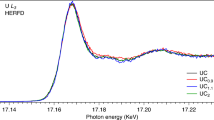
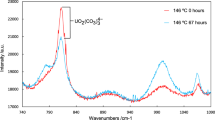
Abstract
It is well established that a significant amount of heat produced in the Earth’s mantle is due to the decay of uranium. However, uranium cannot be incorporated in large amounts into the most common mantle minerals. Here, we suggest that carbonates could be host phases for uranium in carbon-rich mantle lithologies. Two anhydrous uranium carbonates, U2[CO3]3 and U[CO3]2, were simultaneously synthesized by a reaction of UO2 with CO2 in a laser-heated diamond anvil cell at 20(1) GPa and 1800(200) K. Their crystal structures were obtained from synchrotron-based single crystal diffraction data and reproduced by density functional theory-based calculations. In U2[CO3]3 trivalent uranium cations are present, while uranium is four-valent in U[CO3]2. The synthesis of U2[CO3]3 and U[CO3]2 is a significant extension of the chemistry of uranium compounds and we provide a straightforward synthesis route for a UIII-containing compound.
Similar content being viewed by others
Data availability
The X-ray crystallographic coordinates for the structure reported in this study has been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2475926 (U[CO3]2) and 2475927 (U2[CO3]3). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The supplementary material contains additional information to the results of the single crystal structure determination and DFT-based calculations.
References
Sammon, L. G. & McDonough, W. F. Quantifying Earth’s radiogenic heat budget. EPSL 593, 117684 (2022).
Abe, S. et al. Abundances of uranium and thorium elements in Earth estimated by geoneutrino spectroscopy. Geophys. Res. Lett. 49, e2022GL099566 (2022).
Morss, L. R., Edelstein, N. M. & Fuger, J. (eds.) The Chemistry of the Actinide and Transactinide Elements (Springer, 2011).
Dahlkamp, F. J. Uranium Ore Deposits (Springer-Verlag, 1993).
Burns, P. C. U6+ minerals and inorganic compounds: insights into an expanded structural hierarchy of crystal structures. Can. Mineral. 43, 1839–1894 (2005).
Gurzhiy, V. V., Kalashnikova, S. A., Kuporev, I. V. & Pláa̧il, J. Crystal chemistry and structural complexity of the uranyl carbonate minerals and synthetic compounds. Crystals 11, 704 (2021).
Cowie, B. E., Purkis, J. M., Austin, J., Love, J. B. & Arnold, P. L. Thermal and photochemical reduction and functionalization chemistry of the uranyl dication, [UVIO2]2+. Chem. Rev. 119, 10595–10637 (2019).
Gautron, L. et al. Uranium in the Earth’s lower mantle. Geophys. Res. Lett. 43, L23301 (2006).
Perry, S. N., Pigott, J. S. & Panero, W. R. Ab initio calculations of uranium and thorium storage in CaSiO3-perovskite in the Earth’s lower mantle. Am. Mineral. 102, 321–326 (2017).
Kaminsky, F. Mineralogy of the lower mantle: A review of super-deep mineral inclusions in diamond. Earth Sci. Rev. 110, 127–147 (2012).
Deines, P. The carbon isotope geochemistry of mantle xenoliths. Earth-Sci. Rev. 58, 247–278 (2002).
Gu, T. et al. Hydrous peridotitic fragments of Earth’s mantle 660 km discontinuity sampled by a diamond. Nat. Geosci. 15, 950–954 (2022).
Bayarjargal, L., Fruhner, C.-J., Schrodt, N. & Winkler, B. CaCO3 phase diagram studied with Raman spectroscopy at pressures up to 50 GPa and high temperatures and DFT modeling. Phys. Earth Planet. Inter. 281, 31–45 (2018).
Binck, J. et al. Phase stabilities of MgCO3 and MgCO3-II studied by Raman spectroscopy, X-ray diffraction, and density functional theory calculations. Phys. Rev. Mater. 4, 055001 (2020).
Binck, J. et al. Synthesis of calcium orthocarbonate, Ca2CO4-Pnma at p, T-conditions of Earth’s transition zone and lowermantle. Am. Mineral. 107, 336–342 (2022).
König, J. et al. Novel calcium sp3-carbonate CaC2O5-\(I\overline{4}2d\) may be a carbon host in Earth’s lower mantle. Earth. Space Chem. 6, 73–80 (2022).
Spahr, D. et al. Tetrahedrally coordinated sp3-hybridized carbon in Sr2CO4 orthocarbonate at ambient conditions. Inorg. Chem. 60, 5419–5422 (2021).
Spahr, D. et al. Sr3[CO4]O antiperovskite with tetrahedrally-coordinated sp3-hybridized carbon and OSr6-octahedra. Inorg. Chem. 60, 14504–14508 (2021).
Spahr, D. et al. Sr[C2O5] is an inorganic pyrocarbonate salt with [C2O5]2− Complex Anions. J. Am. Chem. Soc. 144, 2899–2904 (2022).
Spahr, D. et al. Ca3[C2O5]2[CO3] is a pyrocarbonate which can be formed at p, T-conditions prevalent in the Earth’s transition zone. Commun. Chem. 7, 238 (2024).
Christ, C. L., Clark, J. R. & Evans Jr, H. T. Crystal Structure of Rutherfordine, U2CO3. Science 121, 472–473 (1995).
Finch, R. J., Cooper, M. A., Hawthorne, F. C. & Ewing, R. C. Refinement of the crystal structure of rutherfordine. Can. Mineral. 37, 929–938 (1999).
Sahoo, B. & Patnaik, D. Carbonates of uranium. Nature 185, 683 (1960).
Dasgupta, R. & Hirschmann, M. M. The deep carbon cycle and melting in Earth’s interior. EPSL 298, 1–13 (2010).
Dasgupta, R. et al. EPSL575, 117181 (2021).
MacDonald, M. R. et al. Identification of the +2 oxidation state for uranium in a crystalline molecular complex, [K(2.2.2-Cryptand)][(C5H4SiMe3)3U]. J. Am. Chem. Soc. 135, 13310–13313 (2013).
Deng, C. et al. Accessing five oxidation states of uranium in a retained ligand framework. Nat. Commun. 14, 4657 (2002).
Barluzzi, L., Giblin, S. R., Mansikkamäki, A. & Layfield, R. A. Identification of Oxidation State +1 in a Molecular Uranium Complex. J. Am. Chem. Soc. 144, 18229–18233 (2022).
Effenberger, H. & Zemann, J. Verfeinerung der Kristallstruktur Des Lithiumkarbonates, Li2CO3. Z. Kristallogr. 150, 133–138 (1979).
Spahr, D. et al. Synthesis and crystal structure of anhydrous di-iodyl carbonate (IO2)2[CO3], Hosting I5+-Cations. JACS Au 5, 4675–4680 (2025).
Bayarjargal, L. et al. High-pressure synthesis of an iron carbonate, Fe2[CO3]3. Inorg. Chem. 63, 21637–21644 (2024).
Wang, Y. et al. Cr3+-containing carbonates and Cr2O3-Pbcn at extreme conditions. Inorg. Chem. 64, 4996–5003 (2025).
Silva, C. L. et al. On the origin of low-valent uranium oxidation state. Nat. Commun. 15, 6861 (2024).
Wooles, A. J. et al. Uranium(III)-carbon multiple bonding supported by arene σ-bonding in mixed-valence hexauranium nanometre-scale rings. Nat. Commun. 9, 2097 (2018).
Keener, M. et al. Multielectron redox chemistry of uranium by accessing the +II oxidation state and enabling reduction to a U(I) Synthon. J. Am. Chem. Soc. 145, 16271–16283 (2023).
Drożdżyński, J. Tervalent uranium compounds. Coord. Chem. Rev. 249, 2351–2373 (2005).
Liu, L.-G. & Lin, C.-C. A calcite → aragonite-type phase transition in CdCO3. Am. Mineral. 82, 643–646 (1997).
Bayarjargal, L. et al. Anhydrous aluminium carbonates and isostructural compounds. Inorg. Chem. 62, 13910–13918 (2023).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A32, 751–767 (1976).
Spahr, D. et al. Synthesis and crystal structure of acentric anhydrous beryllium carbonate Be(CO3). Chem. Commun. 60, 10208–10211 (2024).
Dziewonski, A. M. & Anderson, D. L. Preliminary reference Earth model. Phys. Earth Planet. Inter. 25, 297–356 (1981).
Ritsema, J., Xu, W., Stixrude, L. & Lithgow-Bertelloni, C. Estimates of the transition zone temperature in a mechanically mixed upper mantle. EPSL 277, 244–252 (2009).
Katsura, T., Yoneda, A., Yamazaki, D., Yoshino, T. & Ito, E. Adiabatic temperature profile in the mantle. Phys. Earth Planet. Inter. 183, 212–218 (2010).
Leinders, G., Cardinaels, T., Binnemans, K. & Verwerft, M. Accurate lattice parameter measurements of stoichiometric uranium dioxide. J. Nucl. Mater. 459, 135–142 (2015).
Bruneval, F., Freyss, M. & Crocombette, J.-P. Lattice constant in nonstoichiometric uranium dioxide from first principles. Phys. Rev. Mater. 2, 023801 (2018).
Mao, H. K., Xu, J. & Bell, P. M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 91, 4673–4676 (1986).
Aoki, K., Yamawaki, H., Sakashita, M., Gotoh, Y. & Takemura, K. Crystal structure of the high-pressure phase of solid CO2. Science 263, 356–358 (1994).
Olijnyk, H. & Jephcoat, A. P. Vibrational studies on CO2 up to 40 GPa by Raman spectroscopy at room temperature. Phys. Rev. B 57, 879–888 (1998).
Scelta, D. et al. Extending the stability field of polymeric carbon dioxide phase V beyond the Earth’s geotherm. Phys. Rev. Lett. 126, 065701 (2021).
Yoo, C. S. et al. Crystal structure of pseudo-six-fold carbon dioxide phase II at high pressures and temperatures. Phys. Rev. B 65, 104103 (2002).
Datchi, F., Giordano, F. M., Munsch, P. & Saitta, A. M. Structure of carbon dioxide phase IV: breakdown of the intermediate bonding state scenario. Phys. Rev. Lett. 103, 185701 (2009).
Spahr, D. et al. 6-fold-coordinated beryllium in calcite-type be BeCO3. Inorg. Chem. 63, 19513–19517 (2024).
Hoppe, R. et al. A new route to charge distributions in ionic solids. J. Less Common Met. 156, 105–122 (1989).
Momma, A. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 44, 1272–1276 (2011).
Zhang, F. X. et al. Structural transitions and electron transfer in coffinite, USiO4, at high pressure. Am. Mineral. 94, 916–920 (2009).
Bauer, J. D. et al. High-pressure phase transition of coffinite, USiO4. J. Phys. Chem. C. 118, 25141–25149 (2014).
Redfern, S. A. T. & Angel, R. J. High-pressure behaviour and equation of state of calcite, CaCO3. Contrib. Mineral. Petrol. 134, 102–106 (1999).
Pennacchioni, L., Speziale, S., Bayarjargal, L., Schneider, M. & Winkler, B. Elasticity of amorphous calcium carbonate at high pressure and its dependence on the H2O content: A Brillouin scattering study to 20 GPa. Phys. Earth Planet. Inter. 336, 106984 (2023).
Nguyen-Thanh, T. et al. Lattice dynamics and elasticity of SrCO3. J. Appl. Cryst. 49, 1982–1990 (2016).
Biedermann, N. et al. Equation of state and high-pressure phase behaviour of SrCO3. Eur. J. Mineral. 32, 575–586 (2020).
Wood, B. J., Blundy, J. D. & Robinson, J. A. C. The role of clinopyroxene in generating U-series disequilibrium during mantle melting. Geochim. Cosmochim. Acta 63, 1613–1620 (1999).
Gréaux, S. et al. X-ray absorption near edge structure (XANES) study of the speciation of uranium and thorium in Al-rich CaSiO3 perovskite. Am. Mineral. 96, 100–109 (2012).
Murphy, G. L. et al. Deconvoluting Cr states in Cr-doped UO2 nuclear fuels via bulk and single crystal spectroscopic studies. Nat. Commun. 14, 2455 (2023).
Murphy, G. L., Kegler, P. & Alekseev, E. V. Advances and perspectives of actinide chemistry from ex situ high pressure and high temperature chemical studies. Dalton Trans. 51, 7401–7415 (2022).
Boehler, R. New diamond cell for single–crystal X-ray diffraction. Rev. Sci. Instrum. 77, 115103–1–115103—3 (2006).
Liermann, H.-P. et al. The Extreme Conditions Beamline P02.2 and the Extreme Conditions Science Infrastructure at PETRAIII. J. Synchrotron Radiat. 22, 908–924 (2015).
Hohenberg, P. & Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 136, B864–B871 (1964).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Clark, S. J. et al. First principles methods using CASTEP. Z. Kristallogr. 220, 567–570 (2005).
Tkatchenko, A. & Scheffler, M. Accurate molecular van der waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 102, 073005 (2009).
Acknowledgements
We gratefully acknowledge funding from the DFG (WI1232 and BA4020) and the BMBF (02NUK060).E.B. and M.B. acknowledge the support of the DFG Emmy-Noether Program (projects BY101/2-1 and BY112/2-1) and the Johanna-Quandt-Stiftung. M.B. acknowledges the support by the LOEWE program. B.W. is grateful for support by the Dassault Systémes Science Ambassador program. We acknowledge DESY (Hamburg, Germany), a member of the Helmholtz Association HGF, for the provision of experimental facilities. Parts of this research were carried out at PETRA III, beamline P02.2.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
D.S., L.B., G.L.M., and P.K. performed experiments. V.M. and B.W. performed DFT calculations. N.G.managed the synchrotron beam line. B.W., E.B. and M.B. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spahr, D., Bayarjargal, L., Bykova, E. et al. High-pressure synthesis of U2[CO3]3 and U[CO3]2 as potential host phases for uranium in the Earth’s mantle. Commun Chem (2026). https://doi.org/10.1038/s42004-026-01911-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-026-01911-0