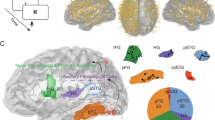
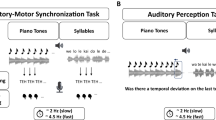
Abstract
According to neural oscillatory accounts, periodicity at the syllabic scale enhances speech comprehension through theta brain rhythms. Natural speech, however, is not strictly periodic and stronger periodicity, such as under conditions of fast speech, may hinder comprehension. Using magnetoencephalography, we investigate how natural variation in syllabic-level periodicity affects comprehension and auditory-motor coupling in brain areas related to temporal speech processing. We model speech periodicity and rate independently. Theta-band phase coupling between the posterior superior temporal gyrus (pSTG) and speech motor areas is assessed using Gaussian-Copula Mutual Information (GCMI). We find that faster syllabic rates and lower periodicity are associated with stronger coupling between the pSTG and inferior precentral gyrus, but also inferior frontal gyrus and supplementary motor areas. Comprehension improves with lower periodicity and declines at faster rates. The syllabic rate and periodicity moderate the coupling-comprehension relationship, possibly reflecting a complex interplay of lower-level auditory processing and higher-level prediction from the speech motor cortices. These findings suggest a sweet spot for natural, less periodic speech rhythms that support optimal processing and emphasize the necessity to investigate natural speech.
Similar content being viewed by others
Data availability
The anonymized preprocessed MEG and behavioral data are available on the Open Science Framework (OSF) as stated in Lubinus et al. 91. Due to restrictions, the pseudonymized raw MRI and MEG data, as well as the unprocessed stimulus material, are not publicly available. The data required to reproduce the results are available on OSF107.
Code availability
All custom code central to the conclusions is available on OSF107.
References
Ahissar, E. et al. Speech comprehension is correlated with temporal response patterns recorded from the auditory cortex. Proc. Natl. Acad. Sci. USA 98, 13367–13372 (2001).
Goswami, U. & Leong, V. Speech rhythm and temporal structure: Converging perspectives? Lab. Phonol. 4, 67–92 (2013).
Greenberg, S., Arai, T. & Silipo, R. Speech intelligibility derived from exceedingly sparse spectral information. In Proc. 5th International Conference on Spoken Language Processing, ICSLP 1998 https://doi.org/10.21437/ICSLP.1998-473 (1998).
Greenberg, S., Carvey, H., Hitchcock, L. & Chang, S. Temporal properties of spontaneous speech—a syllable-centric perspective. J. Phon. 31, 465–485 (2003).
Shannon, R. V., Zeng, F.-G., Kamath, V., Wygonski, J. & Ekelid, M. Speech recognition with primarily temporal cues. Science 270, 303–304 (1995).
Ghitza, O. & Greenberg, S. On the possible role of brain rhythms in speech perception: intelligibility of time-compressed speech with periodic and aperiodic insertions of silence. Phonetica 66, 113–126 (2009).
Ten Oever, S. & Martin, A. E. An oscillating computational model can track pseudo-rhythmic speech by using linguistic predictions. ELife 10, e68066 (2021).
Dellwo, V., Leemann, A., Kolly, M.-J. & Kolly, M.-J. Rhythmic variability between speakers: articulatory, prosodic, and linguistic factors. J. Acoust. Soc. Am. 137, 1513–1528 (2015).
Goswami, U. Language acquisition and speech rhythm patterns: an auditory neuroscience perspective. R. Soc. Open Sci. 9, 211855 (2022).
Varnet, L. et al. A cross-linguistic study of speech modulation spectra. J. Acoust. Soc. Am. 142, 1976–1989 (2017).
Dellwo, V. & Szigeti, I. Rhythm And Speech Rate: A Variation Coefficient for Deltac (Peter Lang, 2006).
Dellwo, V., Wagner, P., Solé, M. J., Recasens, D. & Romero, J. Relations between language rhythm and speech rate. In Proceedings of the International Congress of Phonetic Sciences, 471–474 (International Phonetic Association, 2003).
Quené, H. & Port, R. F. Effects of timing regularity and metrical expectancy on spoken-word perception. Phonetica 62, 1–13 (2005).
Robson, H., Thomasson, H., Upton, E., Leff, A. P. & Davis, M. H. The impact of speech rhythm and rate on comprehension in aphasia. Cortex 180, 126–146 (2024).
Aubanel, V., Davis, C. & Kim, J. Exploring the role of brain oscillations in speech perception in noise: Intelligibility of isochronously retimed speech. Front. Hum. Neurosci. 10, 195284 (2016).
Aubanel, V. & Schwartz, J. L. The role of isochrony in speech perception in noise. Sci. Rep. 10, 19580 (2020).
Ghitza, O. On the role of theta-driven syllabic parsing in decoding speech: Intelligibility of speech with a manipulated modulation spectrum. Front. Psychol. 3, 29386 (2012).
Ghitza, O. Linking speech perception and neurophysiology: speech decoding guided by cascaded oscillators locked to the input rhythm. Front. Psychol. 2, 9259 (2011).
Giraud, A. L. & Poeppel, D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat. Neurosci. 15, 511–517 (2012).
Gross, J. et al. Speech rhythms and multiplexed oscillatory sensory coding in the human brain. PLoS Biol. 11, e1001752 (2013).
Schroeder, C. E. & Lakatos, P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 32, 9–18 (2009).
Lakatos, P. et al. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J. Neurophysiol. 94, 1904–1911 (2005).
Ding, N., Melloni, L., Zhang, H., Tian, X. & Poeppel, D. Cortical tracking of hierarchical linguistic structures in connected speech. Nat. Neurosci. 19, 158–164 (2015).
Doelling, K. B., Arnal, L. H., Ghitza, O. & Poeppel, D. Acoustic landmarks drive delta–theta oscillations to enable speech comprehension by facilitating perceptual parsing. NeuroImage 85, 761–768 (2014).
Luo, H. & Poeppel, D. Phase patterns of neuronal responses reliably discriminate speech in human auditory cortex. Neuron 54, 1001–1010 (2007).
Schönwiesner, M. & Zatorre, R. J. Spectro-temporal modulation transfer function of single voxels in the human auditory cortex measured with high-resolution fMRI. Proc. Natl. Acad. Sci. USA 106, 14611–14616 (2009).
Oderbolz, C., Stark, E., Sauppe, S. & Meyer, M. Concurrent processing of the prosodic hierarchy is supported by cortical entrainment and phase-amplitude coupling. Cereb. Cortex 34, bhae479 (2024).
Large, E. W. & Jones, M. R. The dynamics of attending: how people track time-varying events. Psychol. Rev. 106, 119–159 (1999).
Solli, S. et al. Rhythm-based temporal expectations: unique contributions of predictability and periodicity. J. Cogn. Neurosci. 1–27 https://doi.org/10.1162/JOCN_A_02261. (2024).
Morillon, B. & Baillet, S. Motor origin of temporal predictions in auditory attention. Proc. Natl. Acad. Sci. USA 114, E8913–E8921 (2017).
Keitel, A., Gross, J. & Kayser, C. Perceptually relevant speech tracking in auditory and motor cortex reflects distinct linguistic features. PLoS Biol. 16, e2004473 (2018).
Lamekina, Y., Titone, L., Maess, B. & Meyer, L. Speech prosody serves temporal prediction of language via contextual entrainment. J. Neurosci. 44, e1041232024 (2024).
Gehrig, J. et al. Left perisylvian rhythms encode prosody and syntax during delayed sentence repetition. J. Neurosci. e2160242025 https://doi.org/10.1523/JNEUROSCI.2160-24.2025 (2025).
Arnal, L. H. & Giraud, A. L. Cortical oscillations and sensory predictions. Trends Cogn. Sci. 16, 390–398 (2012).
Park, H., Thut, G. & Gross, J. Predictive entrainment of natural speech through two fronto-motor top-down channels. Lang., Cogn. Neurosci. 35, 739 (2020).
Rimmele, J. M., Morillon, B., Poeppel, D. & Arnal, L. H. Proactive sensing of periodic and aperiodic auditory patterns. Trends Cogn. Sci. 22, 870–882 (2018).
Assaneo, M. F., Rimmele, J. M., Sanz Perl, Y. & Poeppel, D. Speaking rhythmically can shape hearing. Nat. Hum. Behav. 5, 71–82 (2021).
Assaneo, M. F. & Poeppel, D. The coupling between auditory and motor cortices is rate-restricted: evidence for an intrinsic speech-motor rhythm. Sci. Adv. 4, eaao3842 (2018).
Park, H., Ince, R. A. A., Schyns, P. G., Thut, G. & Gross, J. Frontal top-down signals increase coupling of auditory low-frequency oscillations to continuous speech in human listeners. Curr. Biol. 25, 1649–1653 (2015).
Keitel, A. & Gross, J. Individual human brain areas can be identified from their characteristic spectral activation fingerprints. PLoS Biol. 14, e1002498 (2016).
Lubinus, C. et al. Data-driven classification of spectral profiles reveals brain region-specific plasticity in blindness. Cereb. Cortex 31, 2505–2522 (2021).
Morillon, B., Arnal, L. H., Schroeder, C. E. & Keitel, A. Prominence of delta oscillatory rhythms in the motor cortex and their relevance for auditory and speech perception. Neurosci. Biobehav. Rev. 107, 136–142 (2019).
Pefkou, M., Arnal, L. H., Fontolan, L. & Giraud, A. L. θ-Band and β-band neural activity reflects independent syllable tracking and comprehension of time-compressed speech. J. Neurosci. 37, 7930–7938 (2017).
Wang, L. et al. Beta oscillations relate to the N400m during language comprehension. Hum. Brain Mapp. 33, 2898–2912 (2012).
Arnal, L. H., Doelling, K. B. & Poeppel, D. Delta–beta coupled oscillations underlie temporal prediction accuracy. Cereb. Cortex 25, 3077–3085 (2015).
Wilson, S. M., Saygin, A. P., Sereno, M. I. & Iacoboni, M. Listening to speech activates motor areas involved in speech production. Nat. Neurosci. 7, 701–702 (2004).
Grahn, J. A. & Brett, M. Rhythm and beat perception in motor areas of the brain. J. Cogn. Neurosci. 19, 893–906 (2007).
Hoddinott, J. D. & Grahn, J. A. Neural representations of beat and rhythm in motor and association regions. Cereb. Cortex 34, bhae406 (2024).
Hickok, G. & Poeppel, D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition 92, 67–99 (2004).
Hickok, G. & Poeppel, D. The cortical organization of speech processing. Nat. Rev. Neurosci. 8, 393–402 (2007).
Broadmann, K. Vergleichende lokalisationslehre der grosshirnrinde. Comparative Study of Localization in the Cerebral Cortex (Barth, 1909).
Khalighinejad, B. et al. Functional characterization of human Heschl’s gyrus in response to natural speech. NeuroImage 235, 118003 (2021).
Yousry, T. A., Fesl, G., Buttner, A., Noachtar, S. & Schmid, U. D. Heschl's gyrus-Anatomic description and methods of identification on magnetic resonance imaging. Int. J. Neuroradiol. 3, 2–12 (1997).
Blumstein, S. E., Baker, E. & Goodglass, H. Phonological factors in auditory comprehension in aphasia. Neuropsychologia 15, 19–30 (1977).
Wernicke, C. Der aphasische Symptomenkomplex. Der aphasische Symptomenkomplex https://doi.org/10.1007/978-3-642-65950-8 (1974).
Kaas, J. H. & Hackett, T. A. ‘What’ and ‘where’ processing in auditory cortex. Nat. Neurosci. 2, 1045–1047 (1999).
Rauschecker, J. P. Cortical processing of complex sounds. Curr. Opin. Neurobiol. 8, 516–521 (1998).
Romanski, L. M. et al. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat. Neurosci. 2, 1131–1136 (1999).
Hickok, G. & Poeppel, D. Towards a functional neuroanatomy of speech perception. Trends Cogn. Sci. 4, 131–138 (2000).
Saura, D. et al. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. USA 105, 18035–18040 (2008).
Tourville, J. A. & Guenther, F. H. The DIVA model: a neural theory of speech acquisition and production. Lang. Cogn. Process. 26, 952–981 (2011).
Clos, M. et al. Effects of prior information on decoding degraded speech: an fMRI study. Hum. Brain Mapp. 35, 61–74 (2014).
D’Ausilio, A., Bufalari, I., Salmas, P. & Fadiga, L. The role of the motor system in discriminating normal and degraded speech sounds. Cortex 48, 882–887 (2012).
Glanz, O. et al. Real-life speech production and perception have a shared premotor-cortical substrate. Sci. Rep. 8, 1–14 (2018).
Morillon, B., Schroeder, C. E. & Wyart, V. Motor contributions to the temporal precision of auditory attention. Nat. Commun. 5, 1–9 (2014).
Möttönen, R., Dutton, R. & Watkins, K. E. Auditory-motor processing of speech sounds. Cereb. Cortex 23, 1190–1197 (2013).
Oliveira, D. S. et al. Mu rhythm dynamics suggest automatic activation of motor and premotor brain regions during speech processing. J. Neurolinguist. 60, 101006 (2021).
Onojima, T., Kitajo, K. & Mizuhara, H. Ongoing slow oscillatory phase modulates speech intelligibility in cooperation with motor cortical activity. PLoS ONE 12, e0183146 (2017).
Riecker, A., Brendel, B., Ziegler, W., Erb, M. & Ackermann, H. The influence of syllable onset complexity and syllable frequency on speech motor control. Brain Lang. 107, 102–113 (2008).
Stokes, R. C., Venezia, J. H. & Hickok, G. The motor system’s [modest] contribution to speech perception. Psychon. Bull. Rev. 26, 1354–1366 (2019).
Vukovic, N., Feurra, M., Shpektor, A., Myachykov, A. & Shtyrov, Y. Primary motor cortex functionally contributes to language comprehension: an online rTMS study. Neuropsychologia 96, 222–229 (2017).
Geiser, E., Zaehle, T., Jancke, L. & Meyer, M. The neural correlate of speech rhythm as evidenced by metrical speech processing. J. Cogn. Neurosci. 20, 541–552 (2008).
Keitel, A., Ince, R. A. A., Gross, J. & Kayser, C. Auditory cortical delta-entrainment interacts with oscillatory power in multiple fronto-parietal networks. NeuroImage 147, 32–42 (2017).
Bengtsson, S. L. et al. Listening to rhythms activates motor and premotor cortices. Cortex 45, 62–71 (2009).
Chen, J. L., Penhune, V. B. & Zatorre, R. J. Listening to musical rhythms recruits motor regions of the brain. Cereb. Cortex 18, 2844–2854 (2008).
Ding, N. & Simon, J. Z. Emergence of neural encoding of auditory objects while listening to competing speakers. Proc. Natl. Acad. Sci. USA 109, 11854–11859 (2012).
Peelle, J. E., Gross, J. & Davis, M. H. Phase-locked responses to speech in human auditory cortex are enhanced during comprehension. Cereb. Cortex 23, 1378–1387 (2013).
Zion Golumbic, E. M. et al. Mechanisms underlying selective neuronal tracking of attended speech at a ‘cocktail party. Neuron 77, 980–991 (2013).
Pittman-Polletta, B. R. et al. Differential contributions of synaptic and intrinsic inhibitory currents to speech segmentation via flexible phase-locking in neural oscillators. PLoS Comput. Biol. 17, e1008783 (2021).
Poeppel, D. & Assaneo, M. F. Speech rhythms and their neural foundations. Nat. Rev. Neurosci. 21, 322–334 (2020).
Mita, A., Mushiake, H., Shima, K., Matsuzaka, Y. & Tanji, J. Interval time coding by neurons in the presupplementary and supplementary motor areas. Nat. Neurosci.12, 502–507 (2009).
Schwartze, M., Rothermich, K. & Kotz, S. A. Functional dissociation of pre-SMA and SMA-proper in temporal processing. NeuroImage 60, 290–298 (2012).
Hertrich, I., Dietrich, S. & Ackermann, H. The role of the supplementary motor area for speech and language processing. Neurosci. Biobehav. Rev. 68, 602–610 (2016).
Brungart, D. S. Informational and energetic masking effects in the perception of two simultaneous talkers. J. Acoust. Soc. Am. 109, 1101–1109 (2001).
Lubinus, C., Keitel, A., Obleser, J., Poeppel, D. & Rimmele, J. M. Explaining flexible continuous speech comprehension from individual motor rhythms. Proc. R. Soc. B 290, 20222410 (2023).
Nourski, K. V. et al. Temporal envelope of time-compressed speech represented in the human auditory cortex. J. Neurosci. 29, 15564–15574 (2009).
Murakami, T., Kell, C. A., Restle, J., Ugawa, Y. & Ziemann, U. Left dorsal speech stream components and their contribution to phonological processing. J. Neurosci. 35, 1411–1422 (2015).
Kern, P., Assaneo, M. F., Endres, D., Poeppel, D. & Rimmele, J. M. Preferred auditory temporal processing regimes and auditory-motor synchronization. Psychon. Bull. Rev. 28, 1860 (2021).
Baayen, R. H., Davidson, D. J. & Bates, D. M. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 59, 390–412 (2008).
Barr, D. J., Levy, R., Scheepers, C. & Tily, H. J. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lang. 68, 255–278 (2013).
Lubinus, C., Keitel, A., Obleser, J., Poeppel, D. & Rimmele, J. M. Endogenous auditory and motor brain rhythms predict individual speech tracking. Preprint at https://doi.org/10.1101/2025.03.24.644939 (2025).
Brainard, D. H. The psychophysics toolbox. Spat. Vis. 10, 433–436 (1997).
Charpentier, F. Traitement de la parole par analyse-synthèse de Fourier: Application à la synthèse par diphones (Doctoral dissertation, Paris, ENST, 1988).
Moulines, E. & Charpentier, F. Pitch-synchronous waveform processing techniques for text-to-speech synthesis using diphones. Speech Commun. 9, 453–467 (1990).
Keitel, A. et al. Cortical and behavioral tracking of rhythm in music: Effects of pitch predictability, enjoyment, and expertise. Ann. N.Y. Acad. Sci. 1546, 120–135 (2025).
Leys, C., Ley, C., Klein, O., Bernard, P. & Licata, L. Detecting outliers: do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 49, 764–766 (2013).
de Jong, N. H., Pacilly, J. & Heeren, W. PRAAT scripts to measure speed fluency and breakdown fluency in speech automatically. Assess. Educ. Princ. Policy Pract. 28, 456–476 (2021).
Oostenveld, R., Fries, P., Maris, E. & Schoffelen, J. M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell. Neurosci. 2011, 156869 (2011).
Makeig, S., Jung, T.-P., Bell, A. J. & Sejnowski, T. J. Independent component analysis of electroencephalographic data. Adv. Neural Inf. Process. Syst. 8, 145–151 (1995).
Nolte, G. et al. Robustly estimating the flow direction of information in complex physical systems. Phys. Rev. Lett. 100, 234101 (2008).
Westner, B. U. et al. A unified view on beamformers for M/EEG source reconstruction. NeuroImage 246, 118789 (2022).
Bear, M., Connors, B. & Paradiso, M. A. Neuroscience: Exploring the Brain, Enhanced Edition: Exploring the Brain (Jones & Bartlett Learning, 2020).
Chalas, N. et al. Multivariate analysis of speech envelope tracking reveals coupling beyond auditory cortex. NeuroImage 258, 119395 (2022).
Gross, J. et al. Comparison of undirected frequency-domain connectivity measures for cerebro-peripheral analysis. NeuroImage 245, 118660 (2021).
De Clercq, P., Vanthornhout, J., Vandermosten, M. & Francart, T. Beyond linear neural envelope tracking: a mutual information approach. J. Neural Eng. 20, 026007 (2023).
Demirkale, C. Y., Nettleton, D. & Maiti, T. <strong>Linear mixed model selection for false discovery rate control in microarray data analysis</strong>. Biometrics 66, 621–629 (2010).
Kwon, S. Effects of speech periodicity and speech rate on auditory–motor coupling during speech comprehension. Preprint at: https://doi.org/10.17605/OSF.IO/ZDNFV (2025).
Acknowledgements
We thank the Max Planck Institute for Empirical Aesthetics for funding this project. We thank Dr. Klaus Frieler for valuable advice on statistical analysis. AK is supported by the Medical Research Council (grant number MR/W02912X/1).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
S.K.: conceptualization, data curation, formal analysis, methodology, software, visualization, writing—original draft, review and editing; C.L.: data curation, investigation, methodology, project administration, software, writing—review and editing; C.A.K.: methodology, writing—review and editing; A.K.: methodology, visualization, writing—review and editing; J.M.R.: conceptualization, supervision, resources, methodology, writing—original draft, review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Benedikt Zoefel and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Helen Blank and Jasmine Pan. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kwon, S., Lubinus, C., Kell, C.A. et al. Effects of speech periodicity and speech rate on auditory-motor coupling during speech comprehension. Commun Biol (2026). https://doi.org/10.1038/s42003-025-09481-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-09481-y