Abstract
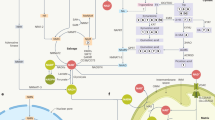
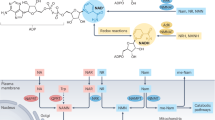
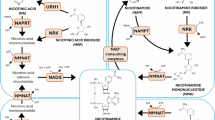
Nicotinamide adenine dinucleotide (NAD+) is an essential molecule involved in cellular metabolism, and its decline has been implicated in ageing and age-related disorders. However, evidence for an age-related decline in NAD+ levels in humans has been consistently observed only in a limited number of studies. Similarly, although preclinical studies support the idea that supplementation with NAD+ precursors is a promising therapeutic strategy to promote healthy ageing, human clinical trials have shown limited efficacy. Therefore, an increasing understanding of how NAD+ metabolism is affected in different tissues during disease and following NAD+ precursor supplementation is crucial to defining the therapeutic value of NAD+-targeted therapies. In this Review, we evaluate the clinical evidence supporting the notion that NAD+ levels decline with age, as well as the tissue-specific effects of NAD+ precursor supplementation. Viewed in perspective, the published body of data on NAD+ dynamics in human tissues remains sparse, and the extrapolation of rodent-based data is not straightforward, underscoring the need for more clinical studies to gain deeper insights into systemic and tissue-specific NAD+ metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Johnson, S. & Imai, S. NAD+ biosynthesis, aging, and disease. F1000Res. 7, 132 (2018).
Houtkooper, R. H., Cantó, C., Wanders, R. J. & Auwerx, J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 31, 194–223 (2010).
Covarrubias, A. J., Perrone, R., Grozio, A. & Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 22, 119–141 (2021).
Zapata-Pérez, R., Wanders, R. J. A., van Karnebeek, C. D. M. & Houtkooper, R. H. NAD+ homeostasis in human health and disease. EMBO Mol. Med. 13, e13943 (2021).
Sauve, A. A. et al. Triple-isotope tracing for pathway discernment of NMN-induced NAD+ biosynthesis in whole mice. Int. J. Mol. Sci. 24, 11114 (2023).
Canto, C. NAD+ precursors: a questionable redundancy. Metabolites 12, 630 (2022).
Garten, A. et al. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 11, 535–546 (2015).
Revollo, J. R., Grimm, A. A. & Imai, S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 279, 50754–50763 (2004).
Bieganowski, P. & Brenner, C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss–Handler independent route to NAD+ in fungi and humans. Cell 117, 495–502 (2004).
Real, A. M., Hong, S. & Pissios, P. Nicotinamide N-oxidation by CYP2E1 in human liver microsomes. Drug Metab. Dispos. 41, 550–553 (2013).
Lenglet, A. et al. N-methyl-2-pyridone-5-carboxamide (2PY)—major metabolite of nicotinamide: an update on an old uremic toxin. Toxins 8, 339 (2016).
Terao, M., Garattini, E., Romão, M. J. & Leimkühler, S. Evolution, expression, and substrate specificities of aldehyde oxidase enzymes in eukaryotes. J. Biol. Chem. 295, 5377–5389 (2020).
Kücükgöze, G. & Leimkühler, S. Direct comparison of the four aldehyde oxidase enzymes present in mouse gives insight into their substrate specificities. PLoS ONE 13, e0191819 (2018).
Mrochek, J. E., Jolley, R. L., Young, D. S. & Turner, W. J. Metabolic response of humans to ingestion of nicotinic acid and nicotinamide. Clin. Chem. 22, 1821–1827 (1976).
Shibata, K. Fate of excess nicotinamide and nicotinic acid differs in rats. J. Nutr. 119, 892–895 (1989).
Berger, F., Lau, C., Dahlmann, M. & Ziegler, M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 280, 36334–36341 (2005).
Cambronne, X. A. et al. Biosensor reveals multiple sources for mitochondrial NAD+. Science 352, 1474–1477 (2016).
Di Lisa, F. & Ziegler, M. Pathophysiological relevance of mitochondria in NAD+ metabolism. FEBS Lett. 492, 4–8 (2001).
Luongo, T. S. et al. SLC25A51 is a mammalian mitochondrial NAD+ transporter. Nature 588, 174–179 (2020).
Girardi, E. et al. Epistasis-driven identification of SLC25A51 as a regulator of human mitochondrial NAD import. Nat. Commun. 11, 6145 (2020).
Kory, N. et al. MCART1/SLC25A51 is required for mitochondrial NAD transport. Sci. Adv. 6, eabe5310 (2020).
Høyland, L. E. et al. Subcellular NAD+ pools are interconnected and buffered by mitochondrial NAD+. Nat. Metab. 6, 2319–2337 (2024).
Nikiforov, A., Dölle, C., Niere, M. & Ziegler, M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J. Biol. Chem. 286, 21767–21778 (2011).
Yang, H. et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130, 1095–1107 (2007).
Pittelli, M. et al. Inhibition of nicotinamide phosphoribosyltransferase: cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J. Biol. Chem. 285, 34106–34114 (2010).
Zhang, T. et al. Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J. Biol. Chem. 284, 20408–20417 (2009).
Ryu, K. W. et al. Metabolic regulation of transcription through compartmentalized NAD+ biosynthesis. Science 360, eaan5780 (2018).
Felici, R., Lapucci, A., Ramazzotti, M. & Chiarugi, A. Insight into molecular and functional properties of NMNAT3 reveals new hints of NAD homeostasis within human mitochondria. PLoS ONE 8, e76938 (2013).
Hikosaka, K. et al. Deficiency of nicotinamide mononucleotide adenylyltransferase 3 (Nmnat3) causes hemolytic anemia by altering the glycolytic flow in mature erythrocytes. J. Biol. Chem. 289, 14796–14811 (2014).
Davila, A. et al. Nicotinamide adenine dinucleotide is transported into mammalian mitochondria. eLife 7, e33246 (2018).
Katsyuba, E., Romani, M., Hofer, D. & Auwerx, J. NAD+ homeostasis in health and disease. Nat. Metab. 2, 9–31 (2020).
Mori, V. et al. Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PLoS ONE 9, e113939 (2014).
Shibata, K., Hayakawa, T. & Iwai, K. Tissue distribution of the enzymes concerned with the biosynthesis of NAD in rats. Agric. Biol. Chem. 50, 3037–3041 (1986).
Bruzzone, S., Guida, L., Zocchi, E., Franco, L. & De Flora, A. Connexin 43 hemichannels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 15, 10–12 (2001).
Billington, R. A. et al. Characterization of NAD uptake in mammalian cells. J. Biol. Chem. 283, 6367–6374 (2008).
Grant, R. et al. A pilot study investigating changes in the human plasma and urine NAD+ metabolome during a 6 hour intravenous infusion of NAD+. Front. Aging Neurosci. 11, 257 (2019).
Karthikeyan, K. & Thappa, D. M. Pellagra and skin. Int. J. Dermatol. 41, 476–481 (2002).
Klaessens, S., Stroobant, V., De Plaen, E. & Van den Eynde, B. J. Systemic tryptophan homeostasis. Front. Mol. Biosci. 9, 897929 (2022).
Palzer, L. et al. Alpha-amino-beta-carboxy-muconate-semialdehyde decarboxylase controls dietary niacin requirements for NAD+ synthesis. Cell Rep. 25, 1359–1370 (2018).
Knopp, R. H. Drug treatment of lipid disorders. N. Engl. J. Med. 341, 498–511 (1999).
Wu, B. J. et al. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids. Arterioscler. Thromb. Vasc. Biol. 30, 968–975 (2010).
Frederick, D. W. et al. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 24, 269–282 (2016).
Tunaru, S. et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9, 352–355 (2003).
Lauring, B. et al. Niacin lipid efficacy is independent of both the niacin receptor GPR109A and free fatty acid suppression. Sci. Transl. Med. 4, 148ra115 (2012).
Burgos, E. S. & Schramm, V. L. Weak coupling of ATP hydrolysis to the chemical equilibrium of human nicotinamide phosphoribosyltransferase. Biochemistry 47, 11086–11096 (2008).
Jacobson, T. A. A “hot” topic in dyslipidemia management—“how to beat a flush”: optimizing niacin tolerability to promote long-term treatment adherence and coronary disease prevention. Mayo Clin. Proc. 85, 365–379 (2010).
Ashihara, H. Metabolism of alkaloids in coffee plants. Braz. J. Plant Physiol. 18, 1–8 (2006).
Membrez, M. et al. Trigonelline is an NAD+ precursor that improves muscle function during ageing and is reduced in human sarcopenia. Nat. Metab. 6, 433–447 (2024).
Zapata-Pérez, R. et al. Reduced nicotinamide mononucleotide is a new and potent NAD+ precursor in mammalian cells and mice. FASEB J. 35, e21456 (2021).
Giroud-Gerbetant, J. et al. A reduced form of nicotinamide riboside defines a new path for NAD+ biosynthesis and acts as an orally bioavailable NAD+ precursor. Mol. Metab. 30, 192–202 (2019).
Chini, C. C. S. et al. Dihydronicotinamide riboside is a potent NAD+ precursor promoting a pro-inflammatory phenotype in macrophages. Front. Immunol. 13, 840246 (2022).
Liu, Y. et al. Reduced nicotinamide mononucleotide (NMNH) potently enhances NAD+ and suppresses glycolysis, the TCA cycle, and cell growth. J. Proteome Res. 20, 2596–2606 (2021).
Shats, I. et al. Bacteria boost mammalian host NAD metabolism by engaging the deamidated biosynthesis pathway. Cell Metab. 31, 564–579 (2020).
Yaku, K. et al. BST1 regulates nicotinamide riboside metabolism via its glycohydrolase and base-exchange activities. Nat. Commun. 12, 6767 (2021).
Chellappa, K. et al. NAD precursors cycle between host tissues and the gut microbiome. Cell Metab. 34, 1947–1959 (2022).
Belenky, P., Christensen, K. C., Gazzaniga, F., Pletnev, A. A. & Brenner, C. Nicotinamide riboside and nicotinic acid riboside salvage in fungi and mammals: quantitative basis for Urh1 and purine nucleoside phosphorylase function in NAD+ metabolism. J. Biol. Chem. 284, 158–164 (2009).
Kropotov, A. et al. Purine nucleoside phosphorylase controls nicotinamide riboside metabolism in mammalian cells. J. Biol. Chem. 298, 102615 (2022).
Liu, L. et al. Quantitative analysis of NAD synthesis–breakdown fluxes. Cell Metab. 27, 1067–1080 (2018).
Ratajczak, J. et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat. Commun. 7, 13103 (2016).
Mukherjee, S. et al. Nicotinamide adenine dinucleotide biosynthesis promotes liver regeneration. Hepatology 65, 616–630 (2017).
Dall, M. et al. Hepatocyte-specific perturbation of NAD+ biosynthetic pathways in mice induces reversible nonalcoholic steatohepatitis-like phenotypes. J. Biol. Chem. 297, 101388 (2021).
Kim, L.-J. et al. Host–microbiome interactions in nicotinamide mononucleotide (NMN) deamidation. FEBS Lett. 597, 2196–2220 (2023).
Grozio, A. et al. CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J. Biol. Chem. 288, 25938–25949 (2013).
Yaku, K. et al. Nicotinamide riboside and nicotinamide mononucleotide facilitate NAD+ synthesis via enterohepatic circulation. Sci. Adv. 11, eadr1538 (2025).
Schmidt, M. S. & Brenner, C. Absence of evidence that Slc12a8 encodes a nicotinamide mononucleotide transporter. Nat. Metab. 1, 660–661 (2019).
Camacho-Pereira, J. et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 23, 1127–1139 (2016).
Madawala, R. et al. CD38 mediates nicotinamide mononucleotide base exchange to yield nicotinic acid mononucleotide. J. Biol. Chem. 301, 108248 (2025).
Yoshida, M. et al. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 30, 329–342 (2019).
Hara, N., Yamada, K., Shibata, T., Osago, H. & Tsuchiya, M. Nicotinamide phosphoribosyltransferase/visfatin does not catalyze nicotinamide mononucleotide formation in blood plasma. PLoS ONE 6, e22781 (2011).
Zapata-Pérez, R. et al. Biotechnological production of reduced and oxidized NAD+ precursors. Food Res. Int. 165, 112560 (2023).
Berven, H. et al. NR-SAFE: a randomized, double-blind safety trial of high dose nicotinamide riboside in Parkinson’s disease. Nat. Commun. 14, 7793 (2023).
Pencina, K. M. et al. MIB-626, an oral formulation of a microcrystalline unique polymorph of β-nicotinamide mononucleotide, increases circulating nicotinamide adenine dinucleotide and its metabolome in middle-aged and older adults. J. Gerontol. A Biol. Sci. Med. Sci. 78, 90–96 (2023).
Wang, P. et al. Fingerstick blood assay maps real-world NAD+ disparity across gender and age. Aging Cell 22, e13965 (2023).
Chaleckis, R., Murakami, I., Takada, J., Kondoh, H. & Yanagida, M. Individual variability in human blood metabolites identifies age-related differences. Proc. Natl Acad. Sci. USA 113, 4252–4259 (2016).
Breton, M. et al. Blood NAD levels are reduced in very old patients hospitalized for heart failure. Exp. Gerontol. 139, 111051 (2020).
Euro, L. et al. Dynamics of blood NAD and glutathione in health, disease, aging and under NAD-booster treatment. Preprint at bioRxiv https://doi.org/10.1101/2025.02.24.639825 (2025).
Yang, F. et al. Association of human whole blood NAD+ contents with aging. Front. Endocrinol. 13, 829658 (2022).
Trammell, S. A. J. et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 7, 12948 (2016).
Song, E.-K. et al. Connexin-43 hemichannels mediate cyclic ADP-ribose generation and its Ca2+-mobilizing activity by NAD+/cyclic ADP-ribose transport. J. Biol. Chem. 286, 44480–44490 (2011).
Montecino-Rodriguez, E., Leathers, H. & Dorshkind, K. Expression of connexin 43 (Cx43) is critical for normal hematopoiesis. Blood 96, 917–924 (2000).
Clement, J., Wong, M., Poljak, A., Sachdev, P. & Braidy, N. The plasma NAD+ metabolome is dysregulated in “normal” aging. Rejuvenation Res. 22, 121–130 (2019).
Schwarzmann, L., Pliquett, R. U., Simm, A. & Bartling, B. Sex-related differences in human plasma NAD+/NADH levels depend on age. Biosci. Rep. 41, BSR20200340 (2021).
Minhas, P. S. et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat. Immunol. 20, 50–63 (2019).
Covarrubias, A. J. et al. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat. Metab. 2, 1265–1283 (2020).
Wagner, S., Manickam, R., Brotto, M. & Tipparaju, S. M. NAD+ centric mechanisms and molecular determinants of skeletal muscle disease and aging. Mol. Cell. Biochem. 477, 1829–1848 (2022).
Sincennes, M.-C. et al. Acetylation of PAX7 controls muscle stem cell self-renewal and differentiation potential in mice. Nat. Commun. 12, 3253 (2021).
Xu, Y. & Xiao, W. NAD+: an old but promising therapeutic agent for skeletal muscle ageing. Ageing Res. Rev. 92, 102106 (2023).
Janssens, G. E. et al. Healthy aging and muscle function are positively associated with NAD+ abundance in humans. Nat. Aging 2, 254–263 (2022).
Strømland, Ø, Diab, J., Ferrario, E., Sverkeli, L. J. & Ziegler, M. The balance between NAD+ biosynthesis and consumption in ageing. Mech. Ageing Dev. 199, 111569 (2021).
de Guia, R. M. et al. Aerobic and resistance exercise training reverses age-dependent decline in NAD+ salvage capacity in human skeletal muscle. Physiol. Rep. 7, e14139 (2019).
Costford, S. R. et al. Skeletal muscle NAMPT is induced by exercise in humans. Am. J. Physiol. Endocrinol. Metab. 298, E117–E126 (2010).
Zhu, X.-H., Lu, M., Lee, B.-Y., Ugurbil, K. & Chen, W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl Acad. Sci. USA 112, 2876–2881 (2015).
Cuenoud, B. et al. Brain NAD is associated with ATP energy production and membrane phospholipid turnover in humans. Front. Aging Neurosci. 12, 609517 (2020).
Bagga, P. et al. Single-voxel 1H MR spectroscopy of cerebral nicotinamide adenine dinucleotide (NAD+) in humans at 7T using a 32-channel volume coil. Magn. Reson. Med. 83, 806–814 (2020).
Lautrup, S., Sinclair, D. A., Mattson, M. P. & Fang, E. F. NAD+ in brain aging and neurodegenerative disorders. Cell Metab. 30, 630–655 (2019).
Zhou, C.-C. et al. Hepatic NAD+ deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br. J. Pharmacol. 173, 2352–2368 (2016).
Franczyk, M. P. et al. Importance of adipose tissue NAD+ biology in regulating metabolic flexibility. Endocrinology 162, bqab006 (2021).
Massudi, H. et al. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS ONE 7, e42357 (2012).
Yalcintepe, L. et al. Changes in NAD/ADP-ribose metabolism in rectal cancer. Braz. J. Med. Biol. Res. 38, 361–365 (2005).
Smits, M. A. J. et al. Human ovarian aging is characterized by oxidative damage and mitochondrial dysfunction. Hum. Reprod. 38, 2208–2220 (2023).
Bai, X. & Wang, P. Relationship between sperm NAD + concentration and reproductive aging in normozoospermia men: a cohort study. BMC Urol. 22, 159 (2022).
Demarest, T. G. et al. Assessment of NAD+ metabolism in human cell cultures, erythrocytes, cerebrospinal fluid and primate skeletal muscle. Anal. Biochem. 572, 1–8 (2019).
Giner, M. P. et al. A method to monitor the NAD+ metabolome—from mechanistic to clinical applications. Int. J. Mol. Sci. 22, 10598 (2021).
Cantó, C. et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15, 838–847 (2012).
Uddin, G. M., Youngson, N. A., Sinclair, D. A. & Morris, M. J. Head to head comparison of short-term treatment with the NAD+ precursor nicotinamide mononucleotide (NMN) and 6 weeks of exercise in obese female mice. Front. Pharmacol. 7, 258 (2016).
Gong, B. et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol. Aging 34, 1581–1588 (2013).
Ramanathan, C. et al. Oral administration of nicotinamide mononucleotide increases nicotinamide adenine dinucleotide level in an animal brain. Nutrients 14, 300 (2022).
de Picciotto, N. E. et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15, 522–530 (2016).
Hou, Y. et al. NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc. Natl Acad. Sci. USA 115, E1876–E1885 (2018).
Kiss, T. et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience 41, 419–439 (2019).
Mills, K. F. et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 24, 795–806 (2016).
Sharma, A., Chabloz, S., Lapides, R. A., Roider, E. & Ewald, C. Y. Potential synergistic supplementation of NAD+ promoting compounds as a strategy for increasing healthspan. Nutrients 15, 445 (2023).
Damgaard, M. V. & Treebak, J. T. What is really known about the effects of nicotinamide riboside supplementation in humans. Sci. Adv. 9, eadi4862 (2023).
Brakedal, B. et al. The NADPARK study: a randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease. Cell Metab. 34, 396–407 (2022).
Elhassan, Y. S. et al. Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 28, 1717–1728 (2019).
Dollerup, O. L. et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 108, 343–353 (2018).
Lapatto, H. A. K. et al. Nicotinamide riboside improves muscle mitochondrial biogenesis, satellite cell differentiation, and gut microbiota in a twin study. Sci. Adv. 9, eadd5163 (2023).
Wu, J. et al. Boosting NAD+ blunts TLR4-induced type I IFN in control and systemic lupus erythematosus monocytes. J. Clin. Invest. 132, e139828 (2022).
Martens, C. R. et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 9, 1286 (2018).
Remie, C. M. E. et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am. J. Clin. Nutr. 112, 413–426 (2020).
Dollerup, O. L. et al. Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. J. Physiol. 598, 731–754 (2020).
Stocks, B. et al. Nicotinamide riboside supplementation does not alter whole-body or skeletal muscle metabolic responses to a single bout of endurance exercise. J. Physiol. 599, 1513–1531 (2021).
Aksoy, S., Szumlanski, C. L. & Weinshilboum, R. M. Human liver nicotinamide N-methyltransferase: cDNA cloning, expression, and biochemical characterization. J. Biol. Chem. 269, 14835–14840 (1994).
Yoshino, M. et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science 372, 1224–1229 (2021).
Yoshino, J., Mills, K. F., Yoon, M. J. & Imai, S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14, 528–536 (2011).
McReynolds, M. R. et al. NAD+ flux is maintained in aged mice despite lower tissue concentrations. Cell Syst. 12, 1160–1172 (2021).
Terpstra, A. H. M. Differences between humans and mice in efficacy of the body fat lowering effect of conjugated linoleic acid: role of metabolic rate. J. Nutr. 131, 2067–2068 (2001).
Wang, H.-L. et al. A luminescent-based protocol for NAD+/NADH detection in C. elegans, mice, and human whole blood. STAR Protoc. 5, 103428 (2024).
Liang, X. et al. Measuring NAD+ levels in mouse blood and tissue samples via a surrogate matrix approach using LC–MS/MS. Bioanalysis 6, 1445–1457 (2014).
Mevenkamp, J. et al. Development of a 31P magnetic resonance spectroscopy technique to quantify NADH and NAD+ at 3 T. Nat. Commun. 15, 9159 (2024).
Hove-Jensen, B. et al. Phosphoribosyl diphosphate (PRPP): biosynthesis, enzymology, utilization, and metabolic significance. Microbiol. Mol. Biol. Rev. 81, e00040-16 (2017).
Pirinen, E. et al. Niacin cures systemic NAD+ deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell Metab. 31, 1078–1090 (2020).
Dolopikou, C. F. et al. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: a double-blind cross-over study. Eur. J. Nutr. 59, 505–515 (2020).
Igarashi, M. et al. Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men. NPJ Aging 8, 5 (2022).
Kim, M. et al. Effect of 12-week intake of nicotinamide mononucleotide on sleep quality, fatigue, and physical performance in older Japanese adults: a randomized, double-blind placebo-controlled study. Nutrients 14, 755 (2022).
Bhullar, K. S. et al. Tripeptide IRW upregulates NAMPT protein levels in cells and obese C57BL/6J mice. J. Agric. Food Chem. 69, 1555–1566 (2021).
Rodríguez-Hernandez, M., Hirano, M., Naini, A. & Santiestéban, R. Biochemical studies of patients with Cuban epidemic neuropathy. Ophthalmic Res. 33, 310–313 (2001).
Katz, A. & Sahlin, K. Effect of decreased oxygen availability on NADH and lactate contents in human skeletal muscle during exercise. Acta Physiol. Scand. 131, 119–127 (1987).
Sahlin, K. NADH and NADPH in human skeletal muscle at rest and during ischaemia. Clin. Physiol. 3, 477–485 (1983).
Balashova, N. V. et al. Efficient assay and marker significance of NAD+ in human blood. Front. Med. 9, 886485 (2022).
Dellinger, R. W. et al. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ Aging Mech. Dis. 3, 17 (2017).
Mierzejewska, P. et al. Nicotinamide metabolism alterations in bladder cancer: preliminary studies. Nucleosides Nucleotides Nucleic Acids 37, 687–695 (2018).
Nagana Gowda, G. A. & Raftery, D. Whole blood metabolomics by 1H NMR spectroscopy provides a new opportunity to evaluate coenzymes and antioxidants. Anal. Chem. 89, 4620–4627 (2017).
Airhart, S. E. et al. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS ONE 12, e0186459 (2017).
Creeke, P. I. et al. Whole blood NAD and NADP concentrations are not depressed in subjects with clinical pellagra. J. Nutr. 137, 2013–2017 (2007).
Formato, M., Masala, B. & De Luca, G. The levels of adenine nucleotides and pyridine coenzymes in red blood cells from the newborn, determined simultaneously by HPLC. Clin. Chim. Acta 189, 131–137 (1990).
Stocchi, V., Cucchiarini, L., Canestrari, F., Piacentini, M. P. & Fornaini, G. A very fast ion-pair reversed-phase HPLC method for the separation of the most significant nucleotides and their degradation products in human red blood cells. Anal. Biochem. 167, 181–190 (1987).
Stocchi, V. et al. Adenine and pyridine nucleotides in the red blood cells of subjects with solid tumors. Tumori 73, 25–28 (1987).
Zerez, C. R., Lee, S. J. & Tanaka, K. R. Spectrophotometric determination of oxidized and reduced pyridine nucleotides in erythrocytes using a single extraction procedure. Anal. Biochem. 164, 367–373 (1987).
Rijksen, G. et al. A new case of purine nucleoside phosphorylase deficiency: enzymologic, clinical, and immunologic characteristics. Pediatr. Res. 21, 137–141 (1987).
Crescentini, G. & Stocchi, V. Fast reversed-phase high-performance liquid chromatographic determination of nucleotides in red blood cells. J. Chromatogr. 290, 393–399 (1984).
Sander, B. J., Oelshlegel, F. J. Jr. & Brewer, G. J. Quantitative analysis of pyridine nucleotides in red blood cells: a single-step extraction procedure. Anal. Biochem. 71, 29–36 (1976).
Aljaser, F. S. et al. Glutathione and oxidized nicotinamide adenine dinucleotide (NAD+) redox status in plasma and placental tissue of Saudi patients with intrauterine growth restriction. Saudi Med. J. 42, 491–498 (2021).
Liu, H. et al. An integrated LC–MS/MS strategy for quantifying the oxidative–redox metabolome in multiple biological samples. Anal. Chem. 92, 8810–8818 (2020).
Shi, H. et al. NAD deficiency, congenital malformations, and niacin supplementation. N. Engl. J. Med. 377, 544–552 (2017).
Braidy, N., Lim, C. K., Grant, R., Brew, B. J. & Guillemin, G. J. Serum nicotinamide adenine dinucleotide levels through disease course in multiple sclerosis. Brain Res. 1537, 267–272 (2013).
Castro-Marrero, J. et al. Does oral coenzyme Q10 plus NADH supplementation improve fatigue and biochemical parameters in chronic fatigue syndrome? Antioxid. Redox Signal. 22, 679–685 (2015).
Weidele, K., Kunzmann, A., Schmitz, M., Beneke, S. & Bürkle, A. Ex vivo supplementation with nicotinic acid enhances cellular poly(ADP-ribosyl)ation and improves cell viability in human peripheral blood mononuclear cells. Biochem. Pharmacol. 80, 1103–1112 (2010).
Liebes, L. F., Krigel, R. L., Conklyn, M., Nevrla, D. R. & Silber, R. Ribonucleotide content of mononuclear cells from normal subjects and patients with chronic lymphocytic leukemia: increased nicotinamide adenine dinucleotide concentration in chronic lymphocytic leukemia lymphocytes. Cancer Res. 43, 5608–5617 (1983).
Parker, R., Schmidt, M. S., Cain, O., Gunson, B. & Brenner, C. Nicotinamide adenine dinucleotide metabolome is functionally depressed in patients undergoing liver transplantation for alcohol-related liver disease. Hepatol. Commun. 4, 1183–1192 (2020).
Azouaoui, D. et al. Meta-analysis of NAD(P)(H) quantification results exhibits variability across mammalian tissues. Sci. Rep. 13, 2464 (2023).
McDermott, M. M. et al. Nicotinamide riboside for peripheral artery disease: the NICE randomized clinical trial. Nat. Commun. 15, 5046 (2024).
Norheim, K. L. et al. Effect of nicotinamide riboside on airway inflammation in COPD: a randomized, placebo-controlled trial. Nat. Aging 4, 1772–1781 (2024).
Orr, M. E. et al. A randomized placebo-controlled trial of nicotinamide riboside in older adults with mild cognitive impairment. Geroscience 46, 665–682 (2024).
Presterud, R. et al. Long-term nicotinamide riboside use improves coordination and eye movements in ataxia telangiectasia. Mov. Disord. 39, 360–369 (2024).
Wang, D. D. et al. Safety and tolerability of nicotinamide riboside in heart failure with reduced ejection fraction. JACC Basic Transl. Sci. 7, 1183–1196 (2022).
Zhou, B. et al. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Invest. 130, 6054–6063 (2020).
Conze, D., Brenner, C. & Kruger, C. L. Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci. Rep. 9, 9772 (2019).
Kimura, S. et al. Nicotinamide mononucleotide is safely metabolized and significantly reduces blood triglyceride levels in healthy individuals. Cureus 14, e28812 (2022).
Okabe, K. et al. Oral administration of nicotinamide mononucleotide is safe and efficiently increases blood nicotinamide adenine dinucleotide levels in healthy subjects. Front. Nutr. 9, 868640 (2022).
Shoji, M. et al. Nicotinamide riboside supplementation benefits in patients with Werner syndrome: a double-blind randomized crossover placebo-controlled trial. Aging Cell 24, e70093 (2025).
Veenhuis, S. J. G. et al. Nicotinamide riboside improves ataxia scores and immunoglobulin levels in ataxia telangiectasia. Mov. Disord. 36, 2951–2957 (2021).
Yamane, T., Imai, M., Bamba, T. & Uchiyama, S. Nicotinamide mononucleotide (NMN) intake increases plasma NMN and insulin levels in healthy subjects. Clin. Nutr. ESPEN 56, 83–86 (2023).
Irie, J. et al. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr. J. 67, 153–160 (2020).
Katayoshi, T. et al. Nicotinamide adenine dinucleotide metabolism and arterial stiffness after long-term nicotinamide mononucleotide supplementation: a randomized, double-blind, placebo-controlled trial. Sci. Rep. 13, 2786 (2023).
Yi, L. et al. The efficacy and safety of β-nicotinamide mononucleotide (NMN) supplementation in healthy middle-aged adults: a randomized, multicenter, double-blind, placebo-controlled, parallel-group, dose-dependent clinical trial. Geroscience 45, 29–43 (2023).
Morita, Y. et al. Clinical evaluation of changes in biomarkers by oral intake of NMN. Glycative Stress Res. 9, 33–41 (2022).
Yamaguchi, S. et al. Safety and efficacy of long-term nicotinamide mononucleotide supplementation on metabolism, sleep, and nicotinamide adenine dinucleotide biosynthesis in healthy, middle-aged Japanese men. Endocr. J. 71, 153–169 (2024).
Qiu, Y. et al. NAD+ exhaustion by CD38 upregulation contributes to blood pressure elevation and vascular damage in hypertension. Signal Transduct. Target. Ther. 8, 353 (2023).
Vreones, M. et al. Oral nicotinamide riboside raises NAD+ and lowers biomarkers of neurodegenerative pathology in plasma extracellular vesicles enriched for neuronal origin. Aging Cell 22, e13754 (2023).
Migaud, M. E., Ziegler, M. & Baur, J. A. Regulation of and challenges in targeting NAD+ metabolism. Nat. Rev. Mol. Cell Biol. 25, 822–840 (2024).
Acknowledgements
The authors gratefully acknowledge the contributions of the NADIS consortium members: F.L. Bertoli (Institut Imagine Paris, France), R.P. Mato (Università degli Studi di Genova, Italy), T. Brochard (University of Oulu, Finland), J. Frank (University of Oslo, Norway), F. Matiyevskaya (University of Copenhagen, Denmark), M.E. Ermert (Khondrion BV, Nijmegen; Amsterdam UMC, Netherlands), Q. Zhang (University of Copenhagen, Denmark), L. Van Gijn (École Polytechnique Fédérale de Lausanne, Switzerland), S. Bruzzone (Università degli Studi di Genova, Italy), M. Deleidi (Institut Imagine Paris, France), M. Scheibye-Knudsen (University of Copenhagen, Denmark), E.F. Fang (University of Oslo, Norway), E. Pirinen (University of Oulu, Finland), H. Renkema (Khondrion BV, Nijmegen, Netherlands) and J. Smeitink (Khondrion BV, Nijmegen, Netherlands). K.T.V., M.M.T., C.C., G.E.J. and R.H.H. received funding from the European Union’s Horizon Europe research and innovation programme through the NADIS project (#101073251). R.Z.-P. is supported by a project (PID2023-147560OA-I00) from the Ministerio de Ciencia, Innovación y Universidades-Agencia Estatal de Investigación and by the Fondo Europeo de Desarrollo Regional (FEDER, EU).
Author information
Authors and Affiliations
Contributions
K.T.V., M.M.T., C.C., R.Z.-P., G.E.J. and R.H.H. conceptualized the article. K.T.V. and M.M.T. wrote the manuscript, with contributions from all coauthors. K.T.V., M.M.T. and E.C. researched data and created the tables. M.v.W. provided substantial input on technical and methodological aspects. All authors reviewed, edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Marie Migaud and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vinten, K.T., Trętowicz, M.M., Coskun, E. et al. NAD+ precursor supplementation in human ageing: clinical evidence and challenges. Nat Metab 7, 1974–1990 (2025). https://doi.org/10.1038/s42255-025-01387-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01387-7
This article is cited by
-
The microbiome at the centre of NAD+ supplementation
Nature Metabolism (2026)