip using a glucose strip and a glucometer after 15, 30, 60, 90, and 120 min after administration. Data are presented as blood glucose concentration (mg/dL) over time.
Mouse cytokine and hormone measurements
Twenty-five microliter mouse plasma was used in a customized U-PLEX Custom Metabolic Group Assay kit (Meso Scale Diagnostics, Rockville, MD) by following the manufacturer’s protocol. Mouse hormones and cytokines were measured: glucagon, insulin, CXCL1, IL-1β, IL-6, IL-10, MCP-1 (CCL-2) and TNF-α, using a MESO SECTOR S 600MM Ultra-Sensitive Plate Imager. Insulin and glucagon levels were presented as ng/mL. Cytokines and chemokines were presented as pg/mL.
Measurement of plasma catecholamines
Plasma catecholamines were quantified using an Atlantis dC18 column (100 Å, 3 µm, 3 × 100 mm; Waters Corp., Milford, MA) on an ACQUITY UPLC H-Class System equipped with an electrochemical detector (ECD) (model 2465; Waters Corp.) as previously described21,67. The isocratic mobile phase consisted of phosphate–citrate buffer and acetonitrile (95:5, v/v) at a flow rate of 0.3 mL/min. For extraction, 2 ng of 3,4-dihydroxybenzylamine was added as an internal standard to 100 µL of plasma. Catecholamines were adsorbed onto ~15 mg of activated aluminum oxide by rotating the mixture for 10 min. Following adsorption, the aluminum oxide was washed with 1 mL of ultrapure water, and catecholamines were eluted with 100 µL of 0.1 N HCl. The ECD was set at a sensitivity of 500 pA for catecholamine detection. Chromatograms were analyzed using Empower software (Waters Corp.). Catecholamine concentrations were normalized to the recovery of the internal standard and expressed as ng/mL.
Immunoblotting
Macrophages grown from mouse bone marrow68, or tissue (medulla, isolated pancreatic islets69) were lysed in lysis buffer (1% SDS, 10 mM TrisHCl, pH 6.8). Protein concentration was determined according to manufacturer’s instructions (Bio-Rad; 500-0114). Afterwards, for each condition 60 µg of protein was loaded and run on 10% mini-protein TGX gels (Bio-Rad; 4561033) in Tris/Tricine/SDS buffer (Bio-Rad; 1610744) followed by transfer for 60 min at 100 V at 4 °C in Tris/Glycine buffer (Bio-Rad; 1610771) with methanol (Supelco; 1263283323) using a PVDF membrane. The membranes were taken out the cassette and washed with distilled water, followed by blocking in 3% BSA buffer for 1 h at r.t. Membranes were stained o/n at 4°C with primary antibody rabbit anti-CgA (1:1000, Invitrogen; PA5-35071) or rabbit anti-CST (1:1000, Proteintech; 289-MM-0288). Next day, the membranes were washed three times quickly and three times for 10 min with TBS-T 0.02%. Followed by staining with secondary goat-a-rabbit antibody 1:5000 dilution (IRDye800; P/N 925-32211) for 1 h at r.t. Afterwards, the membrane was again washed 3 times with TBS-t 0.02%. Both membranes were scanned on a Bio-Rad ChemiDoc MP imager to visualize the fluorescent staining.
Spatial mass spectrometry
Two consecutive 12 µm pancreatic sections were taken containing tissue of 3 WT, 3 CgA-KO, and 3 CST-KO mice. One section was stained with Hematoxylin–Eosin (HE) (Supplementary Method Fig. 2) to annotate the pancreatic islets using QuPath. The other slide was used for neurotransmitter analysis of serotonin (5-HT), GABA, histamine, cysteine, taurine, creatine, spermidine, histidine, NE, tyrosine, spermine and hypoxanthine. Derivatization matrix FMP-10 (4.4 mM in 70% acetonitrile, Tag-ON AB, Uppsala, Sweden)70 (Supplementary Table 2) was applied with TM-Sprayer (HTX-Technologies, Chapel Hill, NC, USA). The spraying method was set up to include 30 passes, with a flow rate of 80 μl/min at a temperature of 80 °C. The nozzle velocity was adjusted to 1100 mm/min, while the track spacing was set at 2.0 mm, and N2 was set 6 psi. Full tissue MSI experiments were performed using a timsTOF fleX MS imaging instrument in positive ion mode (Bruker Daltonics GmbH, Bremen, Germany). Online calibration was performed using m/z 555.2231, an abundant ion cluster signal of FMP-10. Data acquisition was set up in single scan mode at 30 µm lateral resolution, collecting 200 shots per pixel in the m/z range 300–1000 using flexImaging 5.0 and timsControl 6.0 (Bruker Daltonics). Islets were mapped on the tissue with 5-HT signal and overlay with stained HE staining. For each islet a corresponding ROI of exocrine tissue was drawn.
Metabolite identities were validated by MS/MS directly from tissue and compared with MS/MS of FMP-10 derivatized analytical standards (Supplementary Method Figs. 3 and 4). MS/MS data were collected on a MALDI-FTICR MS (solariX, Bruker Daltonics). The isolation window for fragmentation was set to 1 Da and collision energy of 35 eV.
Analysing neurotransmitters in the islets and exocrine pancreas
Islet selections in Qupath were exported using the SCiLS lab extension (Bruker Daltonics) and imported into the flexImaging (Bruker Daltonics, Bremen, v.5.0) software. To match and optimize the pancreatic islet location from the HE staining, the serotonin signal was used. After identifying the pancreatic islets, annotations were made for the matching exocrine tissue (serotonin-negative area). Expression data of neurotransmitters in the pancreatic islet and exocrine tissue were extracted and visualized in graphs. Illustration showing islet selection and data extraction can be found in the supplementary material (Supplementary Method Fig. 2).
Statistics and reproducibility
Data are expressed as mean ± SEM, all datapoints are shown by dots and “n” in the figure legends represents the biological replicates of the group. One-way or two-way ANOVA with Sidak’s post-hoc tests or nonparametric Mann–Whitney test were applied for multiple comparisons. A p-value of less than 0.05 was considered statistically significant. Sample sizes, statistical analyses, and statistical power are indicated in the corresponding figure descriptions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets generated and analyzed in the current study are available in the FigShare repository (https://figshare.com/s/8dace17885070d6916f1, https://doi.org/10.17044/scilifelab.28172552). Raw spatial MS data, slide scans, and human data are available upon request via Gustaf Christoffersson (gustaf.christoffersson@scilifelab.uu.se).
References
Mahata, S. K. & Corti, A. Chromogranin A and its fragments in cardiovascular, immunometabolic, and cancer regulation. Ann. N. Y. Acad. Sci. 1455, 34–58 (2019).
O’Connor, D. T. Plasma chromogranin A. Initial studies in human hypertension. Hypertension 7, I76–I79 (1985).
O’connor, D. T. et al. Heritability and genome-wide linkage in US and Australian twins identify novel genomic regions controlling chromogranin A implications for secretion and blood pressure. https://doi.org/10.1161/CIRCULATIONAHA.107.709105 (2008).
Ceconi, C. Chromogranin A in heart failure. A novel neurohumoral factor and a predictor for mortality. Eur. Heart J. 23, 967–974 (2002).
O’Connor, D. T., Pandian, M. R., Cariton, E., Cervenka, J. H. & Hsiao, R. J. Rapid radioimmunoassay of circulating chromogranin A: in vitro stability, exploration of the neuroendocrine character of neoplasia, and assessment of the effects of organ failure. Clin. Chem. 35, 1631–1637 (1989).
Muntjewerff, E. M. et al. Chromogranin A regulates gut permeability via the antagonistic actions of its proteolytic peptides. Acta Physiol. 232, https://doi.org/10.1111/apha.13655 (2021).
Di Comite, G. et al. Circulating chromogranin A reveals extra-articular involvement in patients with rheumatoid arthritis and curbs TNF-α-elicited endothelial activation. J. Leukoc. Biol. 85, 81–87 (2009).
Hsu, C.-H. et al. Chromogranin A levels and mortality in patients with severe sepsis. Biomarkers 20, 171–176 (2015).
Bousiges, O. et al. Diagnostic value of CSF chromogranin A to discriminate between Alzheimer’s disease and dementia with Lewy bodies. Neuropathol. Appl. Neurobiol. 50, 1–157 (2024).
Jati, S. et al. Chromogranin A deficiency attenuates tauopathy by altering epinephrine-alpha-adrenergic receptor signaling in PS19 mice. Nat. Commun. 16, 4703 (2025).
Muntjewerff, E. M., Christoffersson, G., Mahata, S. K. & van den Bogaart, G. Putative regulation of macrophage-mediated inflammation by catestatin. Trends Immunol. 43, 41–50 (2022).
Lukinius, A., Stridsberg, M. & Wilander, E. Cellular expression and specific intragranular localization of chromogranin A, chromogranin B, and synaptophysin during ontogeny of pancreatic islet cells: an ultrastructural study. Pancreas 27, 38–46 (2003).
Portela-Gomes, G. M. M., Gayen, J. R. R., Grimelius, L., Stridsberg, M. & Mahata, S. K. K. The importance of chromogranin A in the development and function of endocrine pancreas. Regul. Pept. 151, 19–25 (2008).
Maj, M., Hernik, K., & Tyszkiewicz, K. A complex role of chromogranin A and its peptides in inflammation, autoimmunity, and infections. Front. Immunol. 16, 1567874 (2025).
Bandyopadhyay, G. K. & Mahata, S. K. Chromogranin A regulation of obesity and peripheral insulin sensitivity. Front. Endocrinol. 8, 20 (2017).
Muntjewerff, E. M., Dunkel, G., Nicolasen, M. J. T., Mahata, S. K. & van den Bogaart, G. Catestatin as a target for treatment of inflammatory diseases. Front. Immunol. 9, 1–10 (2018).
Bandyopadhyay, G. K. et al. Pancreastatin-dependent inflammatory signaling mediates obesity-induced insulin resistance. Diabetes 64, 104–116 (2015).
Bandyopadhyay, G. K. et al. Catestatin (chromogranin A352-372) and novel effects on mobilization of fat from adipose tissue through regulation of adrenergic and leptin signaling. J. Biol. Chem. 287, 23141–23151 (2012).
Ying, W. et al. Catestatin inhibits obesity-induced macrophage infiltration and inflammation in the liver and suppresses hepatic glucose production, leading to improved insulin sensitivity. Diabetes 67, 841–848 (2018).
Kojima, M. et al. Catestatin prevents macrophage-driven atherosclerosis but not arterial injury–induced neointimal hyperplasia. Thromb. Haemost. 118, 182–194 (2018).
Ying, W. et al. Immunosuppression of macrophages underlies the cardioprotective effects of CST (catestatin). Hypertension 77, 1670–1682 (2021).
Mahata, S. K., Kiranmayi, M. & Mahapatra, N. R. Catestatin: a master regulator of cardiovascular functions. Curr. Med. Chem. 25, 1352–1374 (2018).
Pankova, O. & Korzh, O. Plasma catestatin levels are related to metabolic parameters in patients with essential hypertension and type 2 diabetes mellitus. Heart Vessels 39, 144–159 (2024).
Simac, P. et al. Serum catestatin levels in patients with rheumatoid arthritis. Sci. Rep. 12, 3812 (2022).
Schneider, F. et al. Assessment of plasma catestatin in COVID-19 reveals a hitherto unknown inflammatory activity with impact on morbidity-mortality. Front. Immunol. 13, 985472 (2022).
Borovac, J. A. et al. Catestatin in acutely decompensated heart failure patients: insights from the CATSTAT-HF study. J. Clin. Med. 8, 1132 (2019).
Xu, W. et al. Plasma catestatin: a useful biomarker for coronary collateral development with chronic myocardial ischemia. PLoS ONE 11, e0149062 (2016).
Meng, L. et al. Plasma catecholamine release-inhibitory peptide catestatin in patients with essential hypertension. J. Cardiovasc. Med. 12, 643–647 (2011).
Liu, L. et al. Plasma levels and potential roles of catestatin in patients with coronary heart disease. Scand. Cardiovasc. J. 47, 217–224 (2013).
Zhu, D. et al. Correlation of plasma catestatin level and the prognosis of patients with acute myocardial infarction. PLoS ONE 10, e0122993 (2015).
Mahapatra, N. R. et al. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J. Clin. Investig. 115, 1942–1952 (2005).
Gayen, J. R. et al. Role of reactive oxygen species in hyperadrenergic hypertension: biochemical, physiological, and pharmacological evidence from targeted ablation of the chromogranin a (Chga) gene. Circ. Cardiovasc. Genet. 3, 414–425 (2010).
Avolio, E. et al. Antihypertensive and neuroprotective effects of catestatin in spontaneously hypertensive rats: interaction with GABAergic transmission in amygdala and brainstem. Neuroscience 270, 48–57 (2014).
Fung, M. M. et al. Direct vasoactive effects of the chromogranin A (CHGA) peptide catestatin in humans in vivo. Clin. Exp. Hypertens. 32, 278–287 (2010).
Rabbi, M. F. et al. Reactivation of intestinal inflammation is suppressed by catestatin in a murine model of colitis via M1 macrophages and not the gut microbiota. Front. Immunol. 8, https://doi.org/10.3389/FIMMU.2017.00985 (2017).
Rabbi, M. F., Labis, B., Metz-Boutigue, M. H., Bernstein, C. N. & Ghia, J. E. Catestatin decreases macrophage function in two mouse models of experimental colitis. Biochem. Pharm. 89, 386–398 (2014).
Dasgupta, A. et al. Catestatin improves insulin sensitivity by attenuating endoplasmic reticulum stress: In vivo and in silico validation. Comput. Struct. Biotechnol. J. 18, 464–481 (2020).
Gayen, J. R. et al. A novel pathway of insulin sensitivity in chromogranin A null mice: a crucial role for pancreastatin in glucose homeostasis. J. Biol. Chem. 284, 28498–28509 (2009).
Wolkersdorfer, M., Laslop, A., Lazure, C., Fischer-Colbrie, R. & Winkler, H. Processing of chromogranins in chromaffin cell culture: effects of reserpine and alpha-methyl-p-tyrosine. Biochem. J. 316, 953–958 (1996).
Biswas, N., Curello, E., O’Connor, D. T. & Mahata, S. K. Chromogranin/secretogranin proteins in murine heart: myocardial production of chromogranin A fragment catestatin (Chga(364-384)). Cell Tissue Res. 342, 353–361 (2010).
Brereton, M. F. et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat. Commun. 5, 1–11 (2014).
Nakagawa, R. et al. Circularity of islets is a distinct marker for the pathological diagnosis of adult non-neoplastic hyperinsulinemic hypoglycemia using surgical specimens. Diagn. Pathol. 18, https://doi.org/10.1186/S13000-023-01403-Y (2023).
Bak, D. W., Bechtel, T. J., Falco, J. A. & Weerapana, E. Cysteine reactivity across the sub-cellular universe. Curr. Opin. Chem. Biol. 48, 96 (2019).
Marselli, L. et al. Arginase 2 and polyamines in human pancreatic beta cells: possible role in the pathogenesis of type 2 diabetes. Int J. Mol. Sci. 22, 12099 (2021).
Abramov, G. et al. Intermediate products of purine metabolism in an experimental model of pancreatic necrosis. Acta Bio. Med. Atenei Parm. 93, 2022298 (2022).
Sharp, G. W. G. & Straub, S. G. Evolving insights regarding mechanisms for the inhibition of insulin release by norepinephrine and heterotrimeric G proteins. Am. J. Physiol. Cell Physiol. 302, 1687–1698 (2012).
Hampton, R. F., Jimenez-Gonzalez, M. & Stanley, S. A. Unravelling innervation of pancreatic islets. Diabetologia 65, 1069 (2022).
Tatemoto, K. et al. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature 324, 476–478 (1986).
Mahata, S. K. et al. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J. Clin. Investig. 100, 1623–1633 (1997).
Sluga, N. et al. Physiological levels of adrenaline fail to stop pancreatic beta cell activity at unphysiologically high glucose levels. Front. Endocrinol. 13, 1013697 (2022).
Strubbe, J. H. & Steffens, A. B. Neural control of insulin secretion. Horm. Metab. Res. 25, 507–512 (1993).
Mick, G. J. & McCormick, K. L. The role of GABA in type 1 diabetes. Front. Endocrinol. 15, 1–15 (2024).
Hougaard, D. M. & Larsson, L. I. Localization and possible function of polyamines in protein and peptide secreting cells. Med. Biol. 64, 89–94 (1986).
Hougaard, D. M., Nielsen, J. H. & Larsson, L. I. Localization and biosynthesis of polyamines in insulin-producing cells. Biochem. J. 238, 43–47 (1986).
Welsh, N. & Sjöholm, A. Polyamines and insulin production in isolated mouse pancreatic islets. Biochem. J. 252, 701–707 (1988).
Welsh, N. A role for polyamines in glucose-stimulated insulin-gene expression. Biochem. J. 271, 393–397 (1990).
Sjöholm, Å., Arkhammae, P., Berggren, P. O. & Andersson, A. Polyamines in pancreatic islets of obese-hyperglycemic (ob/ob) mice of different ages. Am. J. Physiol. Cell Physiol. 280, https://doi.org/10.1152/AJPCELL.2001.280.2.C317/ASSET/IMAGES/LARGE/H00210308003.JPEG (2001).
Lenzen, S. & Rustenbeck, I. Effects of IP3, spermine, and Mg2+ on regulation of Ca2+ transport by endoplasmic reticulum and mitochondria in permeabilized pancreatic islets. Diabetes 40, 323–326 (1991).
Mahata, S. K., Mahata, M., Wakade, A. R. & O’Connor, D. T. Primary structure and function of the catecholamine release inhibitory peptide catestatin (chromogranin A344−364): identification of amino acid residues crucial for activity. Mol. Endocrinol. 14, 1525–1535 (2000).
Mahata, S. K. et al. Catecholamine secretory vesicle stimulus-transcription coupling in vivo. J. Biol. Chem. 278, 32058–32067 (2003).
Arrojo e Drigo, R. et al. New insights into the architecture of the islet of Langerhans: a focused cross-species assessment. Diabetologia 58, 2218–2228 (2015).
Guyot, M. et al. Pancreatic nerve electrostimulation inhibits recent-onset autoimmune diabetes. Nat. Biotechnol. 37, 1446–1451 (2019).
Christoffersson, G., Ratliff, S. S. & von Herrath, M. G. Interference with pancreatic sympathetic signaling halts the onset of diabetes in mice. Sci. Adv. 6, https://doi.org/10.1126/sciadv.abb2878 (2020).
Taborsky, G. J., Mei, Q., Bornfeldt, K. E., Hackney, D. J. & Mundinger, T. O. The p75 neurotrophin receptor is required for the major loss of sympathetic nerves from islets under autoimmune attack. Diabetes 63, 2369–2379 (2014).
Grisanti, L. A. et al. Leukocyte-expressed β2-adrenergic receptors are essential for survival after acute myocardial injury. Circulation 134, 153–167 (2016).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 1–7 (2017).
Tang, K. & Mahata, S. K. Determination of catecholamines in a small volume (25 μL) of plasma from conscious mouse tail vein. In Methods in Molecular Biology. Chromaffin Cells: Methods and Protocols. (ed. Borges, R.) 331–342 (Springer, 2022).
Haag, S. M. & Murthy, A. Murine monocyte and macrophage culture. Bio Protoc. 11, https://doi.org/10.21769/BIOPROTOC.3928 (2021).
Bohman, S., Andersson, A. & King, A. No differences in efficacy between noncultured and cultured islets in reducing hyperglycemia in a nonvascularized islet graft model. Diabetes Technol. Ther. 8, 536–545 (2006).
Shariatgorji, M. et al. Comprehensive mapping of neurotransmitter networks by MALDI–MS imaging. Nat. Methods 16, 1021–1028 (2019).
Jati, S. https://biorender.com/gsezj37 (2025).
Jati, S. https://BioRender.com/ip4b77m (2025).
Jati, S. https://BioRender.com/bxbimfu (2025).
Acknowledgements
E.M. is funded by a Rubicon grant from the Dutch Organisation for Knowledge and Innovation in health, healthcare and well-being (ZonMw: 452021310) and the Swedish Research Council (2023-05647). G.C. is supported by grants from the Swedish Research Council (2018-02314 and 2023-02900), the Swedish Society for Medical Research, the Child Diabetes Foundation, and the Göran Gustafsson Foundation. K.S. is supported by Swedish Diabetes Foundation, O.E. och Edla Johanssons, Sederholm, Magnus Bergvalls Stiftelse, the Ernfors Fund, Nils Erik Holmstens, Diabetesfonden, P.O. Zetterlings stiftelse. T.V.R. is supported by the Swiss National Science Foundation (P2BSP3_200177 to T.V.R.) and Larry L. Hillblom Foundation (2023-D-012-FEL to T.V.R.). S.K.M. is supported by grants from the National Institutes of Health (1 R21 AG072487-01, 1 R21 AG080246-01, 1 R21 AG078635-01A1). This study was supported by the Science for Life Laboratory (SciLifeLab) and Uppsala University Research Infrastructure funding.
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
S.K.M. was responsible for mouse husbandry, peptide treatments, and harvesting the pancreata. He conducted GTT, in vivo hormone measurements, and PTT experiments with help from K.T., analyzed the data and made the graphics. K.T. and M.R. ran the MSD kit on the mouse samples. S.K. performed H&E staining on histological slides. S.K. and S.J. ran Western blot on pancreatic islets. E.M.M., H.I., D.Ep., and M.B. have performed H&E stainings, fluorescent stainings, and image analysis. T.V.R. performed and analysed the islet GSIS. D.Ep. performed immunoblotting. M.N. and A.M.N. sectioned, prepared, and ran the pancreatic slices in the Maldi-MS. M.N., A.M.N., A.M.M., and E.T.J. performed data analysis. D.Es. provided human plasma samples and K.S. provided NOD mice material. S.J. made the working hypothesis cartoon. E.M.M. assisted with data interpretation and compiled the manuscript. G.C., P.E.A., S.K.M. and E.T.J. provided expertise. The manuscript was written by E.M.M., G.C., and S.K.M. All authors contributed in writing and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following competing interests: A.M.N. and P.E.A. are cofounders of Tag-On AB, and S.K.M. is the founder of CgA Therapeuticals, Inc. and co-founder of Siraj Therapeutics, Inc. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Peer review
Peer review information
Communications Biology thanks Ron Piran and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muntjewerff, E.M., Epremidze, D., Nezhyva, M. et al. Chromogranin A and catestatin regulate pancreatic islet homeostasis, endocrine function, and neurotransmitter signaling. Commun Biol 8, 1684 (2025). https://doi.org/10.1038/s42003-025-09135-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-09135-z