Abstract
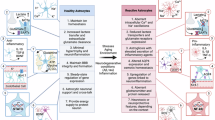
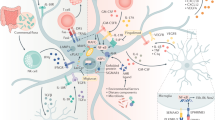
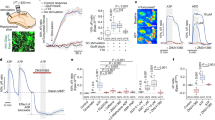
Over the past years, substantial advances have deepened our understanding of the cellular and molecular drivers of brain energy metabolism. Enabled by transformative technologies offering cellular-level resolution, these insights have revealed a highly regulated and dynamic metabolic interplay among brain cell types, particularly between neurons and astrocytes. In this Review, we shed light on the intricate ways in which neurons and astrocytes operate as a metabolically coupled unit, optimized to sustain the energetic demands of neurotransmission while ensuring neuroprotection. We highlight intercellular cooperation as a key determinant of brain function and provide examples of how disruption of the neuron–astrocyte metabolic unit contributes to numerous diseases of the nervous system, underscoring the critical importance of continued fundamental research to dissect the regulatory principles and vulnerabilities of this intercellular metabolic axis and identify potential therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fox, P. T. & Raichle, M. E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl Acad. Sci. USA 83, 1140–1144 (1986).
Fox, P. T., Raichle, M. E., Mintun, M. A. & Dence, C. Nonooxidative glucose consumption during focal physiologic neural activity. Science 241, 462–464 (1988).
Kety, S. S. Circulation and metabolism of the human brain in health and disease. Am. J. Med. 8, 205–217 (1950).
Cummins, C. J., Glover, R. A. & Sellinger, O. Z. Neuronal cues regulate uptake in cultured astrocytes. Brain Res. 170, 190–193 (1979).
Cummins, C. J., Lust, W. D. & Passonneau, J. V. Regulation of glycogen metabolism in primary and transformed astrocytes in vitro. J. Neurochem. 40, 128–136 (1983).
Magistretti, P. J., Morrison, J. H., Shoemaker, W. J., Sapin, V. & Bloom, F. E. Vasoactive intestinal polypeptide induces glycogenolysis in mouse cortical slices: a possible regulatory mechanism for the local control of energy metabolism. Proc. Natl Acad. Sci. USA 78, 6535–6539 (1981).
Kao-Jen, J. & Wilson, J. E. Localization of hexokinase in neural tissue: electron microscopic studies of rat cerebellar cortex. J. Neurochem. 35, 667–678 (1980).
Kadekaro, M., Crane, A. M. & Sokoloff, L. Differential effects of electrical stimulation of sciatic nerve on metabolic activity in spinal cord and dorsal root ganglion in the rat. Proc. Natl Acad. Sci. USA 82, 6010–6013 (1985).
Pellerin, L. & Magistretti, P. J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl Acad. Sci. USA 91, 10625–10629 (1994).
McIlwain, H. A transitory, rapid, production of lactate in electrically excited cerebral tissues. Biochem. J. 60, xxxi (1955).
Schurr, A., West, C. A. & Rigor, B. M. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 240, 1326–1328 (1988).
Voutsinos-Porche, B. et al. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron 37, 275–286 (2003).
Cholet, N. et al. Local injection of antisense oligonucleotides targeted to the glial glutamate transporter GLAST decreases the metabolic response to somatosensory activation. J. Cereb. Blood Flow Metab. 21, 404–412 (2001).
Zimmer, E. R. et al. [18F]FDG PET signal is driven by astroglial glutamate transport. Nat. Neurosci. 20, 393–395 (2017).
Loaiza, A., Porras, O. H. & Barros, L. F. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J. Neurosci. 23, 7337–7342 (2003).
Bittner, C. X. et al. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J. Neurosci. 31, 4709–4713 (2011).
Barros, L. F., Ruminot, I., Sotelo-Hitschfeld, T., Lerchundi, R. & Fernandez-Moncada, I. Metabolic recruitment in brain tissue. Annu. Rev. Physiol. 85, 115–135 (2023).
Bonvento, G. & Bolaños, J. P. Astrocyte–neuron metabolic cooperation shapes brain activity. Cell Metab. 33, 1546–1564 (2021).
Almeida, A., Moncada, S. & Bolaños, J. P. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 6, 45–51 (2004).
Herrero-Mendez, A. et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C–Cdh1. Nat. Cell Biol. 11, 747–752 (2009).
Jimenez-Blasco, D. et al. Weak neuronal glycolysis sustains cognition and organismal fitness. Nat. Metab. 7, 1253–1267 (2024).
Lopez-Fabuel, I. et al. Aberrant upregulation of the glycolytic enzyme PFKFB3 in CLN7 neuronal ceroid lipofuscinosis. Nat. Commun. 13, 536 (2022).
Woo, M. S. et al. The immunoproteasome disturbs neuronal metabolism and drives neurodegeneration in multiple sclerosis. Cell 188, 4567–4585 (2025).
Belanger, M. et al. Role of the glyoxalase system in astrocyte-mediated neuroprotection. J. Neurosci. 31, 18338–18352 (2011).
Cai, X. et al. Lactate activates the mitochondrial electron transport chain independently of its metabolism. Mol. Cell 83, 3904–3920 (2023).
Bolaños, J. P., Peuchen, S., Heales, S. J., Land, J. M. & Clark, J. B. Nitric oxide-mediated inhibition of the mitochondrial respiratory chain in cultured astrocytes. J. Neurochem. 63, 910–916 (1994).
Bolaños, J. P., Heales, S. J. R., Land, J. M. & Clark, J. B. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary cultures. J. Neurochem. 64, 1965–1972 (1995).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Dembitskaya, Y. et al. Lactate supply overtakes glucose when neural computational and cognitive loads scale up. Proc. Natl Acad. Sci. USA 119, e2212004119 (2022).
Roumes, H. et al. Lactate transporters in the rat barrel cortex sustain whisker-dependent BOLD fMRI signal and behavioral performance. Proc. Natl Acad. Sci. USA 118, e2112466118 (2021).
Zuend, M. et al. Arousal-induced cortical activity triggers lactate release from astrocytes. Nat. Metab. 2, 179–191 (2020).
Machler, P. et al. In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. 23, 94–102 (2016).
Suzuki, A. et al. Astrocyte–neuron lactate transport is required for long-term memory formation. Cell 144, 810–823 (2011).
Akter, M. et al. Astrocyte and L-lactate in the anterior cingulate cortex modulate schema memory and neuronal mitochondrial biogenesis. eLife 12, e85751 (2023).
Muraleedharan, R. et al. AMPK-regulated astrocytic lactate shuttle plays a non-cell-autonomous role in neuronal survival. Cell Rep. 32, 108092 (2020).
Bhatti, M. S. & Frostig, R. D. Astrocyte–neuron lactate shuttle plays a pivotal role in sensory-based neuroprotection in a rat model of permanent middle cerebral artery occlusion. Sci. Rep. 13, 12799 (2023).
Petit, J. M. & Magistretti, P. J. Regulation of neuron–astrocyte metabolic coupling across the sleep–wake cycle. Neuroscience 323, 135–156 (2016).
Carrard, A. et al. Role of adult hippocampal neurogenesis in the antidepressant actions of lactate. Mol. Psychiatry 26, 6723–6735 (2021).
Rabah, Y. et al. Glycolysis-derived alanine from glia fuels neuronal mitochondria for memory in Drosophila. Nat. Metab. 5, 2002–2019 (2023).
Volkenhoff, A. et al. Glial glycolysis is essential for neuronal survival in Drosophila. Cell Metab. 22, 437–447 (2015).
Gonzalez-Gutierrez, A., Ibacache, A., Esparza, A., Barros, L. F. & Sierralta, J. Neuronal lactate levels depend on glia-derived lactate during high brain activity in Drosophila. Glia 68, 1213–1227 (2020).
Li, H. et al. Neurons require glucose uptake and glycolysis in vivo. Cell Rep. 42, 112335 (2023).
Cohen, J. & Torres, C. Astrocyte senescence: evidence and significance. Aging Cell 18, e12937 (2019).
Aron, L., Zullo, J. & Yankner, B. A. The adaptive aging brain. Curr. Opin. Neurobiol. 72, 91–100 (2022).
Amaral, D. G., Scharfman, H. E. & Lavenex, P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 163, 3–22 (2007).
Diaz-Garcia, C. M. et al. Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab. 26, 361–374 (2017).
Karsten, S. L. et al. Global analysis of gene expression in neural progenitors reveals specific cell-cycle, signaling, and metabolic networks. Dev. Biol. 261, 165–182 (2003).
Rafalski, V. A. & Brunet, A. Energy metabolism in adult neural stem cell fate. Prog. Neurobiol. 93, 182–203 (2011).
Baeza-Lehnert, F. et al. Non-canonical control of neuronal energy status by the Na+ pump. Cell Metab. 29, 668–680 (2019).
Santos, R. et al. Local glycolysis fuels actomyosin contraction during axonal retraction. J. Cell Biol. 222, e202206133 (2023).
Ketschek, A., Sainath, R., Holland, S. & Gallo, G. The axonal glycolytic pathway contributes to sensory axon extension and growth cone dynamics. J. Neurosci. 41, 6637–6651 (2021).
Wei, Y. et al. Aerobic glycolysis is the predominant means of glucose metabolism in neuronal somata, which protects against oxidative damage. Nat. Neurosci. 26, 2081–2089 (2023).
Blazquez, C., Sanchez, C., Velasco, G. & Guzman, M. Role of carnitine palmitoyltransferase I in the control of ketogenesis in primary cultures of rat astrocytes. J. Neurochem. 71, 1597–1606 (1998).
Eraso-Pichot, A. et al. GSEA of mouse and human mitochondriomes reveals fatty acid oxidation in astrocytes. Glia 66, 1724–1735 (2018).
Fecher, C. et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat. Neurosci. 22, 1731–1742 (2019).
Morant-Ferrando, B. et al. Fatty acid oxidation organizes mitochondrial supercomplexes to sustain astrocytic ROS and cognition. Nat. Metab. 5, 1290–1302 (2023).
Lopez-Fabuel, I. et al. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc. Natl Acad. Sci. USA 113, 13063–13068 (2016).
Vicente-Gutierrez, C. et al. Astrocytic mitochondrial ROS modulate brain metabolism and mouse behaviour. Nat. Metab. 1, 201–211 (2019).
Jimenez-Blasco, D. et al. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature 583, 603–608 (2020).
Blazquez, C., Woods, A., de Ceballos, M. L., Carling, D. & Guzman, M. The AMP-activated protein kinase is involved in the regulation of ketone body production by astrocytes. J. Neurochem. 73, 1674–1682 (1999).
Guzman, M. & Blazquez, C. Is there an astrocyte–neuron ketone body shuttle? Trends Endocrinol. Metab. 12, 169–173 (2001).
Silva, B. et al. Glia fuel neurons with locally synthesized ketone bodies to sustain memory under starvation. Nat. Metab. 4, 213–224 (2022).
McMullen, E. et al. Glycolytically impaired Drosophila glial cells fuel neural metabolism via β-oxidation. Nat. Commun. 14, 2996 (2023).
Liu, L. et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 160, 177–190 (2015).
Liu, L., MacKenzie, K. R., Putluri, N., Maletic-Savatic, M. & Bellen, H. J. The glia–neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metab. 26, 719–737 (2017).
Kumar, M. et al. Triglycerides are an important fuel reserve for synapse function in the brain. Nat. Metab. 7, 1392–1403 (2025).
Saber, S. H. et al. DDHD2 provides a flux of saturated fatty acids for neuronal energy and function. Nat. Metab. https://doi.org/10.1038/s42255-025-01367-x (2025).
Greda, A. K. et al. Interaction of sortilin with apolipoprotein E3 enables neurons to use long-chain fatty acids as alternative metabolic fuel. Nat. Metab. https://doi.org/10.1038/s42255-025-01389-5 (2025).
Yang, J. et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl Acad. Sci. USA 111, 12228–12233 (2014).
Neame, S. et al. The NMDA receptor activation by d-serine and glycine is controlled by an astrocytic Phgdh-dependent serine shuttle. Proc. Natl Acad. Sci. USA 116, 20736–20742 (2019).
Le Douce, J. et al. Impairment of glycolysis-derived L-serine production in astrocytes contributes to cognitive deficits in Alzheimer’s disease. Cell Metab. 31, 503–517 (2020).
Magistretti, P. J. & Allaman, I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 19, 235–249 (2018).
Matsui, T. et al. Astrocytic glycogen-derived lactate fuels the brain during exhaustive exercise to maintain endurance capacity. Proc. Natl Acad. Sci. USA 114, 6358–6363 (2017).
Gao, V. et al. Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc. Natl Acad. Sci. USA 113, 8526–8531 (2016).
Fiumelli, H. et al. Lactate potentiates NMDA receptor currents via an intracellular redox mechanism targeting cysteines in the C-terminal domain of GluN2B subunits: implications for synaptic plasticity. Preprint at bioRxiv https://doi.org/10.1101/2024.11.21.624499 (2024).
Bonvento, G., Oliet, S. H. R. & Panatier, A. Glycolysis-derived L-serine levels versus PHGDH expression in Alzheimer’s disease. Cell Metab. 34, 654–655 (2022).
Fernández-Moncada, I. et al. A lactate-dependent shift of glycolysis mediates synaptic and cognitive processes. Nat. Commun. 15, 6842 (2024).
Brandebura, A. N., Paumier, A., Onur, T. S. & Allen, N. J. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat. Rev. Neurosci. 24, 23–39 (2023).
Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 539, 180–186 (2016).
Goyal, M. S. et al. Loss of brain aerobic glycolysis in normal human aging. Cell Metab. 26, 353–360 (2017).
Goyal, M. S. et al. Brain aerobic glycolysis and resilience in Alzheimer disease. Proc. Natl Acad. Sci. USA 120, e2212256120 (2023).
Hipkiss, A. R. Aging, Alzheimer’s disease and dysfunctional glycolysis; similar effects of too much and too little. Aging Dis. 10, 1328–1331 (2019).
Boumezbeur, F. et al. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab. 30, 211–221 (2010).
Beard, E., Lengacher, S., Dias, S., Magistretti, P. J. & Finsterwald, C. Astrocytes as key regulators of brain energy metabolism: new therapeutic perspectives. Front. Physiol. 12, 825816 (2021).
Tefera, T. W., Steyn, F. J., Ngo, S. T. & Borges, K. CNS glucose metabolism in amyotrophic lateral sclerosis: a therapeutic target? Cell Biosci. 11, 14 (2021).
Maksimovic, K., Youssef, M., You, J., Sung, H. K. & Park, J. Evidence of metabolic dysfunction in amyotrophic lateral sclerosis (ALS) patients and animal models. Biomolecules 13, 863 (2023).
Weerasekera, A. et al. Non-invasive characterization of amyotrophic lateral sclerosis in a hTDP-43A315T mouse model: a PET–MR study. Neuroimage Clin. 27, 102327 (2020).
Browne, S. E. et al. Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A SOD1 mouse model of ALS. Neurobiol. Dis. 22, 599–610 (2006).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012).
Sonninen, T. M. et al. Metabolic alterations in Parkinson’s disease astrocytes. Sci. Rep. 10, 14474 (2020).
Rappold, P. M. & Tieu, K. Astrocytes and therapeutics for Parkinson’s disease. Neurotherapeutics 7, 413–423 (2010).
Damier, P., Hirsch, E. C., Zhang, P., Agid, Y. & Javoy-Agid, F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 52, 1–6 (1993).
Zeevalk, G. D., Razmpour, R. & Bernard, L. P. Glutathione and Parkinson’s disease: is this the elephant in the room?. Biomed. Pharmacother. 62, 236–249 (2008).
Proschel, C., Stripay, J. L., Shih, C. H., Munger, J. C. & Noble, M. D. Delayed transplantation of precursor cell-derived astrocytes provides multiple benefits in a rat model of Parkinsons. EMBO Mol. Med. 6, 504–518 (2014).
Butterfield, D. A. & Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 20, 148–160 (2019).
Mosconi, L., Pupi, A. & De Leon, M. J. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1147, 180–195 (2008).
Minhas, P. S. et al. Restoring hippocampal glucose metabolism rescues cognition across Alzheimer’s disease pathologies. Science 385, eabm6131 (2024).
Williams, H. C. et al. APOE alters glucose flux through central carbon pathways in astrocytes. Neurobiol. Dis. 136, 104742 (2020).
Pascual, J. M. et al. GLUT1 deficiency and other glucose transporter diseases. Eur. J. Endocrinol. 150, 627–633 (2004).
Xu, W. & Borges, K. Case for supporting astrocyte energetics in glucose transporter 1 deficiency syndrome. Epilepsia 65, 2213–2226 (2024).
Tsacopoulos, M. & Magistretti, P. J. Metabolic coupling between glia and neurons. J. Neurosci. 16, 877–885 (1996).
Welch, G. R. & Easterby, J. S. Metabolic channeling versus free diffusion: transition-time analysis. Trends Biochem. Sci. 19, 193–197 (1994).
Berridge, M. V., Schneider, R. T. & McConnell, M. J. Mitochondrial transfer from astrocytes to neurons following ischemic insult: guilt by association? Cell Metab. 24, 376–378 (2016).
Dong, L. F. et al. Mitochondria on the move: horizontal mitochondrial transfer in disease and health. J. Cell Biol. 222, e202211044 (2023).
Boudreau, L. H. et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 124, 2173–2183 (2014).
Makar, T. K. et al. Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurones: evidence that astrocytes play an important role in antioxidative processes in the brain. J. Neurochem. 62, 45–53 (1994).
Bolaños, J. P. et al. Nitric oxide-mediated mitochondrial damage: a potential neuroprotective role for glutathione. Free Radic. Biol. Med. 21, 995–1001 (1996).
Dringen, R., Kussmaul, L., Gutterer, J. M., Hirrlinger, J. & Hamprecht, B. The glutathione system of peroxide detoxification is less efficient in neurons than in astrocytes. J. Neurochem. 72, 2523–2530 (1999).
Jimenez-Blasco, D., Santofimia-Castano, P., Gonzalez, A., Almeida, A. & Bolaños, J. P. Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5–Nrf2 pathway. Cell Death Differ. 22, 1877–1889 (2015).
Bell, K. F. et al. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat. Commun. 6, 7066 (2015).
Dringen, R., Pfeiffer, B. & Hamprecht, B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J. Neurosci. 19, 562–569 (1999).
Funfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Looser, Z. J. et al. Oligodendrocyte–axon metabolic coupling is mediated by extracellular K+ and maintains axonal health. Nat. Neurosci. 27, 433–448 (2024).
Spate, E. et al. Downregulated expression of lactate dehydrogenase in adult oligodendrocytes and its implication for the transfer of glycolysis products to axons. Glia 72, 1374–1391 (2024).
Asadollahi, E. et al. Oligodendroglial fatty acid metabolism as a central nervous system energy reserve. Nat. Neurosci. 27, 1934–1944 (2024).
Ramos-Cabrer, P. et al. Reversible reduction in brain myelin content upon marathon running. Nat. Metab. 7, 697–703 (2025).
Acknowledgements
J.P.B. is funded by MICIU/AEI (PID2022-138813OB-I00/10.13039/501100011033 and FEDER, UE), la Caixa Foundation (grant agreement LCF/PR/HR23/52430016), the European Union’s Horizon Europe research and innovation programme under the MSCA Doctoral Networks 2021 (101072759, fuel the brain in healthy ageing and age-related diseases (ETERNITY)) and the European Research Council Advanced Grant NeuroSTARS (reference 101199747). Research in P.J.M.’s laboratory is funded by King Abdullah University of Science and Technology.
Author information
Authors and Affiliations
Contributions
J.P.B. and P.J.M. conceived the idea and wrote, edited and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
P.J.M. is the scientific founder of GliaPharm. J.P.B declares no competing interests.
Peer review
Peer review information
Nature Metabolism thanks L. Felipe Barros and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Gimenez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bolaños, J.P., Magistretti, P.J. The neuron–astrocyte metabolic unit as a cornerstone of brain energy metabolism in health and disease. Nat Metab 7, 2414–2423 (2025). https://doi.org/10.1038/s42255-025-01404-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01404-9
This article is cited by
-
Signaling roles for astrocytic lipid metabolism in brain function
EMBO Reports (2026)