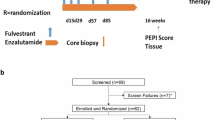
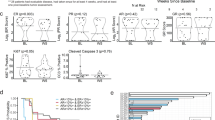
Abstract
Breast cancer ranks highest globally in terms of both incidence and mortality rates among female malignancies. Elucidating the molecular mechanisms driving breast cancer initiation and progression, as well as identifying novel therapeutic agents, remains a critical unmet medical need. This study aimed to identify FDA-approved CYP4Z1 inhibitors with anti-breast cancer activity through a drug repurposing strategy, thereby providing preclinical evidence for potential clinical adjuvant therapies. Fluvastatin was identified as a concentration-dependent CYP4Z1 inhibitor through molecular docking and site-directed mutagenesis studies, binding to critical residues Lys109, Pro444, and Arg450 in the enzyme’s active site. Functional studies demonstrated that Fluvastatin significantly attenuated cancer stem cell properties, migratory/invasive capacities, and epithelial-mesenchymal transition in breast cancer cell lines. In vivo experiments revealed that fluvastatin suppressed primary tumor growth and lung metastasis in xenograft models, while delaying mammary tumorigenesis in PyMT-MMTV-CYP4Z1 transgenic mice. Notably, this effect was less pronounced in PyMT-MMTV wild-type controls. This study establishes Fluvastatin as a novel CYP4Z1-targeted therapeutic candidate for breast cancer, providing preclinical validation for its potential use in combination therapies.

Similar content being viewed by others
Data availability
The RNA-seq data used in this study were previously generated and published in our prior work14. The raw data have been deposited in the GEO database under accession codes GSE116984. In this study, these data were reanalyzed to identify biological metabolism associated with CYP4Z1. The lipidomics data have been deposited in the Figshare database under accession https://doi.org/10.6084/m9.figshare.30866477. The numerical source data for the graphs is found in Supplementary Data. Unedited western blots is found in Supplementary Fig. 10–13. All data are presented within the article and supplementary online data. All other data are available from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 72, 409–436 (2022).
Clara, J. A., Monge, C., Yang, Y. & Takebe, N. Targeting signalling pathways and the immune microenvironment of cancer stem cells-a clinical update. Nat. Rev. Clin. Oncol. 17, 204–232 (2020).
Zhou, H. M., Zhang, J. G., Zhang, X. & Li, Q. Targeting cancer stem cells for reversing therapy resistance: mechanism, signaling, and prospective agents. Signal Transduct. Target Ther. 6, 62 (2021).
Jewer, M. et al. Translational control of breast cancer plasticity. Nat. Commun. 11, 2498 (2020).
Lu, H. et al. Chemotherapy-induced S100A10 recruits KDM6A to facilitate OCT4-mediated breast cancer stemness. J. Clin. Invest. 130, 4607–4623 (2020).
Rieger, M. A. et al. Identification of a novel mammary-restricted cytochrome P450, CYP4Z1, with overexpression in breast carcinoma. Cancer Res. 64, 2357–2364 (2004).
Al-Saraireh, Y. M. et al. Screening of cytochrome 4Z1 expression in human non-neoplastic, pre-neoplastic and neoplastic tissues. Ecancermedicalscience 14, 1114 (2020).
Yang, X., Hutter, M., Goh, W. W. B. & Bureik, M. CYP4Z1-A human cytochrome P450 enzyme that might hold the key to curing breast cancer. Curr. Pharm. Des. 23, 2060–2064 (2017).
Khayeka-Wandabwa, C. et al. Plasma membrane localization of CYP4Z1 and CYP19A1 and the detection of anti-CYP19A1 autoantibodies in humans. Int Immunopharmacol. 73, 64–71 (2019).
Nunna, V., Jalal, N. & Bureik, M. Anti-CYP4Z1 autoantibodies detected in breast cancer patients. Cell Mol. Immunol. 14, 572–574 (2017).
Al-Saraireh, Y. M. et al. Cytochrome 4Z1 expression is correlated with poor prognosis in patients with cervical cancer. Curr. Oncol. 28, 3573–3584 (2021).
Yu, W. et al. Increased expression of CYP4Z1 promotes tumor angiogenesis and growth in human breast cancer. Toxicol. Appl. Pharm. 264, 73–83 (2012).
Zheng, L. et al. Transcriptional factor six2 promotes the competitive endogenous RNA network between CYP4Z1 and pseudogene CYP4Z2P responsible for maintaining the stemness of breast cancer cells. J. Hematol. Oncol. 12, 23 (2019).
Zheng, L., Li, X., Gu, Y., Lv, X. & Xi, T. The 3’UTR of the pseudogene CYP4Z2P promotes tumor angiogenesis in breast cancer by acting as a ceRNA for CYP4Z1. Breast Cancer Res. Treat. 150, 105–118 (2015).
Li, C. et al. The competing endogenous RNA network of CYP4Z1 and pseudogene CYP4Z2P exerts an anti-apoptotic function in breast cancer. FEBS Lett. 591, 991–1000 (2017).
Santo, L., Ward, B. W., Rui, P. & Ashman, J. J. Antineoplastic drugs prescription during visits by adult cancer patients with comorbidities: findings from the 2010-2016 National Ambulatory Medical Care Survey. Cancer Causes Control 31, 353–363 (2020).
He, T. et al. Adjuvant chemotherapy-associated lipid changes in breast cancer patients: a real-word retrospective analysis. Medicine 99, e21498 (2020).
Arpino, G. et al. Metabolic and anthropometric changes in early breast cancer patients receiving adjuvant therapy. Breast Cancer Res. Treat. 154, 127–132 (2015).
Dieli-Conwright, C. M. et al. An observational study to examine changes in metabolic syndrome components in patients with breast cancer receiving neoadjuvant or adjuvant chemotherapy. Cancer 122, 2646–2653 (2016).
Qi, A. et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg. 10, 1470–1477 (2021).
Liu, H. et al. HET0016 attenuates the stemness of breast cancer cells through targeting CYP4Z1. Mol. Carcinog. 60, 413–426 (2021).
Qin, H. et al. 1-Benzylimidazole attenuates the stemness of breast cancer cells through partially targeting CYP4Z1. Environ. Toxicol. 39, 1505–1520 (2024).
Yuan, Y. et al. Identification of a novel potent CYP4Z1 inhibitor attenuating the stemness of breast cancer cells through lead optimization. J. Med Chem. 65, 15749–15769 (2022).
Ashburn, T. T. & Thor, K. B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 3, 673–683 (2004).
Zheng, C. et al. Targeting PFKL with penfluridol inhibits glycolysis and suppresses esophageal cancer tumorigenesis in an AMPK/FOXO3a/BIM-dependent manner. Acta Pharm. Sin. B 12, 1271–1287 (2022).
Pushpakom, S. et al. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 18, 41–58 (2019).
Corsini, A., Jacobson, T. A. & Ballantyne, C. M. Fluvastatin: clinical and safety profile. Drugs 64, 1305–1323 (2004).
Guo, Q. et al. Transcriptional factor Yin Yang 1 promotes the stemness of breast cancer cells by suppressing miR-873-5p transcriptional activity. Mol. Ther. Nucleic Acids 21, 527–541 (2020).
Zhao, Q. et al. MiR-375 inhibits the stemness of breast cancer cells by blocking the JAK2/STAT3 signaling. Eur. J. Pharm. 884, 173359 (2020).
Liu, Y. et al. A positive TGF-β/miR-9 regulatory loop promotes the expansion and activity of tumour-initiating cells in breast cancer. Br. J. Pharm. 180, 2280–2297 (2023).
Johnson, S., Chen, H. & Lo, P.-K. In vitro tumorsphere formation assays. Bio-Protoc. 3, e325 (2013).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 100, 3983–3988 (2003).
Khayeka-Wandabwa, C., Zhao, J., Pathak, J. L., Wu, H. & Bureik, M. Upregulation of estrogen receptor alpha (ERα) expression in transgenic mice expressing human CYP4Z1. Breast Cancer Res. Treat. 191, 319–326 (2022).
Britschgi, A. et al. The Hippo kinases LATS1 and 2 control human breast cell fate via crosstalk with ERα. Nature 541, 541–545 (2017).
Yan, Q. et al. Efficient substrate screening and inhibitor testing of human CYP4Z1 using permeabilized recombinant fission yeast. Biochem Pharm. 146, 174–187 (2017).
Zhao, Y., Chen, Q., Chen, L., Shen, S. G. F. & Dai, J. Thalidomide leads to mandible hypoplasia through inhibiting angiogenesis and secondary hemorrhage in the fetal craniofacial region in rabbits. Toxicol. Lett. 319, 250–255 (2020).
Lu, C., Li, X., Ren, Y. & Zhang, X. Disulfiram: a novel repurposed drug for cancer therapy. Cancer Chemother. Pharm. 87, 159–172 (2021).
Zhu, Z. et al. Identification of lysine isobutyrylation as a new histone modification mark. Nucleic Acids Res. 49, 177–189 (2021).
Bi, Y. Y., Chen, Q., Yang, M. Y., Xing, L. & Jiang, H. L. Nanoparticles targeting mutant p53 overcome chemoresistance and tumor recurrence in non-small cell lung cancer. Nat. Commun. 15, 2759 (2024).
Mohapatra, D. et al. Fluvastatin sensitizes pancreatic cancer cells toward radiation therapy and suppresses radiation- and/or TGF-β-induced tumor-associated fibrosis. Lab. Investig. 102, 298–311 (2022).
Xu, W. H., Zhang, T., Zhou, Y. & Mao, Y. Fluvastatin prevents lung metastasis in triple-negative breast cancer by triggering autophagy via the RhoB/PI3K/mTOR pathway. Exp. Cell Res. 435, 113893 (2024).
Yi, H. et al. Reversal of HER2 negativity: an unexpected role for lovastatin in triple-negative breast cancer stem cells. J. Cancer 11, 3713–3716 (2020).
Ginestier, C. et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1, 555–567 (2007).
Yuan, S. et al. Ras drives malignancy through stem cell crosstalk with the microenvironment. Nat 612, 555–563 (2022).
Weina, K. & Utikal, J. SOX2 and cancer: current research and its implications in the clinic. Clin. Transl. Med 3, 19 (2014).
Chen, Y. Transwell cell migration assay using human breast epithelial cancer cell. Bio-Protoc. 2, e99 (2012).
Martinez Molina, D. et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341, 84–87 (2013).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 82204432, 82473955, 82173842), Henan Province Science and Technology Research Project(No.252102311191), Henan provincial Medical Science and Technology Research Project (No.SBGJ202502035). The Opening Foundation of State Key Laboratory of Neurology and Oncology Drug Development. WU JIEPING Medical Foundation (No. 320.6750.2023-05-7), Guizhou Provincial Basic Research Program(Natural Science) (Qian Ke He Ji Chu-[2024] Youth 020), 2025 Hospital-Level Scientific Research Fund of Beiiing Jishuitan Hospital Guizhou Hospital (JGYYK[2025]02), the Fundamental Research Funds for the Central Universities (2632025TD04), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions. The graphical abstract was drawn by Figdraw.
Author information
Authors and Affiliations
Contributions
Lufeng Zheng, Qianqian Guo and Hai Qin designed the research. Huilong Li and Ying Chen analyzed the data. Huilong Li, Ying Chen, Wanjin Shi, Zheng Miao, Yu Lu, Xuedan Han, Haitao Chen, Yunnan Zhang, Miaomiao Niu and Shengtao Xu performed the research. Huilong Li and Ying Chen wrote the paper. Lufeng Zheng, Qianqian Guo and Hai Qin reviewed this paper. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Hexin Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Kaliya Georgieva. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Chen, Y., Shi, W. et al. Fluvastatin suppresses breast cancer initiation and progression via targeting CYP4Z1. Commun Biol (2026). https://doi.org/10.1038/s42003-026-09532-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-026-09532-y