Abstract
Sleep benefits memory consolidation through periodic sleep spindle activity and associated memory reactivations. The temporal organization of spindles in “trains” is considered a critical sleep mechanism for the timed and repeated reactivation of memories. Evidence suggests that a timely phase-locking between slow oscillations (SO) and spindles facilitates learning-related synaptic plasticity. Here, we investigated the contribution of spindles’ clustering and coupling with SO in motor memory consolidation by promoting local synaptic depression in sensorimotor cortical regions through upper-limb immobilization following motor sequence learning. Our results reveal that the cluster-based organization of spindles is independent of daytime sensorimotor experience, while leading to distinct overnight behavioral outcomes. Interestingly, immobilization induced a phase shift in the SO-spindle coupling for spindles grouped in trains, but not when isolated outside trains. We demonstrate that spindle trains may promote skill-specific strengthening of motor memories, while isolated spindles may instead create memory-instability conditions leading to enhanced skill generalization.

Similar content being viewed by others
Introduction
Behind every learned motor skill lies a period of practice and rehearsal1,2. However, although the repeated practice of a motor skill is crucial for its initial acquisition, developing an effective movement representation is not only a result of practice3. The newly formed memory continues to be processed “offline” during waking and sleeping hours following practice. This offline period offers a privileged time window for memory consolidation, which relates to the process whereby newly acquired and relatively labile memories are transformed into enhanced and more stable memory traces4,5. Following its initial acquisition, the memory trace is thought to be dynamically maintained during wakefulness and actively reprocessed during a subsequent sleep period, allowing its strengthening and thus influencing its generalizability3,6,7,8. A daytime nap or a night of sleep has long been shown to play a crucial role in the strengthening and transformation of motor memories during consolidation9,10,11,12.
Another important aspect of motor learning involves the ability to generalize the acquired skill to another one or another effector, such as inter-limb transfer. This fundamental principle underlies our capacity to perform unpracticed motor skills or learn new ones more easily7. While the role of sleep in the skill-specific strengthening of motor memories during consolidation has been widely studied, evidence also showed that it can promote their generalization7,13,14. Indeed, recent theoretical evidence suggests that learning contexts creating conditions of memory instability may be critical for the generalization of motor skills7,14. Instability is thought to make a memory vulnerable to interference and even forgetting, following its initial formation or reactivation, with the loss of detailed knowledge reducing memory specificity and enabling the development of generalizable knowledge7. Hence, it remains to be determined whether sleep consolidation mechanisms may offer optimal conditions for motor memory strengthening and memory-instability conditions supporting the creation of generalized knowledge4,7,15.
Growing evidence suggests that brief bursts of sigma-like thalamocortical oscillations, known as sleep spindles, during non-rapid eye movement (NREM) sleep play a critical role in the offline covert reactivation of motor memories during consolidation, resulting in overnight/nap memory improvements9,11,14,16,17,18,19. Sleep spindle activity is an electrophysiological hallmark of NREM-stage 2 (NREM2) sleep involving short (0.3–2 s) synchronous bursts of waxing and waning 11–16 Hz oscillations8,14,18. Boutin and colleagues (2020)8 recently developed a framework for motor memory consolidation that outlines the essential contribution of the clustering and hierarchical rhythmicity of spindle activity during this sleep-dependent process. More specifically, sleep spindles tend to cluster in “trains” on a low-frequency time scale of approximately 50 s (~0.02 Hz infraslow rhythm) during NREM sleep, interspersed with relative spindle-free periods where sporadic isolated spindles may occur. This infraslow periodicity has been considered a crucial neurophysiological regulatory mechanism providing transient temporal windows of optimal neural conditions for the reactivation of memory traces through spindle activity20,21,22. In addition to the periodic clustering of spindles, recent findings unveiled a recurring pattern where spindles tend to reoccur approximately every 3–4 s during trains (~0.2–0.3 Hz mesoscale rhythm). In theory, such inter-spindle intervals during trains represent periods of refractoriness that are assumed to be crucial for the timely and organized segregation of spindle-induced memory reactivations, thus regulating the cyclic interference-free reprocessing of memory traces for efficient memory consolidation8,18,23,24. However, recent evidence indicates that spindle trains during NREM2 sleep contribute more significantly to motor memory consolidation after motor sequence learning than those occurring during NREM3 sleep18. Importantly, the contribution and dynamics of sleep spindles appear to depend on the nature of the practiced task25. Therefore, in this study, we employed a motor sequence learning paradigm to further investigate the specific role of NREM2 sleep spindle trains.
Accompanying this multi-scale rhythmicity of spindle activity, it has been proposed that cross-frequency coupling and hierarchical nesting of NREM sleep rhythms may be critical for memory consolidation. Current trends postulate that slow oscillations (SO) (SO; 0.5–1.25 Hz) confer a temporal window for spindles to occur in their excitable up-states and that a timely phase-locking facilitates the induction of persistent synaptic plastic changes26,27,28,29,30,31,32,33 (see34 for a review). At the synaptic level, post-learning neuroplasticity has further been shown to be mediated by inter-hemispheric sleep regulations over localized sensorimotor region19,35,36, suggesting that local regulations of both SOs and spindles reflect learning-dependent brain plasticity and skill consolidation during sleep. Indeed, slow-wave activity (0.5–4 Hz) is now established as a marker of homeostatically regulated sleep pressure and synaptic downscaling during NREM sleep37. In an elegant study, Huber and colleagues (2006)36 transiently promoted local synaptic depression in sensorimotor regions using a short-term upper-limb immobilization paradigm. Results revealed that the immobilization procedure induced local homeostatic changes (i.e., reduced spectral power mainly in the SO frequency band) in the contralateral affected-limb sensorimotor cortex that were associated with deteriorations in motor performance. Moreover, Debarnot and colleagues (2021)38 revealed that sleep spindle activity is also locally affected by immobilization-induced synaptic depression. Thus, upper-limb immobilization has proven to be an effective paradigm for investigating the causal link between daytime sensorimotor experience and sleep-dependent memory consolidation, as it induces cortical synaptic changes that modulate sleep characteristics36,38,39,40. However, the behavioral effects of sensorimotor restriction on motor sequence learning remain unclear. In a recent study, King et al.41 used a similar paradigm following motor sequence learning but, unexpectedly, did not observe any performance deterioration after 6 h of immobilization. As shown for visuomotor adaptation tasks, the immobilization procedure may not have been long enough to produce measurable effects42. Nevertheless, changes in sleep-EEG dynamics have been reported across different types of tasks, even with reduced practice36,38, suggesting that these effects may stem primarily from synaptic efficiency changes induced by sensorimotor restriction rather than from task-specific features. Recent studies further demonstrated that a fine-tuned SO-spindle coupling depends on prior sensorimotor experience and structural brain integrity25,29,30. Considering that SOs and spindles are involved in synaptic consolidation, the potential contribution of spindles’ clustering and the precision of the SO-spindle coupling in the strengthening and generalization of motor skills during consolidation remains to be explored.
Using a short-term upper-limb immobilization procedure, combined with the recording of behavioral and electroencephalographic (EEG) night sleep measures, the aim of the present study was twofold: (i) to determine the effects of daytime sensorimotor experience on local sleep spindle expression (i.e., clustering and rhythmicity) and the integrity of the cross-frequency coupling between SOs and spindles, and (ii) to evaluate their functional contribution in the consolidation and generalization of motor skills. Given that upper-limb immobilization disrupts the balance of inter-hemispheric inhibition40, a process closely tied to motor learning and generalization43, it can be hypothesized that transient upper-limb immobilization would impact the consolidation and generalization of memory traces. Adopting a time-based clustering approach, we hypothesized that the rhythmic occurrence of spindles in trains would confer favorable conditions for efficient reprocessing and consolidation of the memory trace, supported by an effective plasticity-dependent SO-spindle coupling compared to spindles occurring in isolation. Isolated spindles are instead expected to impair skill-specific motor memory strengthening through ineffective, non-recurring memory reactivations18 or impaired SO-spindle coupling, thus rendering the memory trace unstable and prone to generalization.
Results
Thirty right-handed participants were equally divided into a left upper-limb immobilization group (IMMO) or a control group without immobilization (CTRL). A 13-h immobilization procedure was administered immediately following the practice of a finger motor-sequence task to transiently promote local synaptic depression in contralateral sensorimotor cortical regions. Sleep EEG recordings were collected the night before (acclimatization night) and after motor sequence learning (experimental night). Participants were instructed to repeat an 8-element motor sequence task as quickly and accurately as possible. Based on evidence that speed is a sensitive measure of sleep-dependent motor memory consolidation44, motor performance was assessed using the tapping speed of complete sequences, reflected by the response time (RT) (the interval between two consecutive keypresses). The mean RT was computed for each block composing the motor sequence learning phase, as well as during test blocks before and after the experimental night (Fig. 1). The ability to transfer or generalize the newly acquired skill to another one (new sequence) or another effector (i.e., inter-limb transfer) was also assessed after the experimental night. Analyses were conducted on the temporal cluster-based organization of sleep spindles, SO-spindle coupling, and their relationship with behavioral performance. As described by Huber et al.36, the effect of the immobilization procedure on sleep-EEG activity is expected to occur mainly during the first 20 min of sleep, returning to baseline within the first hour. Accordingly, we analyzed sleep spindle and SO activity in NREM2 epochs both during the first 20 min of sleep and across the whole night to capture potential temporal dynamics of the induced changes. See “Methods” for further details.
Sleep EEG recordings of 30 participants were acquired during two consecutive nights. Between the two nights, in the morning, participants were trained on an 8-element finger movement sequence with the left hand. Immediately after training, one-half of the participants were left-hand immobilized (n = 15, IMMO group) for a period of 13 h, while the other half were not (n = 15, CTRL group). Following the immobilization or control procedure, all participants were tested on the trained motor sequence during pre-night and post-night tests. They were then evaluated on two transfer tests: an inter-manual transfer test (same motor sequence performed with the right-hand fingers), and a new motor-sequence test (unpracticed motor sequence performed with the left-hand fingers). EEG electroencephalography.
Control of the immobilization procedure
To control for the correct immobilization of the left upper limb in the IMMO group, a mixed ANOVA was performed on the mean velocity score computed from the accelerometer with the between-subject factor CONDITION (IMMO, CTRL) and the within-subject factor LATERALITY (left, right). The analysis revealed significant main effects for the factor CONDITION (F(1, 28) = 21.0, p < 0.001, ɳ2p = 0.43), and LATERALITY (F(1, 28) = 164, p < 0.001, ɳ2p = 0.85), as well as a significant CONDITION × LATERALITY interaction (F(1, 28) = 111, p < 0.001, ɳ2p = 0.80). Holm post-hoc tests revealed that the velocity score of the left limb was lower in the IMMO group than in the CTRL group (p < 0.001), as well as compared to the right limb of the CTRL group (p < 0.001) and IMMO group (p < 0.001), which did not differ from each other (p = 0.75). These findings thus guarantee compliance with the immobilization procedure in the IMMO group, whose participants used their left upper limb significantly less than their right upper limb, and less than both upper limbs in the CTRL group.
Behavioral results
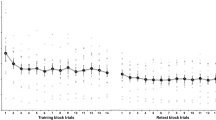
The upper panel of the Fig. 2 shows the mean RTs measured for each test block (Fig. 2a). Analyses regarding the number of accurately typed sequences are available in Supplementary Information (Supplementary Fig. 1). A mixed ANOVA using a CONDITION (IMMO, CTRL) × BLOCK (pre-test, post-test) factorial design, with repeated measures on the BLOCK factor, was first performed to ensure that the two groups did not differ in terms of performance before and after the acquisition phase. The analysis revealed a significant effect of the factor BLOCK (F(1, 28) = 102, p < 0.001, ɳ2p = 0.79), indicating performance improvements during training for both groups. The analysis failed to detect a significant effect of the factor CONDITION (F(1, 28) = 0.93, p = 0.34, ɳ2p = 0.03), nor a BLOCK × CONDITION interaction (F(1, 28) = 0.44, p = 0.51, ɳ2p = 0.02), suggesting that performance before the immobilization procedure did not differ between the IMMO and CTRL groups. Analyses of the learning curves for both groups during acquisition, in terms of RT and tapping speed, are presented in the Supplementary Information (Supplementary Fig. 2). To compare changes in performance over the 13-h retention interval, a mixed ANOVA was performed using a CONDITION (IMMO, CTRL) × BLOCK (post-test, pre-night) factorial design, with repeated measures on the factor BLOCK. The analysis revealed a significant main effect of the factor BLOCK (F(1, 28) = 7.30, p = 0.012, ɳ2p = 0.21), suggesting a deterioration in performance during the day for both groups. The analysis failed to detect a main effect of the factor CONDITION (F(1, 28) = 0.60, p = 0.44, ɳ2p = 0.02) and a CONDITION × BLOCK interaction (F(1, 28) = 0.04, p = 0.84, ɳ2p < 0.01).
a Mean response time for each test block in the IMMO group (purple) and CTRL group (pink). The colored circles with a black border represent the group average, and the bar represents the standard error of the mean. Individual data points are displayed as small colored circles (n = 15 for each group and each test block, with the exception of the transfer test blocks where n = 13 for the IMMO group). The stars represent p-values associated with mixed ANOVA tests. b Performance changes between each test block in the IMMO and CTRL groups. The curved lines indicate the distribution of data, the dark bars represent the mean of the distribution, and the lighter areas surrounding the mean represent the standard error of the means. Individual data points are displayed as colored circles (n = 15 for each group, except for new-sequence skill transfer, where n = 13 for the IMMO group). The star represents the p-value associated with one-sample ANOVA tests. *p < 0.05, **p < 0.01, ***p < 0.001. Inter-manual T inter-manual transfer; New seq T new-sequence transfer; IM inter-manual; NS new sequence.
To compare changes in performance after the experimental night, a mixed ANOVA was performed using a CONDITION (IMMO, CTRL) × BLOCK (pre-night, post-night) factorial design, with repeated measures on the factor BLOCK. The analysis revealed a significant main effect of the factor BLOCK (F(1, 28) = 29.4, p < 0.001, ɳ2p = 0.51) but failed to detect a main effect of the factor CONDITION (F(1, 28) = 0.25, p = 0.62, ɳ2p < 0.01) nor a CONDITION × BLOCK interaction (F(1, 28) = 0.74, p = 0.40, ɳ2p = 0.03). Thus, both groups improved after the night, but overnight skill consolidation were not significantly different.
To compare skill generalizability between groups, a mixed ANOVA was performed using a CONDITION (IMMO, CTRL) × BLOCK (post-night, inter-manual transfer, new-sequence transfer) factorial design, with repeated measures on the factor BLOCK. Two participants in the IMMO group were removed from this analysis as they did not perform the new-sequence transfer test. The analysis revealed a significant effect of the factor BLOCK (F(2, 52) = 30.9, p < 0.001, ɳ2p = 0.54) and a significant CONDITION × BLOCK interaction (F(2, 52) = 4.64, p = 0.014, ɳ2p = 0.15) without detecting a significant main effect of the factor CONDITION (F(1, 26) = 0.19, p = 0.67, ɳ2p < 0.01). Holm’s post-hoc comparisons revealed significant performance decreases from the post-night test to the inter-manual transfer test in the CTRL group (p < 0.001), but not in the IMMO group (p = 0.17). Also, skill generalization toward a new motor sequence was limited in both the CTRL (p < 0.001) and IMMO (p < 0.001) groups, as expressed by significant performance decreases from the post-night test to the new-sequence transfer test. The IMMO group showed a significant decrease in performance between the inter-manual transfer test and the new-sequence transfer (p = 0.002), in contrast to the CTRL group (p = 1). Finally, to thoroughly evaluate new-sequence skill generalization, a mixed ANOVA was performed using a CONDITION (IMMO, CTRL) × BLOCK (pre-test, new-sequence transfer) factorial design, with repeated measures on the factor BLOCK. The analysis revealed a significant effect of the factor BLOCK (F(1, 26) = 22.4, p < 0.001, ɳ2p = 0.46) and a significant CONDITION × BLOCK interaction (F(1, 26) = 5.69, p = 0.025, ɳ2p = 0.18), but no significant main effect of the factor CONDITION (F(1, 26) = 0.28, p = 0.61, ɳ2p = 0.01). Holm’s post-hoc comparisons indicated a significant performance increase from the pre-test to the new-sequence transfer test in the CTRL group (p < 0.001), but not in the IMMO group (p = 0.48). These results suggest that the CTRL group exhibited partial skill generalization, performing the new unpracticed sequence better than at pre-test, whereas the IMMO group showed no such improvement.
The lower panel of Fig. 2 depicts performance changes between each test blocks (Fig. 2b). One-way ANOVAs with the between-participant factor CONDITION (IMMO, CTRL) were performed respectively to assess performance changes during acquisition (post-test–pre-test), daytime changes (pre-night–post-test), skill consolidation (post-night–pre-night), inter-manual skill transfer (inter-manual transfer test–post-night), and new-sequence skill transfer (new-sequence transfer test–post-night). For the acquisition, the analysis failed to detect a significant CONDITION effect (F(1, 28) = 0.002, p = 0.96), revealing similar gains acquired during training for the IMMO (M = 39.5 ± 2.4%) and CRTL (M = 39.7 ± 3.1%) groups. Regarding daytime changes, the analysis also failed to detect a significant CONDITION effect (F(1, 28) < 0.001, p = 0.99), revealing similar performance changes throughout the day for the IMMO (M = −11.8 ± 6.4%) and CTRL (M = −11.9 ± 3.6%) groups. For skill consolidation, the analysis failed to detect a significant CONDITION effect (F(1, 28) = 3.48, p = 0.074), though there was a trend toward greater gains in the CTRL group (M = 16.9 ± 2.3%) compared to the IMMO group (M = 10.7 ± 2.4%). For the inter-manual skill transfer, the analysis failed to detect a significant CONDITION effect (F(1, 28) = 1.01, p = 0.32), with both the IMMO (M = −19.5 ± 7.2%) and CTRL (M = −30.0 ± 7.6%) groups showing similar performance declines when performing the sequence with the unpracticed right hand compared to the trained left hand. Finally, regarding new-sequence skill transfer, the analysis revealed a significant CONDITION effect (F(1, 26) = 7.08, p = 0.013), indicating a larger performance decrease in the IMMO group (M = −54.4 ± 7.9%) when performing an unpracticed motor sequence compared to the CTRL group (M = −31.6 ± 4.1%).
Effect of sensorimotor restriction on slow oscillation spectral power
To assess whether the short-term sensorimotor restriction led to a reduction in SO spectral power, which reflects synaptic efficacy35,36, a Student’s t permutation test for independent samples (1000 permutations) was performed to obtain the statistical topographic map of the spectral power difference in the SO frequency band between the IMMO and CTRL groups (Fig. 3). The analysis revealed significant decreases in SO power in the IMMO group compared to the CTRL group on multiple electrodes, which are primarily located over sensorimotor cortical regions contralateral to the immobilized limb, after correction for multiple comparisons using the Benjamini–Hochberg procedure45 to control the false discovery rate (Ntests = 63). Hence, the present result shows a general decrease in the spectral power of SOs during the first 20 min of NREM2 sleep following the 13 h of upper-limb immobilization. We also performed supplementary analyses on the C4 derivation, showing that the difference in the SO power between the two groups fades when considering the whole night (Supplementary Fig. 3). To control for baseline differences between groups in SO spectral power, we performed the same analyses on the acclimatization night. No group differences were found in SO power during the first 20 min of NREM2 sleep or across the whole night, after correction for multiple comparisons using the Benjamini–Hochberg procedure to control the false discovery rate (Ntests = 63).
Topographical statistical map of the difference in SO (0.5–1.25 Hz) spectral power between the IMMO and CTRL groups during the first 20 min of NREM2 sleep (IMMO-CTRL contrast). The color bar represents the t-test values. Negative t values (blue) represent lower spectral power for the IMMO group (n = 15) compared with the CTRL group (n = 15). White areas represent the non-significant differences between groups after the Student’s t permutation test (1000 permutations). The red dots highlight the electrodes with statistical differences after correction for multiple comparisons using the Benjamini–Hochberg procedure to control the false discovery rate (Ntests = 63).
Sleep spindle correlates of skill consolidation and generalization
To assess the specific role of grouped and isolated sleep spindles in skill consolidation, correlation analyses were conducted between the proportion of grouped spindles relative to the total number of spindles during the first 20 min of NREM2 sleep, extracted at scalp derivation C4, and the magnitude of skill consolidation (percentage of performance changes from the pre-night test to the post-night test). One participant in the CTRL group did not show grouped spindles in the first 20 min of NREM2 sleep and was therefore excluded from the correlation analyses. A significant positive relationship was observed between the proportion of grouped spindles during the first 20 min of NREM2 sleep and the magnitude of skill consolidation in the CTRL group only (r = 0.58, p = 0.031) but not in the IMMO group (r = −0.10, p = 0.72) (Fig. 4a). We also aimed to compare the coefficient correlations between groups by conducting Fisher’s r to z transform and computing the statistical significance of the observed z-test statistic from the differences of the transformed z scores. No significant difference was found between the IMMO and CTRL groups (Z = 1.83, p = 0.07). However, it is noteworthy that large sample sizes (N = 66) are needed to detect large-sized differences of the Pearson correlation coefficient (Δr ~ 0.5)46. Considering r values of 0.10, 0.30, and 0.50 as the thresholds for small, medium, and large effect sizes, respectively46, our correlation analyses are in favor of greater involvement of grouped than isolated spindles in the memory consolidation process for the CTRL group. No significant relationship was found when the proportion of grouped spindles was computed over the whole night in the CTRL (r = −0.23, p = 0.41) and IMMO groups (r = 0.03, p = 0.92).
a Relationship between the proportion of grouped spindles (unitless), extracted at scalp derivation C4 during the first 20 min of NREM2 sleep, and the magnitude of overnight skill consolidation (in %) on the learned motor sequence in the IMMO (n = 15) and CTRL (n = 14) groups. b Relationship between the proportion of grouped spindles (unitless), extracted at scalp derivation C4 during the first 20 min of NREM2 sleep, and the magnitude of inter-manual skill transfer (in %) in the IMMO (n = 15) and CTRL (n = 14) groups. c Relationship between the proportion of grouped spindles (unitless), extracted at scalp derivation C4 during the first 20 min of NREM2 sleep, and the magnitude of new-sequence skill transfer (in %) in the IMMO (n = 13) and CTRL (n = 14) groups. Individual data (colored circles) and trend lines are provided, along with Pearson’s r and associated p-values. Maps show correlations between the proportion of grouped spindles of the first 20 min of sleep computed at multiple scalp derivations with skill consolidation (d), inter-manual skill transfer (e) and new-sequence skill transfer (f) for the IMMO (right) and CTRL (left) groups. Colors represent Pearson’s correlation coefficient values, and white dots indicate significant-level p-values uncorrected for multiple comparisons. The red dot represents significant-level p-value after correction for multiple comparisons using the Benjamini–Hochberg procedure (Ntests = 30).
We also assessed the role of grouped spindles in the ability to generalize the acquired skill to another one or another effector (inter-limb transfer). To that end, we separately correlated the proportion of grouped spindles during the first 20 min of NREM2 sleep, extracted at scalp derivation C4, with the magnitude of (i) inter-manual skill transfer (percentage of performance changes from the post-night test to the inter-manual transfer test) and (ii) new-sequence skill transfer (percentage of performance changes from the post-night test to the new-sequence transfer test). As mentioned previously, two participants of the IMMO group did not undergo the new-sequence transfer test and were therefore excluded from this analysis. No significant relationship was found between the proportion of grouped spindles and the magnitude of inter-manual skill transfer in the IMMO group (r = −0.20, p = 0.47) and in the CTRL group (r = −0.21, p = 0.48) (Fig. 4b). Similarly, no significant relationship was found when the proportion of grouped spindles was computed over the whole night in the CTRL (r = 0.23, p = 0.41) and IMMO groups (r = −0.27, p = 0.33). Interestingly, though, the analysis revealed a significant negative relationship between the proportion of grouped spindles and the magnitude of new-sequence skill transfer in the IMMO group only (r = −0.70, p = 0.008) but not in the CTRL group (r = −0.33, p = 0.26) (Fig. 4c). Hence, we also aimed to compare the coefficient correlations between groups by conducting Fisher’s r to z transform. No significant difference was found between the IMMO and CTRL groups (Z = 1.20, p = 0.23). Considering r values of 0.10, 0.30, and 0.50 as the thresholds for small, medium, and large effect sizes, respectively46, our correlation analyses are in favor of greater involvement of isolated than grouped spindles in the memory generalization process for the IMMO group; a higher proportion of isolated spindles enhances the ability to transfer or generalize the newly acquired skill to another one (new sequence). No significant relationship was found when considering the whole night, in the CTRL (r = 0.04, p = 0.89) and IMMO groups (r = −0.32, p = 0.29).
The same analyses were conducted across multiple scalp derivations (Fig. 4d, e, f), illustrating the topographical distribution and spatial dynamics of the relationship between the proportion of grouped spindles during the first 20 min of NREM2 sleep and measures of skill consolidation, inter-manual skill transfer and new-sequence skill transfer. Correlation analyses of the proportion of grouped spindles across different scalp derivations are also provided in the Supplementary Information (Supplementary Fig. 4).
Additional analyses regarding the effect of sensorimotor restriction on sleep and spindle characteristics are provided in the Supplementary Information (see Supplementary Tables 1 and 2 for details on the sleep architecture and spindle characteristics of the experimental and acclimatization nights, respectively). An independent-samples Student’s t-test revealed no significant difference in the proportion of grouped spindles during the first 20 min of NREM2 sleep on the experimental night between the IMMO (M = 55.7 ± 3.8%) and CTRL (M = 49.3 ± 4.8%) groups (t(27) = −1.06, p = 0.30). Similarly, no significant difference was found in the proportion of grouped spindles across the whole experimental night between the IMMO (M = 43.4 ± 1.5%) and CTRL (M = 42.2 ± 1.9%) groups (t(28) = −0.50, p = 0.63).
SO-spindle phase-amplitude coupling
Phase-amplitude coupling analyses were performed to assess whether the upper limb’s transient immobilization influences the coupling between SOs and sleep spindles. These analyses were first performed on the SO-spindle concomitant events detected during the first 20 min of NREM2 sleep, at scalp derivation C4 (Fig. 5a, b). A minimum threshold of two coupled SO-spindle was set, and participants who did not meet this criterion were excluded from the current analysis (2 in the IMMO group, 4 in the CTRL group). The Rayleigh and the Watson-Williams tests were applied to test the non-uniformity of the preferred coupling phases and to compare them between the IMMO and CTRL groups. Analyses indicated a non-uniform coupling in both IMMO (θ = 0 rad, Rayleigh Z = 9.54, p < 0.001) and CTRL groups (θ = −0.83 rad, Rayleigh Z = 7.97, p < 0.001). A significant difference in the preferred coupling phase was observed (F(1, 23) = 11.9, p = 0.002) during the first 20 min of NREM2 sleep, showing a delay in the coupling of SOs and sleep spindles for participants in the IMMO group compared to their CTRL group counterparts.
a Preferred phase in radians (and mean length of the resultant vector) of slow oscillations at the peak power of sleep spindles, detected at scalp derivation C4, for the IMMO (purple) and CTRL (pink) groups during NREM2 sleep when considering the first 20 min (left) and the whole night (right). Probability density plots of phases (histograms) are also provided. b Top representation of the mean preferred phase and the circular standard deviation of the SO-spindle coupling for the IMMO and CTRL groups on a simulated slow oscillation. Bottom. Peak-locked sleep spindle group average across all concomitant events during the first 20 min of NREM2 sleep, filtered in the sleep spindle frequency band (purple and pink lines), with the bandpass-filtered SO trace (in gray) superimposed to highlight the SO-spindle coupling in both the IMMO (left) and CTRL (right) groups. c Time-frequency maps (in dB) in the 10–20 Hz frequency range of all concomitant events detected at scalp derivation C4 during all NREM2 epochs over the whole night, using epoch windows ranging from −1 to +2.5 s around slow oscillation onset. Time-frequency maps were displayed with the average bandpass-filtered SO trace superimposed to illustrate the high synchronization between sleep spindle power and slow oscillation phase in the IMMO (top) and CTRL (bottom) groups. The stars represent p-values associated with the Watson-William test. **p < 0.01.
It is noteworthy that these analyses were performed on a limited number of coupled events (see Supplementary Table 1), with an average of 6.5 ± 1.0 events in the IMMO group and 7.9 ± 1.3 events in the CTRL group. The limited number of participants showing SO-spindle coupling with both types of spindles (grouped and isolated) during the first 20 min of NREM2 sleep does not allow us to perform such clustering analyses. Therefore, separate analyses of the SO-spindle coupling for grouped and isolated spindles were only performed on all artifact-free NREM2 epochs over the entire sleep recording (whole night).
First, for all NREM2 sleep spindles, a non-uniform SO-spindle coupling was found for participants in both the IMMO (θ = 0 rad, Rayleigh Z = 13.8, p < 0.001) and CTRL groups (θ = −0.22 rad, Rayleigh Z = 13.6, p < 0.001). No significant group difference in the preferred phase was observed (F(1, 28) = 3.77, p = 0.062), showing the absence of phase shift in the SO-spindle coupling between the IMMO and CTRL groups when considering all NREM2 sleep spindles of the whole night (Fig. 5a, c).
Second, for all NREM2 grouped spindles, a non-uniform SO-spindle coupling was found for participants in both the IMMO (θ = 0.24 rad, Rayleigh Z = 8.90, p < 0.001) and CTRL groups (θ = −0.23 rad, Rayleigh Z = 12.2, p < 0.001). A significant group difference in the preferred phase was observed (F(1,28) = 4.25, p = 0.049), suggesting that a phase shift in the SO-spindle coupling between the IMMO and CTRL groups is present when considering only grouped spindles (Fig. 6a).
Preferred phase in radians (and mean length of the resultant vector) of slow oscillations at the peak power of sleep spindles, detected at scalp derivation C4, for the IMMO (purple) and CTRL (pink) groups during NREM2 sleep of the whole experimental night, when considering only grouped spindles (a) or isolated spindles (b). Probability density plots of phases (histograms) are also provided. The star represents the p-value associated with the Watson-William test. *p < 0.05.
Third, for all NREM2 isolated spindles, a non-uniform SO-spindle coupling was found for participants in both the IMMO (θ = −0.08 rad, Rayleigh Z = 13.9, p < 0.001) and CTRL groups (θ = −0.23 rad, Rayleigh Z = 13.6, p < 0.001). Interestingly, though, no significant group difference in the preferred phase was observed (F(1,28) = 1.76, p = 0.20), showing the absence of phase shift in the SO-spindle coupling between the IMMO and CTRL groups when considering only isolated spindles (Fig. 6b).
Additional analyses regarding the number of coupled events of the experimental and acclimatization nights are provided in the Supplementary Information (see Supplementary Tables 1 and 2).
These results suggest that synaptic changes induced by transient immobilization delayed the onset of the power peak of spindles occurring in trains (grouped spindles) but not when occurring in isolation. No difference was observed between the IMMO and CTRL groups during the acclimatization night (Supplementary Fig. 5). These analyses were also extended to additional electrodes (Supplementary Fig. 6). This SO-spindle shift between the IMMO and CTRL groups was found at a subset of electrodes, depending on whether spindles occurred in trains or in isolation. These findings suggest that sensorimotor restriction may have affected the SO-spindle coupling for both grouped and isolated spindles, albeit in a spatially distinct manner.
Multi-scale fluctuations of spindle-band power
The temporal organization of sleep spindles is governed by an infraslow (~0.02 Hz) and a mesoscale rhythm (~0.2–0.3 Hz). The infraslow and mesoscale spectral profiles were computed for all artifact-free NREM2 sleep epochs during the whole experimental night (Fig. 7). An independent Student’s t-test was then performed between the IMMO and CTRL groups to compare the effect of the immobilization on the peak power frequency of the infralow periodicity. The analyses failed to reveal a significant difference (t(28) = 1.05, p = 0.30, d = 0.39), suggesting that the periodicity of spindle trains (infraslow rhythm) did not differ between the IMMO (P = 0.017 ± 0.004 Hz) and CTRL groups (P = 0.019 ± 0.005 Hz).
Grand average of the spectral profile at the scalp derivation C4, ranging from 0.001 to 0.12 Hz (a) and 0.1 to 0.6 Hz (b) frequency range of the sleep spindle frequency band (11–16 Hz) power fluctuation during NREM2 sleep episodes across the whole night. The peaks around 0.02 and 0.2 Hz for both the IMMO (purple) and CTRL (pink) groups highlight the multi-scale periodicity of spindle-band power at an infraslow and mesoscale rhythms, respectively. The lighter areas surrounding the mean represent the standard error of the means.
An independent Student’s t-test was also performed between the IMMO and CTRL groups to compare the effect of the immobilization on the peak power frequency of the mesoscale periodicity. The analyses failed to reveal a significant difference (t(28) < 0.01, p = 1, d < 0.01), suggesting that the periodicity of spindles within trains (mesoscale rhythm) did not differ between the IMMO (P = 0.21 ± 0.03 Hz) and CTRL groups (P = 0.21 ± 0.04 Hz). The present results accord with previous findings, confirming the hierarchical multi-scale periodicity in sleep spindle activity with spindle-band power fluctuations at frequencies close to 0.02 Hz and 0.2–0.3 Hz. Here, the immobilization procedure did not affect the double periodicity of the spindle-band power during NREM2 sleep.
Similar analyses were performed for the acclimatization night (Supplementary Fig. 7) and failed to detect significant differences, highlighting the stability of the multi-scale sleep spindle periodicity before and after the immobilization procedure.
Discussion
In the current study, we examined (i) the effects of daytime sensorimotor experience on local sleep spindle expression (i.e., clustering and rhythmicity) and the integrity of the cross-frequency coupling between SOs and spindles, and (ii) their functional contribution in the consolidation and generalization of motor skills. On the one hand, our findings corroborate previous work35,36 by showing significant local decreases in the SO spectral power during the first 20 min of sleep in the IMMO group (IMMO-CTRL contrast), mainly over the affected sensorimotor cortex (i.e., contralateral to the immobilized limb), reflecting the induction of local synaptic depression following immobilization36. Behaviorally, this manifested as a heterogeneous skill generalization capacity, with the IMMO group showing superior transfer to another effector (inter-manual skill transfer) compared to the CTRL group, but poorer transfer to a new motor sequence (new-sequence skill transfer). Although immobilization was expected to induce synaptic depression, our results did not reveal any significant difference between the IMMO and CTRL groups regarding spindle clustering and rhythmicity: sleep spindles tend to cluster on a low-frequency time scale of about 50 s (~0.02 Hz rhythm), during which spindles iterate every 3–4 s (~0.2–0.3 Hz rhythm), irrespective of daytime sensorimotor experience. On the other hand, alterations of the coupling between SOs and spindles during NREM2 sleep have been shown to occur locally over the affected sensorimotor cortex mainly during the first 20 min of sleep but not when considering the whole night. Interestingly, immobilization induced a phase shift in the SO-spindle coupling for grouped spindles but not for isolated spindles, supporting the hypothesis of their functional differences in memory consolidation. More specifically, our results accord with recent theories assuming that sleep spindles occurring in trains (grouped spindles) play a more critical role than isolated ones in motor skill consolidation. In contrast, isolated spindles may impair skill consolidation by providing conditions of memory instability that support the creation of generalized knowledge.
At the behavioral level, our findings revealed that both groups deteriorated performance over the day. Early learning results in a degradation of performance throughout the day, potentially due to the “early-boost” phenomenon47, or a reduction in the signal-to-noise ratio of the newly acquired memory trace48. However, immobilization of the upper limb did not significantly worsen performance deterioration during the day. This result corroborates previous findings reporting no deterioration after 6 h of immobilization following implicit motor sequence learning41. One potential explanation is that 6 h of immobilization may have been insufficient42, or alternatively, that the use of an implicit paradigm engaged consolidation mechanisms49 that are less sensitive to limb immobilization. In our study, however, even with the use of an explicit motor sequence task and 13 h of immobilization, no significant deterioration in performance was observed by the end of the day. Therefore, given that deleterious effects of immobilization have been demonstrated for other tasks, such as mental rotation50,51 and pointing tasks36,42, the limited behavioral impact observed here at the group level may stem from the specific properties of motor sequence tasks. For this type of task, the primary motor cortex appears to play a functional role during the early stages of consolidation but not at later stages (a few hours after learning)52. Thus, the delayed synaptic depression expected from immobilization over the contralateral sensorimotor cortex may not have occurred early enough to interfere with the initial, early phase of motor skill consolidation. Overall, sensorimotor restriction does not appear to be as effective for inducing within-day behavioral changes in motor sequence tasks as it is for mental rotation or motor adaptation tasks36,42,50,51. In contrast, transient limb immobilization appears to have affected the later stages of motor skill consolidation, expressed behaviorally by the magnitude of overnight skill retention performance, with a trend toward greater consolidation in the CTRL group compared to the IMMO group. This interpretation is further supported by the analyses based on the number of correct sequences, a measure previously shown to be a reliable indicator of sleep-dependent memory consolidation53,54. In the present study, this measure revealed greater skill consolidation for the CTRL group than for the IMMO group (Supplementary Fig. 1). Altogether, these findings suggest that sensorimotor restriction may have disrupted sleep-dependent memory consolidation, likely by limiting the local reactivation of task-related brain regions during sleep.
Regarding skill generalization, and consistent with previous work41,55,56, our results indicate that the IMMO group did not perform significantly worse on the inter-manual transfer test than on the post-night test, whereas the CTRL group did. The potential overuse of the non-immobilized limb in the IMMO group may have reduced the inter-hemispheric inhibition exerted by the affected hemisphere40 and, in turn, increased the excitability of the contralateral hemisphere, thereby leading to greater inter-manual skill transfer capacity compared to the CTRL group. Both groups, however, showed reduced performance on the new-sequence transfer test, highlighting a limited capacity to generalize the acquired skill to an unpracticed motor sequence. Importantly, some degree of such skill generalization was still observed in the CTRL group, whose performance on the new motor sequence exceeded pre-test levels, whereas no such effect was evident in the IMMO group. Although task difficulty between the practiced and new motor sequences was not directly controlled, the similarity of finger transitions—albeit in a different order—suggests that the observed limitation in skill generalization is unlikely to stem from differences in sequence complexity. It is worth noting that no significant between-group difference was observed in skill generalization based on the number of correct sequences performed, suggesting that RT (speed) may represent a more sensitive measure of the disruptive effects of sensorimotor restriction on skill generalization.
In line with previous work35,36, a significant decrease in the SO spectral power was found over the sensorimotor cortex of the immobilized limb during the first 20 min of NREM2 sleep in the IMMO group (IMMO-CTRL contrast). Interestingly, this early-night difference in SO power between the two groups fades when considering the whole night (Supplementary Fig. 3). Hence, SO activity may be viewed as a sensitive marker of synaptic efficacy, modulated by daytime sensorimotor experience35,36,57. In our study, the decrease in SO activity suggests that the immobilization procedure likely induced local synaptic depression during the following night, reducing synaptic efficacy in the sensorimotor cortex of the immobilized limb, as previously described36,38,39,40.
Although immobilization was expected to induce synaptic depression, our results did not reveal any significant difference between the IMMO and CTRL groups regarding spindle clustering and rhythmicity. As previously shown, current results confirmed the clustering and temporal organization of spindle activity during NREM2 sleep, irrespective of daytime sensorimotor experience: spindles tend to cluster on a low-frequency time scale of about 50 s (~0.02 Hz rhythm), during which spindles iterate every 3–4 s (~0.2–0.3 Hz rhythm)8,14,18,20,58. Thus, the temporal organization of spindles in trains appears to be an inherent clocking sleep mechanism independent of prior sensorimotor experience, regulated in a learning-independent manner and coordinated with autonomic nervous system-dependent parameters8,14,18,20,22,59. Yet, in line with recent findings of Boutin and colleagues8,14,18,19, an in-depth analysis of spindle clustering revealed a significant positive relationship between the proportion of grouped spindles over the sensorimotor cortex of the left limb during the first 20 min of sleep and the overnight skill consolidation for the CTRL group but not for the IMMO group. The findings suggest that the expected local synaptic depression caused by immobilization may have impaired the efficacy of grouped spindles, thereby disrupting the beneficial effects of repeated memory trace reactivations. Interestingly, correlation analyses were significant only when considering the proportion of grouped spindles at the beginning of the night. Indeed, daytime activity and everyday learning experiences may have reduced the incidence of spindles associated with the reactivation of the memory trace of interest across the night10,60. From a theoretical perspective, because the relevant memory trace was acquired in the morning, we may propose that sleep spindles selectively reactivate memory traces in a (pre-)defined temporal order that depends on the degree of synaptic plasticity induced by daytime sensorimotor experience. In line with the synaptic homeostasis hypothesis37 that emphasizes the role of sleep in regulating synaptic weight in the brain, supposed to be maximal at the beginning of sleep and then progressively returning to baseline, brain regions with higher synaptic efficacy following learning should be processed first during subsequent sleep. However, this remains speculative, and future studies should investigate the temporal dynamics of spindle-related memory trace reactivations throughout the night.
In contrast, however, we observed a significant negative relationship between the proportion of grouped spindles and the magnitude of new-sequence skill transfer in the IMMO group, but not in the CTRL group. Interestingly, sensorimotor restriction did not affect the proportion of grouped (and isolated) spindles (see Supplementary Table 1) but instead amplified the relative functional contribution of isolated spindles. Skill generalization to a new (related) motor skill may thus rely primarily on isolated than grouped sleep spindles, likely contributing to the impaired new-sequence skill transfer observed in the IMMO group. This finding aligns with recent studies supporting the hypothesis that sleep spindles might also be related to forgetting18,61,62,63. More specifically, Boutin and colleagues (2024)18 recently suggested that spindles occurring in trains can strengthen memory representations through their timed reactivations, while in contrast, spindles occurring in isolation may instead activate sleep mechanisms promoting memory-instability conditions leading to the clearance or decreased accessibility of the memory content. Furthermore, since the infraslow rhythm is also related to arousability, segmenting sleep into periods of high arousability (for environmental alertness) and low arousability (for memory consolidation)20, it is tempting to suggest that sporadic reactivations triggered by isolated spindles during periods of heightened alertness might result in inefficient reactivations, which could ultimately destabilize and weaken the memory trace7. At the same time, recent theoretical evidence suggests that memory instability may be critical for motor skill generalization7. Thus, the hypothesized immobilization-induced synaptic depression may have impaired the reprocessing of the memory trace during sleep through local alterations related to spindle clustering, thereby promoting its weakening by isolated spindles and, consequently, its generalization. Altogether, our results accord with recent theories assuming that sleep spindles occurring in trains (grouped spindles) play a more critical role than isolated ones in motor skill consolidation by offering optimal conditions for motor memory strengthening, while isolated spindles may instead promote memory-instability conditions supporting the creation of generalized knowledge4,7. It should be noted that no significant correlation was found between the proportion of grouped spindles and performance changes based on the number of correct sequences performed. While this measure, combining both speed and accuracy11,64, is commonly used in the sleep research literature, it may reflect distinct consolidation and generalization processes compared to those captured by speed alone. Therefore, consistent with Lustenberger et al.44, speed (RT) may represent a more sensitive measure of the disruptive effects of sensorimotor restriction on the roles of grouped and isolated spindles in memory processing.
Finally, alterations in the coupling between SOs and spindles during NREM2 sleep have been shown to occur locally over the affected sensorimotor cortex, mainly during the first 20 min of sleep, suggesting that the presumed synaptic depression induced by transient immobilization delayed the onset of the power peak of sleep spindles. Interestingly, this delay is only observed for grouped spindles and not for isolated spindles, and it persists throughout the night. Solano et al.65 recently demonstrated that the proportion of grouped spindles coupled to SOs increased locally in the sensorimotor cortex of the trained effector, thus suggesting that the SO-spindle coupling relies on previous sensorimotor experience. Hence, it is likely that altered local synaptic efficacy at the cortical level disrupted the SO-spindle coupling mostly during spindle trains. However, the origin of this altered phase shift is uncertain, as immobilization-induced cortical neuroplasticity is assumed to occur early in the night36. In a previous study, Helfrich and colleagues (2018)30 reported a phase shift in the SO-spindle coupling for older adults, compared to young adults, associated with a memory consolidation deficit. This phase shift was linked to a structural alteration of gray matter in the medial prefrontal cortex. In addition, it was described that the structural integrity of the thalamocortical fascicle influences sleep spindle density66. For example, daytime learning and sensorimotor experience can rapidly induce white matter changes67, increasing sleep spindle production during the subsequent night66. Based on the observation that sensorimotor restriction also induces white matter structural changes68, the transient alteration of white matter bundles may be a potential cause of the SO-spindle phase shift. This latter hypothesis remains speculative, and further evaluations of the effects of sensorimotor restriction on sleep spindle expression are needed. Nevertheless, the specificity of this effect on grouped spindles highlights the potential existence of distinct neurobiological mechanisms underlying and modulating the generation of grouped and isolated spindles, supporting the hypothesis of their functional differences in memory consolidation69. However, it should be noted that no relationship was found between the preferred phase of SO-spindle coupling and behavioral outcomes. Practicing the motor task in the morning may not be optimal for assessing memory consolidation60, as multiple interfering tasks and events throughout the day could weaken the memory trace of interest and reduce its synaptic strength10. Given that the engagement of spindle-related memory consolidation mechanisms depend on daytime-induced plasticity20, this may have led to a reduced incidence of spindles associated with the reactivation of the newly formed memory trace, thereby mitigating the link between spindle activity and behavioral outcomes. A study involving evening practice of the motor task, followed by synaptic depression induced via repetitive transcranial magnetic stimulation52,70, could help address this issue by minimizing daytime interference, thus enabling a more accurate assessment of spindle activity and its behavioral correlates.
Our findings confirm the multiscale periodicity of sleep spindle activity regarding spindle clustering and rhythmicity. We revealed that this temporal cluster-based organization of sleep spindles is not dependent on daytime sensorimotor experience. Furthermore, we experimentally induced a phase shift in the coupling between SOs and sleep spindles occurring in trains, while the coupling of isolated spindles remained locally largely unaffected. In line with recent evidence18, this suggests that grouped and isolated spindles exhibit different coalescence patterns and may contribute differently to memory processing, underscoring their potential distinct functional roles, as previously demonstrated for slow and fast spindles69. While sleep spindles occurring in trains may be involved in motor skill consolidation by offering optimal conditions for memory strengthening, isolated spindles may instead be involved in the weakening or even forgetting of memories by creating conditions of memory instability, which may in turn favor the development of generalized knowledge. This dissociation suggests that grouped and isolated spindles may play distinct mechanistic roles in memory processing, warranting further investigation.
Methods
Participants
Thirty healthy volunteers (16 females, mean age: 25.4 ± 4 years) were recruited by local advertisements and were randomly and equally divided into two groups: an immobilization group (IMMO; n = 15, 7 females, mean age: 25.9 ± 4 years) and a control group without immobilization (CTRL; n = 15, 9 females, mean age: 25.0 ± 4 years). All participants met the following inclusion criteria: aged between 18 and 35 years, right-handed (Edinburgh Handedness Inventory71), medication-free, without history of mental illness, epilepsy, or head injury with loss of consciousness, sleep or neurologic disorders, and no recent upper extremity injuries. The experimental protocol was approved by the “Comité de Protection des Personnes Sud-Ouest et Outre-Mer III” (ID-RCB: 2020-A01465-34) and conformed to relevant guidelines and regulations. All ethical regulations relevant to human research participants were followed. All participants gave written informed consent before inclusion. Participants were asked to maintain a regular sleep-wake cycle and to refrain from all caffeine- and alcohol-containing beverages 24 h prior to the experimentation.
Experimental design
Participants sat on a chair at a distance of 50 cm in front of a computer screen. The motor task consisted of performing as quickly and accurately as possible an 8-element finger movement sequence by pressing the appropriate response keys on a standard French AZERTY keyboard using their left, non-dominant hand fingers. The sequence to be performed (B-C-N-V-C-B-V-N, where C corresponds to the little finger and N to the index finger) was explicitly taught to the participant before training.
Test and practice blocks consisted of repeating the 8-element sequence for 30 s. Each block began with the presentation of a green cross in the center of the screen, accompanied by a brief 50-ms tone. In case of occasional errors, participants were asked “not to correct errors and to continue the task from the beginning of the sequence” (see14 for a similar procedure). At the end of each block, the color of the green imperative stimulus turned red, and participants were then required to look at the fixation cross during the 30-s rest period. This experimental protocol was designed to control the duration of practice during the test and practice blocks. Stimuli presentation and response registration were controlled using the MATLAB R2019b software from The MathWorks (Natick, MA) and the Psychophysics Toolbox extensions72.
Each participant completed two visits at the Sleep and Vigilance Center of the Hotel-Dieu Hospital (Fig. 1). The first visit served as a polysomnographic screening and acclimatization night, whereas the second visit consisted of the experimental night following motor sequence learning and the daytime immobilization or non-immobilization control procedure. Assignment to the immobilization or control group was randomized across participants. The first visit started at 9:00 pm; the experimental design was explained, and all the forms were provided. After the participants had prepared for the night, the EEG equipment was set up, and they were invited to sleep. The sleep EEG recording started approximately at 10:30 pm. The EEG cap was removed the next morning at 6:30 am, just after awakening. The experimental procedure began at 7:30 am for all participants to minimize the possible impact of circadian and homeostatic factors on individual performance and give them time to shower and have breakfast. The experimental procedure for the first visit comprised three main phases: familiarization, acquisition, and post-test (Fig. 1). First, participants underwent a brief familiarization phase during which they were instructed to repeatedly and slowly perform the 8-element sequence until they accurately reproduced the sequence three consecutive times. This familiarization was intended to ensure that participants understood the instructions and explicitly memorized the sequence of finger movements. The actual acquisition phase consisted of physically performing 16 blocks of the 8-element motor sequence with their left (non-dominant) hand fingers. The first two blocks of this acquisition phase were used as a pre-test to evaluate baseline performance. Approximately 5 min after the end of the acquisition phase, all participants performed a post-test phase consisting of two blocks. This test was briefly preceded by a physical warm-up phase to ensure that the correct sequence had been practiced. During this brief phase, participants were instructed to produce the sequence correctly once, without any speed constraints. Data from these phases are presented in the Supplementary Information (Supplementary Fig. 8). After the post-test (at 8:00 am), one-half of the participants had their left upper limb immobilized for 13 h (IMMO group), while the other-half (CTRL group) had no restrictions on the use of their left limb. The immobilization kit consisted of an orthopedic splint immobilizing the wrist and 4 fingers (DONJOY brand, “Comfort Digit” model) and an immobilization sling (DONJOY brand, GCI model). To ensure that immobilization was carried out correctly and maintained throughout the day, an accelerometer (GENEactiv Original) was placed on each participant’s wrists, whether immobilized or not.
The second visit took place at 9:00 pm following the immobilization (or control) procedure at the Sleep and Vigilance Center of the Hôtel-Dieu hospital. After removing the immobilization for the IMMO group, all participants were administered another physical warm-up phase before performing a pre-night test consisting of two blocks of the 8-element sequence before sleep time. The EEG equipment was then set up for the second recording night. The next morning, at around 7:30 am and approximately 1 h after awakening, participants carried out a physical warm-up phase before performing a post-night test consisting of two blocks of the 8-element sequence. Finally, the generalizability of the learned motor skill was assessed by conducting two transfer tests. After a warm-up phase performed with the right hand, an inter-manual transfer test was first administered to all participants to investigate skill generalizability toward the transfer from one limb to another. In this inter-manual transfer test, participants were required to perform two blocks on the original 8-element motor sequence with their unpracticed, dominant right-hand fingers (same keypresses). A transfer test with a new (unpracticed) 8-element sequence of stimuli (V-C-N-V-N-B-C-B) was then presented to differentiate sequence learning from generalized practice effects73,74. All participants were requested to perform this new motor sequence using their left (non-dominant) hand fingers. The new-sequence transfer test was preceded by a brief familiarization phase during which participants were asked to perform the new 8-element sequence with the left hand slowly until they reproduced it correctly three times in a row.
EEG-EMG data acquisition and pre-processing
EEG was acquired using a 64-channel EEG cap (actiCAP snap, BrainProducts Inc.) with slim-type electrodes (5 kΩ safety resistor) suitable for sleep recordings. For reliable sleep stage scoring, electrooculography (EOG) and electromyography (EMG) recordings were added using bipolar Ag-AgCl electrodes. The EOG components were recorded by placing a pair of electrodes laterally to both eyes. EMG bipolar electrodes were placed over the chin. All EEG, EMG, and EOG data were recorded using two battery-powered 32-channel amplifiers and a 16-channel bipolar amplifier (respectively, BrainAmp and BrainAmp ExG, Brain Products Inc.). All signals were recorded at a 1-kHz sampling rate with a 100-nV resolution. Electrode-skin impedance was kept below 5 kΩ using Abralyt HiCl electrode paste to ensure stable recordings throughout all experimental phases.
EEG data were down-sampled to 250 Hz, bandpass filtered between 0.5 and 50 Hz to remove low-frequency drift and high-frequency noise; and re-referenced to TP9 and TP10. EOG and EMG data were respectively bandpass filtered between 0.3–35 Hz and 10–100 Hz.
Statistics and reproducibility
All subjects were included in statistical analyses (IMMO: n = 15; CTRL: n = 15), except as noted. Indeed, due to the absence of events of interest during some sleep EEG epochs, statistical power may be reduced for some specific analyses; this will be outlined where appropriate. The significant threshold was set at 0.05 for all analyses. Multiple comparisons were corrected using the Benjamini–Hochberg procedure to control the false discovery rate45.
The sample size in each group was determined based on previous studies9,75,76. Error measurements refer to the standard error of the mean for non-circular data, and the circular standard deviation otherwise.
Behavioral analysis
RT was measured as the interval between two consecutive keypresses during each practice block. This performance index was described as more sensitive to the effects of sleep and the expression of relative performance gains44,77. Analyses regarding the number of accurately typed sequences are available in Supplementary Information (Supplementary Fig. 1). Also, since participants were asked to start over from the beginning of the sequence if they made any error during task production, RTs from error trials (i.e., erroneous key presses) were excluded from the analyses. To better reflect individual performance on the motor sequence task, mean RT performance was computed on accurately typed sequences (see12,78 for a similar procedure). Individual RTs were then averaged to obtain an overall estimation of the performance for each block. Acquisition of the motor sequence task was assessed by analyzing the performance changes (in percentages) from the pre-test to the post-test blocks. Daytime performance changes were computed from the post-test to the pre-night test blocks. Motor skill consolidation was assessed by analyzing the overnight RT performance changes (in percentages) from the pre-night to the post-night test blocks. The inter-manual skill transfer was quantified by the RT performance changes (in percentages) between the post-night and the inter-manual transfer test blocks. Finally, the new-sequence skill transfer, or the capacity of transferring sequence knowledge to a new (unpracticed) sequence, was quantified by the RT performance changes (in percentages) between the post-night and the new-sequence transfer test blocks.
Mixed ANOVAs with the between-subject factor CONDITION (IMMO, CTRL) and the within-subject factor BLOCK were performed on the RT data to assess the difference between groups in the magnitude of overnight motor skill consolidation and generalization. Holm post-hoc comparisons were performed in case of significant effects or interaction.
Actimetry analysis
To ensure that the upper limb was correctly immobilized, two accelerometers were placed on the participants’ wrists. The positions of both wrists were collected during the day between the acclimatization and experimental nights at a rate of 100 Hz. The velocity was computed across the day and averaged, giving us a mean velocity score for each upper limb. This score allows us to compare the degree of use of the immobilized left limb with the contralateral right limb for each participant in the IMMO group, as well as with the non-immobilized upper limbs of participants in the CTRL group. A mixed ANOVA was performed with the between-subject factor CONDITION (IMMO, CTRL) and the within-subject factor LATERALITY (left, right). Holm post-hoc comparisons were performed in case of significant effects or interaction.
EEG analysis
The artifact-free EEG signal was sleep-stage scored according to AASM guidelines79. Each 30-s epoch was visually scored as either NREM stages 1–3, REM, or wake. As described by Huber et al.36, the immobilization procedure should induce local power decreases over sensorimotor regions, mainly in the SO frequency band (0.5–1.25 Hz) during the experimental night, at least in the first 20 min of sleep. Hence, for each participant and all electrodes, we restricted the EEG power spectrum density to the SO frequency band (0.5–1.25 Hz in steps of 0.25 Hz), computed using the Welch method (4-s Hamming window with 50% overlap between windows). The average power of the SO frequency band was obtained for all artifact-free NREM2 epochs of the first 20 min of sleep. A Student’s t permutation test (1000 permutations) was performed to compare the power of the SO frequency band on each channel between the IMMO and CTRL groups. The statistical map was then corrected for multiple comparisons using the Benjamini–Hochberg procedure to control the false discovery rate45 (Ntests = 63).
Recent work has emphasized the greater contribution of NREM2 sleep spindle trains in motor memory consolidation following motor sequence learning18. Hence, the detection of sleep spindle events was conducted using all artifact-free NREM2 sleep epochs over electrode C4 of the acclimatization and experimental night. This electrode was chosen based on the a priori hypothesis that immobilization of the left upper limb should affect the right sensorimotor cortex36,38. Discrete sleep spindle events (i.e., onset and offset) were automatically detected using a wavelet-based algorithm (see18,19 for further details). Spindles were detected at the C4 derivation by applying a dynamic thresholding algorithm (based on the mean absolute deviation of the power spectrum) to the extracted wavelet scale corresponding to the 11–16 Hz frequency range and a minimum window duration set at 300 ms18,19,80 (see8 for a review). Events were considered sleep spindles only if they lasted 0.3–2 s, occurred within the 11–16 Hz frequency range, and with onsets during NREM2 sleep periods. Visual inspections in random samples were done to ensure the correct detection of sleep spindles.
Sleep spindles may be split into two categories: clusters of two or more consecutive and electrode-specific spindle events interspaced by less than or equal to 6 s were categorized as trains, in comparison to those occurring in isolation (i.e., more than 6 s between two consecutive spindles detected on the same electrode)8,18. Hence, spindles belonging to trains were categorized as grouped spindles, and those occurring in isolation were categorized as isolated spindles. To evaluate the role of this temporal organization regarding sleep-related skill consolidation and generalizability, the proportion of grouped spindles defined by the total number of grouped spindles divided by the total number of spindles was computed. Hence, the greater the number of isolated spindles, the lower the proportion of grouped spindles. A minimum threshold of one grouped spindle and one isolated spindle was set to compute this metric, to reliably reflect the balance between grouped and isolated reactivations. Pearson correlation analyses were performed to evaluate the relationship between the proportion of grouped spindles and the magnitude of overnight skill consolidation, inter-manual skill transfer, and new-sequence skill transfer. Fisher’s r to z transform was then performed to compare Pearson’s coefficients between groups.
SO events were detected for each participant separately from sleep spindle events. The method used is similar to that described by Staresina et al.31. First, the EEG data were bandpass filtered between 0.5 and 1.25 Hz (two-pass finite impulse response [FIR] bandpass filter). Only the artifact-free NREM2 and NREM3 epochs were considered. All zero-crossings were determined in the filtered signal, and SO candidates were identified as two successive positive-to-negative zero-crossings (i.e., down-states followed by up-states). SO events were retained if their duration was between 0.8 and 2 s. Finally, the amplitude of the remaining SO events was determined. They were classified as SO if they met the amplitude criteria (≥75% percentile of all SO amplitudes). Visual inspections in random samples were done to ensure the correct SO detection.
Sleep spindles and SOs were classified as concomitant events if the onset of a sleep spindle occurred within the time interval of a SO (i.e., co-occurrence of SO and spindles). For each of these concomitant events, a Hilbert transform was applied to the extracted time window surrounding the SO onset (−1 to 2.5 s around the SO onset) to extract the instantaneous phase angle of the SO component (0.5–1.25 Hz) corresponding to the spindle power peak (11–16 Hz). For each participant, the average (preferred) phase between all concomitant events was given by the synchronization index (SI)81.
Where n is the number of concomitant events, θk is the SO phase value corresponding to the spindle’s power peak of the concomitant event k. The SI is a complex number whose angle corresponds to the preferred (average) phase of this synchronization, and its absolute value gives the average length of the resultant vector, from which the circular standard deviation is calculated. For illustration purposes, time-frequency maps in the sleep spindle frequency band were provided with the superimposed SO-filtered signal. For each extracted time window (−1 to 2.5 s around the SO onset), a time-frequency decomposition across the 10–20 Hz frequency band was performed using an eight-cycle Morlet wavelet (full width at half maximum; FWHM: 3 s for a central frequency of 1 Hz). Finally, the grand average TF map and SO-filtered signal were computed for each participant.
A minimum threshold criterion for this analysis of two coupled SO-spindle events was set to prevent the resultant vector from being artificially inflated by a single event. For each group, the distribution of the preferred phase was tested by the Rayleigh test. It is noteworthy that a non-uniform distribution of the preferred phase is an indicator of coupling82. Watson-Williams tests were then used to compare the preferred phase of SO-spindle coupling between groups.
Two rhythms govern the temporal dynamics of sleep spindle activity: spindles tend to cluster on a low-frequency time scale of about 50 s (~0.02 Hz infraslow rhythm), during which individual spindles iterate every 3–4 s (~0.2–0.3 Hz mesoscale rhythm). Infraslow rhythm: the procedure to highlight the infraslow oscillation is similar to that described by Lecci et al.22. To summarize, a continuous wavelet transform was performed on the artifact-free EEG data during the full night of sleep. The power time course was calculated in the 11–16 Hz range in steps of 0.2 Hz using a four-cycle Morlet wavelet (FWHM: 1.5 s for a central frequency of 1 Hz) and extracted for all artifact-free NREM2 sleep epochs. The average power in the spindle frequency band was calculated at each time point. A symmetric 4-s moving average was applied on the power time course to all consecutive 30-s NREM2 epochs, followed by its standardization, to reduce the temporal resolution and highlight the infraslow oscillation. A second continuous wavelet transform was performed on the power time course of the spindle frequency band, at a frequency resolution of 0.001 Hz in the 0.001–0.12 Hz range, and applied to all NREM2 epochs lasting more than 120 s (constituting a NREM2 period). Lastly, the spectral profile for each participant was computed by averaging the spectrum across all NREM2 periods with a 0.5-s time step, weighted by their duration. The final spectral profile per subject was obtained by normalizing it to its own mean. To gather the spectral profile peak for each participant and its corresponding frequency, a Gaussian fit (three terms) was performed, and the maximal value of the fitted curve was extracted. This maximal value, corresponding to the infraslow rhythm peak, was then compared between groups. Mesoscale rhythm: the same procedure was applied to study the mesoscale rhythm, except for the symmetric moving average, which was not applied. After computation of the average power time course in the spindle frequency band, a second continuous wavelet transform was applied to all consecutive 30-s NREM2 epochs at a frequency resolution of 0.01 Hz in the 0.1–0.6 Hz range. Lastly, the spectral profile for each participant was computed by averaging the spectrum across all NREM2 periods with a 0.5-s time step, weighted by their duration. The final spectral profile was obtained by normalizing it to its own mean. A Gaussian fit (three terms) was performed, and the maximal value of the fitted curve was extracted. This maximal value, corresponding to the mesoscale rhythm peak, was then compared between groups.
Independent Student’s t-tests were performed between the IMMO and CTRL groups to compare the effects of the short-term upper-limb immobilization on the infralow and mesoscale rhythm peaks, respectively.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data underlying the graphs presented in the figures are available as Supplementary Data. Additional data that support the results of this study are available from the corresponding author upon reasonable request and under a formal data-sharing agreement.
Code availability
Sleep EEG data were processed using the MATLAB R2021b software from the MathWorks (Natick, MA) and the open-source Brainstorm software83. The codes for the detection and clustering of sleep spindles are available at the following GitHub repositories: https://github.com/arnaudboutin/Spindle-detection and https://github.com/arnaudboutin/Spindle-clustering. The codes for slow oscillations and infraslow-mesoscale analyses are available at https://github.com/arnaudboutin/Spindle-SO-package. The codes used to perform other analyses are available from the corresponding author upon request.
References
Krakauer, J. W., Hadjiosif, A. M., Xu, J., Wong, A. L. & Haith, A. M. Motor learning. Compr. Physiol. 9, 613–663 (2019).
Schmidt, R. A., Lee, T. D., Winstein, C. J., Wulf, G. & Zelaznik, H. N. Motor Control and Learning: A Behavioral Emphasis (Human Kinetics, 2019).
Doyon, J., Gabitov, E., Vahdat, S., Lungu, O. & Boutin, A. Current issues related to motor sequence learning in humans. Curr. Opin. Neurol. 20, 89–97 (2018).
Dudai, Y., Karni, A. & Born, J. The consolidation and transformation of memory. Neuron 88, 20–32 (2015).
Klinzing, J. G., Niethard, N. & Born, J. Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 22, 1598–1610 (2019).
Genzel, L. & Robertson, E. M. To replay, perchance to consolidate. PLoS Biol. 13, e1002285 (2015).
Robertson, E. M. Memory instability as a gateway to generalization. PLoS Biol. 16, e2004633 (2018).
Boutin, A. & Doyon, J. A sleep spindle framework for motor memory consolidation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190232 (2020).
Fogel, S. et al. Reactivation or transformation? Motor memory consolidation associated with cerebral activation time-locked to sleep spindles. PLoS ONE 12, e0174755 (2017).
Korman, M. et al. Daytime sleep condenses the time course of motor memory consolidation. Nat. Neurosci. 10, 1206–1213 (2007).
Nishida, M. & Walker, M. P. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE 2, e341 (2007).
Vahdat, S., Fogel, S., Benali, H. & Doyon, J. Network-wide reorganization of procedural memory during NREM sleep revealed by fMRI. eLife 6, e24987 (2017).
Stickgold, R. & Walker, M. P. Sleep-dependent memory triage: evolving generalization through selective processing. Nat. Neurosci. 16, 139–145 (2013).
Conessa, A., Debarnot, U., Siegler, I. & Boutin, A. Sleep-related motor skill consolidation and generalizability after physical practice, motor imagery, and action observation. iScience 26, 107314 (2023).
Dudai, Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 55, 51–86 (2004).
Barakat, M. et al. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav. Brain Res. 217, 117–121 (2011).
Barakat, M. et al. Sleep spindles predict neural and behavioral changes in motor sequence consolidation. Hum. Brain Mapp. 34, 2918–2928 (2013).
Boutin, A. et al. Temporal cluster-based organization of sleep spindles underlies motor memory consolidation. Proc. R. Soc. B Biol. Sci. 291, 20231408 (2024).
Boutin, A. et al. Transient synchronization of hippocampo-striato-thalamo-cortical networks during sleep spindle oscillations induces motor memory consolidation. Neuroimage 169, 419–430 (2018).
Fernandez, L. M. J. & Lüthi, A. Sleep spindles: mechanisms and functions. Physiol. Rev. 100, 805–868 (2020).
Lázár, Z. I., Dijk, D.-J. & Lázár, A. S. Infraslow oscillations in human sleep spindle activity. J. Neurosci. Methods 316, 22–34 (2019).
Lecci, S. et al. Coordinated infraslow neural and cardiac oscillations mark fragility and offline periods in mammalian sleep. Sci. Adv. 3, e1602026 (2017).
Schönauer, M. Sleep spindles: timed for memory consolidation. Curr. Biol. 28, R656–R658 (2018).
Antony, J. W., Schönauer, M., Staresina, B. P. & Cairney, S. A. Sleep spindles and memory reprocessing. Trends Neurosci. 42, 1–3 (2019).
Solano, A., Riquelme, L. A., Perez-Chada, D. & Della-Maggiore, V. Motor learning promotes the coupling between fast spindles and slow oscillations locally over the contralateral motor network. Cereb. Cortex 32, 2493–2507 (2022).
Chauvette, S., Seigneur, J. & Timofeev, I. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron 75, 1105–1113 (2012).
Latchoumane, C.-F. V., Ngo, H.-V. V., Born, J. & Shin, H.-S. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95, 424–435.e6 (2017).
Bergmann, T. O. & Born, J. Phase-amplitude coupling: a general mechanism for memory processing and synaptic plasticity? Neuron 97, 10–13 (2018).
Hahn, M. A. et al. Slow oscillation–spindle coupling strength predicts real-life gross-motor learning in adolescents and adults. eLife 11, e66761 (2022).
Helfrich, R. F., Mander, B. A., Jagust, W. J., Knight, R. T. & Walker, M. P. Old brains come uncoupled in sleep: slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron 97, 221–230.e4 (2018).
Staresina, B. P. et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 18, 1679–1686 (2015).
Hahn, M. A., Heib, D., Schabus, M., Hoedlmoser, K. & Helfrich, R. F. Slow oscillation-spindle coupling predicts enhanced memory formation from childhood to adolescence. eLife 9, e53730 (2020).
Nicolas, J. et al. Sigma oscillations protect or reinstate motor memory depending on their temporal coordination with slow waves. eLife 11, e73930 (2022).
Staresina, B. P. Coupled sleep rhythms for memory consolidation. Trends Cogn. Sci. 28, 339–351 (2024).
Huber, R., Felice Ghilardi, M., Massimini, M. & Tononi, G. Local sleep and learning. Nature 430, 78–81 (2004).
Huber, R. et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat. Neurosci. 9, 1169–1176 (2006).
Tononi, G. & Cirelli, C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34 (2014).
Debarnot, U. et al. Motor imagery practice benefits during arm immobilization. Sci. Rep. 11, 8928 (2021).
Burianová, H. et al. Adaptive motor imagery: a multimodal study of immobilization-induced brain plasticity. Cereb. Cortex 26, 1072–1080 (2016).
Avanzino, L., Bassolino, M., Pozzo, T. & Bove, M. Use-dependent hemispheric balance. J. Neurosci. 31, 3423–3428 (2011).
King, E. M., Edwards, L. L. & Borich, M. R. Effects of short-term arm immobilization on motor skill acquisition. PLoS ONE 17, e0276060 (2022).
Moisello, C. et al. Short-term limb immobilization affects motor performance. J. Motor Behav. 40, 165–176 (2008).
Takeuchi, N., Oouchida, Y. & Izumi, S.-I. Motor control and neural plasticity through interhemispheric interactions. Neural Plast. 2012, 1–13 (2012).
Lustenberger, C. et al. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr. Biol. 26, 2127–2136 (2016).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Cohen, J. A power primer. Psychol. Bull. 112, 155–159 (1992).
Hotermans, C., Peigneux, P., Maertens de Noordhout, A., Moonen, G. & Maquet, P. Early boost and slow consolidation in motor skill learning. Learn. Mem. 13, 580–583 (2006).
Brawn, T. P., Fenn, K. M., Nusbaum, H. C. & Margoliash, D. Consolidating the effects of waking and sleep on motor-sequence learning. J. Neurosci. 30, 13977–13982 (2010).
King, B. R., Hoedlmoser, K., Hirschauer, F., Dolfen, N. & Albouy, G. Sleeping on the motor engram: the multifaceted nature of sleep-related motor memory consolidation. Neurosci. Biobehav. Rev. 80, 1–22 (2017).
Toussaint, L. & Meugnot, A. Short-term limb immobilization affects cognitive motor processes. J. Exp. Psychol. Learn. Mem. Cogn. 39, 623–632 (2013).
Meugnot, A., Almecija, Y. & Toussaint, L. The embodied nature of motor imagery processes highlighted by short-term limb immobilization. Exp. Psychol. 61, 180–186 (2014).
Hotermans, C., Peigneux, P., de Noordhout, A. M., Moonen, G. & Maquet, P. Repetitive transcranial magnetic stimulation over the primary motor cortex disrupts early boost but not delayed gains in performance in motor sequence learning. Eur. J. Neurosci. 28, 1216–1221 (2008).
Walker, M. P., Brakefield, T., Morgan, A., Hobson, J. A. & Stickgold, R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron 35, 205–211 (2002).
Walker, M. P. & Stickgold, R. Sleep, memory, and plasticity. Annu. Rev. Psychol. 57, 139–166 (2006).
Meugnot, A. & Toussaint, L. Functional plasticity of sensorimotor representations following short-term immobilization of the dominant versus non-dominant hands. Acta Psychol. 155, 51–56 (2015).
Bruno, V. et al. Long-term limb immobilization modulates inhibition-related electrophysiological brain activity. NeuroImage 218, 116911 (2020).
Kattler, H., Dijk, D. J. & Borbély, A. A. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J. Sleep Res. 3, 159–164 (1994).
Champetier, P. et al. Age-related changes in fast spindle clustering during non-rapid eye movement sleep and their relevance for memory consolidation. Sleep 46, zsac282 (2023).
Osorio-Forero, A. et al. Noradrenergic circuit control of non-REM sleep substates. Curr. Biol. 31, 5009–5023 (2021).
Truong, C. et al. Time of day and sleep effects on motor acquisition and consolidation. NPJ Sci. Learn. 8, 30 (2023).
Saletin, J. M., Goldstein, A. N. & Walker, M. P. The role of sleep in directed forgetting and remembering of human memories. Cereb. Cortex 21, 2534–2541 (2011).
Hoedlmoser, K. et al. The impact of diurnal sleep on the consolidation of a complex gross motor adaptation task. J. Sleep Res. 24, 100–109 (2015).
Dehnavi, F., Moghimi, S., Sadrabadi Haghighi, S., Safaie, M. & Ghorbani, M. Opposite effect of motivated forgetting on sleep spindles during stage 2 and slow wave sleep. Sleep 42, zsz085 (2019).
Stickgold, R. & Walker, M. P. Memory consolidation and reconsolidation: what is the role of sleep? Trends Neurosci. 28, 408–415 (2005).
Solano, A., Riquelme, L. A., Perez-Chada, D. & Della-Maggiore, V. Visuomotor adaptation modulates the clustering of sleep spindles into trains. Front. Neurosci. 16, 803387 (2022).
Vien, C. et al. Thalamo-cortical white matter underlies motor memory consolidation via modulation of sleep spindles in young and older adults. Neuroscience 402, 104–115 (2019).
Sampaio-Baptista, C. & Johansen-Berg, H. White matter plasticity in the adult brain. Neuron 96, 1239–1251 (2017).
Langer, N., Hanggi, J., Muller, N. A., Simmen, H. P. & Jancke, L. Effects of limb immobilization on brain plasticity. Neurology 78, 182–188 (2012).
Mölle, M., Bergmann, T. O., Marshall, L. & Born, J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep 34, 1411–1421 (2011).
Jannati, A., Oberman, L. M., Rotenberg, A. & Pascual-Leone, A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology 48, 191–208 (2023).
Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Kleiner, M., Brainard, D. & Pelli, D. What’s new in Psychtoolbox-3? Sci. Res. 36, 1–16 (2007).
Abrahamse, E. L., Jiménez, L., Verwey, W. B. & Clegg, B. A. Representing serial action and perception. Psychon. Bull. Rev. 17, 603–623 (2010).
Boutin, A., Blandin, Y., Massen, C., Heuer, H. & Badets, A. Conscious awareness of action potentiates sensorimotor learning. Cognition 133, 1–9 (2014).
Nettersheim, A., Hallschmid, M., Born, J. & Diekelmann, S. The role of sleep in motor sequence consolidation: stabilization rather than enhancement. J. Neurosci. 35, 6696–6702 (2015).
Antony, J. W. et al. Sleep spindle refractoriness segregates periods of memory reactivation. Curr. Biol. 28, 1736–1743 (2018).
Albouy, G. et al. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PLoS ONE 8, e52805 (2013).
Bönstrup, M. et al. A rapid form of offline consolidation in skill learning. Curr. Biol. 29, 1346–1351 (2019).
Iber, C., Ancoli-Israel, S., Chesson, A. L. & Quan, S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. (American Academy of Sleep Medicine, Westchester, IL, 2007).
Lustenberger, C. et al. High-density EEG characterization of brain responses to auditory rhythmic stimuli during wakefulness and NREM sleep. NeuroImage 169, 57–68 (2018).
Cohen, M. X. Analyzing Neural Time Series Data: Theory and Practice (MIT Press, 2014).
Berens, P. CircStat: a MATLAB toolbox for circular statistics. J. Stat. Softw. 31, 1–21 (2009).
Tadel, F., Baillet, S., Mosher, J. C., Pantazis, D. & Leahy, R. M. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, e879716 (2011).
Acknowledgements
We thank the medical and technician staff of the Hôtel-Dieu Sleep Center for providing facilities and for their assistance in organizing the study. This work was partly supported by a research grant from the “Société Française de Physiothérapie” to financially compensate participants for their involvement in this study.
Author information
Authors and Affiliations
Contributions
Author contributions: A.C., A.B., and L.D. conceived the experiment; A.C. collected the data; A.C. and A.B. analyzed the data and discussed the results; A.C. and A.B. wrote the manuscript; all authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Dimitrios Mylonas and the other anonymous reviewer for their contribution to the peer review of this work. Primary handling editors: Enzo Tagliazucchi and Jooa Valente. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Conessa, A., Léger, D. & Boutin, A. Temporal sleep spindle clustering and slow-oscillation coupling in motor memory consolidation and generalization. Commun Biol 9, 145 (2026). https://doi.org/10.1038/s42003-025-09425-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-09425-6