Abstract
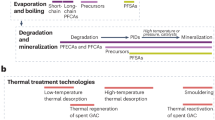
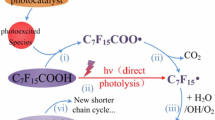
Per- and polyfluoroalkyl substances (PFASs) are synthetic chemicals used across numerous industrial and consumer applications. Their persistence and toxicological impacts necessitate their removal from the environment and their complete destruction; however, many PFAS destruction technologies release gas-phase and aerosol-phase fluorinated products of incomplete destruction (PIDs). In this Review, we discuss the PIDs released by PFAS destruction methods and approaches to categorize and measure them. Existing and emerging technologies use thermal, chemical, electrical or biological approaches to degrade PFASs, with varying degrees of success. Although many technologies achieve destruction and removal efficiencies of more than 99.99%, this metric does not account for the formation of airborne PIDs. Methods are being developed to measure PIDs in air emissions to protect exposed communities and facilitate the closure of fluorine mass balances. The choice of sampling and analytical methods depends on the polarity and volatility of the PIDs. Closing the mass balance is important because PIDs such as CF4 can have unintended global impacts, and potential localized risks from exposure to PIDs remain uncertain. Therefore, diverse analytical methods are needed to comprehensively characterize PIDs; such characterization is important to identify PFAS destruction technologies that minimize atmospheric emissions and their associated environmental and human health risks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kwiatkowski, C. F. et al. Scientific basis for managing PFAS as a chemical class. Environ. Sci. Technol. Lett. 7, 532–543 (2020).
Glüge, J. et al. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 22, 2345–2373 (2020).
Ng, C. et al. Addressing urgent questions for PFAS in the 21st century. Environ. Sci. Technol. https://doi.org/10.1021/acs.est.1c03386 (2021).
Zhang, Z., Sarkar, D., Biswas, J. K. & Datta, R. Biodegradation of per- and polyfluoroalkyl substances (PFAS): a review. Bioresour. Technol. 344, 126223 (2022).
Cousins, I. T. et al. The high persistence of PFAS is sufficient for their management as a chemical class. Environ. Sci. Process. Impacts 22, 2307–2312 (2020).
Hopkins, Z. R., Sun, M., DeWitt, J. C. & Knappe, D. R. U. Recently detected drinking water contaminants: GenX and other per- and polyfluoroalkyl ether acids. J. AWWA 110, 13–28 (2018).
Benskin, J. P., Li, B., Ikonomou, M. G., Grace, J. R. & Li, L. Y. Per- and polyfluoroalkyl substances in landfill leachate: patterns, time trends, and sources. Environ. Sci. Technol. 46, 11532–11540 (2012).
Oliaei, F., Kriens, D., Weber, R. & Watson, A. PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA). Environ. Sci. Pollut. Res. 20, 1977–1992 (2013).
Human Health Toxicity Assessment for Perfluorooctanoic Acid (PFOA) and Related Salts (USEPA, 2024).
Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) Vol. 135 (International Agency for Research on Cancer, 2025).
Wanzek, T. et al. A multiple lines of evidence approach to demonstrate effectiveness of PFAS remediation technologies. Groundw. Monit. Remediation 44, 30–38 (2024).
Smith, S. J. et al. The need to include a fluorine mass balance in the development of effective technologies for PFAS destruction. Environ. Sci. Technol. 58, 2587–2590 (2024).
Houtz, E. F., Higgins, C. P., Field, J. A. & Sedlak, D. L. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ. Sci. Technol. 47, 8187–8195 (2013).
Liu, S. et al. Perfluoroalkyl substances (PFASs) in leachate, fly ash, and bottom ash from waste incineration plants: implications for the environmental release of PFAS. Sci. Total. Environ. 795, 148468 (2021).
Björklund, S., Weidemann, E. & Jansson, S. Emission of per- and polyfluoroalkyl substances from a waste-to-energy plant—occurrence in ashes, treated process water, and first observation in flue gas. Environ. Sci. Technol. 57, 10089–10095 (2023).
Block, C., Van Caneghem, J., Van Brecht, A., Wauters, G. & Vandecasteele, C. Incineration of hazardous waste: a sustainable process? Waste Biomass Valoriz. 6, 137–145 (2015).
Winchell, L. J. et al. Fate of perfluoroalkyl and polyfluoroalkyl substances (PFAS) through two full-scale wastewater sludge incinerators. Water Environ. Res. 96, 11009 (2024).
Weber, N. H. et al. Thermal mineralization of perfluorooctanesulfonic acid (PFOS) to HF, CO2, and SO2. Ind. Eng. Chem. Res. 62, 881–892 (2023).
Lim, X. How to get rid of toxic ‘forever chemical’ pollution. Nature 640, 22–24 (2025).
Longendyke, G. K., Katel, S. & Wang, Y. PFAS fate and destruction mechanisms during thermal treatment: a comprehensive review. Environ. Sci. Process. Impacts 24, 196–208 (2022).
Weber, N. H. et al. Thermal decomposition of perfluorooctanesulfonic acid (PFOS) in the presence of water vapor. Ind. Eng. Chem. Res. 61, 15146–15155 (2022).
Kuepouo, G., Jelinek, N., Bell, L., Petrlik, J. & Grechko, V. Trials of Burning PFASs Containing Wastes in a Waste Incinerator and Cement Kiln Assessed against Stockholm Convention Objectives (IPEN, 2022); https://ipen.org/sites/default/files/documents/dioxin2022_pfas-australia_f.pdf.
Watanabe, N., Takemine, S., Yamamoto, K., Haga, Y. & Takata, M. Residual organic fluorinated compounds from thermal treatment of PFOA, PFHxA and PFOS adsorbed onto granular activated carbon (GAC). J. Mater. Cycles Waste Manag. 18, 625–630 (2016).
Austin, C. et al. Hydrothermal destruction and defluorination of trifluoroacetic acid (TFA). Environ. Sci. Technol. 58, 8076–8085 (2024).
Austin, C. et al. Destruction and defluorination of PFAS matrix in continuous-flow supercritical water oxidation reactor: effect of operating temperature. Chemosphere 327, 138358 (2023).
Cheng, J., Vecitis, C. D., Park, H., Mader, B. T. & Hoffmann, M. R. Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in landfill groundwater: environmental matrix effects. Environ. Sci. Technol. 42, 8057–8063 (2008).
Stratton, G. R. et al. Plasma-based water treatment: efficient transformation of perfluoroalkyl substances in prepared solutions and contaminated groundwater. Environ. Sci. Technol. 51, 1643–1648 (2017).
Park, H. et al. Reductive defluorination of aqueous perfluorinated alkyl surfactants: effects of ionic headgroup and chain length. J. Phys. Chem. A 113, 690–696 (2009).
Nzeribe, B. N., Crimi, M., Mededovic Thagard, S. & Holsen, T. M. Physico-chemical processes for the treatment of per- and polyfluoroalkyl substances (PFAS): a review. Crit. Rev. Environ. Sci. Technol. 49, 866–915 (2019).
Sühnholz, S., Gawel, A., Kopinke, F.-D. & Mackenzie, K. Evidence of heterogeneous degradation of PFOA by activated persulfate — FeS as adsorber and activator. Chem. Eng. J. 423, 130102 (2021).
Yi, L. et al. Enhanced degradation of perfluorooctanoic acid by a genome shuffling-modified Pseudomonas parafulva YAB-1. Environ. Technol. 40, 3153–3161 (2018).
Sikder, S., Toha, M. & Rahman, M. M. in Technical Landfills and Waste Management (eds Souabi, S. & Anouzla, A.) 169–188 (Springer, 2024).
Thermal oxidizer performance test report. Chemours https://www.chemours.com/en/-/media/files/corporate/fayetteville-works/2020-03-thermal-oxidizer-test-report.pdf (2020).
SW-846 Compendium: Incineration https://www.epa.gov/sites/default/files/2015-10/documents/chap13.pdf (USEPA, 1986).
Weber, N. H. et al. Formation of products of incomplete destruction (PID) from the thermal oxidative decomposition of perfluorooctanoic acid (PFOA): measurement, modeling, and reaction pathways. J. Phys. Chem. A 128, 5362–5373 (2024).
Alinezhad, A. et al. Mechanistic investigations of thermal decomposition of perfluoroalkyl ether carboxylic acids and short-chain perfluoroalkyl carboxylic acids. Environ. Sci. Technol. 57, 8796–8807 (2023).
Mattila, J. M. et al. Characterizing volatile emissions and combustion byproducts from aqueous film-forming foams using online chemical ionization mass spectrometry. Environ. Sci. Technol. 58, 3942–3952 (2024).
Shields, E. P. et al. Pilot-scale thermal destruction of per- and polyfluoroalkyl substances in a legacy aqueous film forming foam. ACS ES&T Eng. 3, 1308–1317 (2023).
Galloway, J. E. et al. Evidence of air dispersion: HFPO–DA and PFOA in Ohio and West Virginia surface water and soil near a fluoropolymer production facility. Environ. Sci. Technol. 54, 7175–7184 (2020).
D’Ambro, E. L. et al. Characterizing the air emissions, transport, and deposition of per- and polyfluoroalkyl substances from a fluoropolymer manufacturing facility. Environ. Sci. Technol. 55, 862–870 (2021).
Zhou, J. et al. PFOS dominates PFAS composition in ambient fine particulate matter (PM2.5) collected across North Carolina nearly 20 years after the end of its US production. Environ. Sci. Process. Impacts 23, 580–587 (2021).
Abou-Khalil, C., Sarkar, D., Braykaa, P. & Boufadel, M. C. Mobilization of per- and polyfluoroalkyl substances (PFAS) in soils: a review. Curr. Pollut. Rep. 8, 422–444 (2022).
Boyer, T. H. et al. Anion exchange resin removal of per- and polyfluoroalkyl substances (PFAS) from impacted water: a critical review. Water Res. 200, 117244 (2021).
Sleep, J. A. & Juhasz, A. L. A review of immobilisation-based remediation of per- and poly-fluoroalkyl substances (PFAS) in soils. Curr. Pollut. Rep. 7, 524–539 (2021).
Liu, C. et al. Evaluating the efficiency of nanofiltration and reverse osmosis membrane processes for the removal of per- and polyfluoroalkyl substances from water: a critical review. Sep. Purif. Technol. 302, 122161 (2022).
Wallace, J. S. et al. Burning questions: current practices and critical gaps in evaluating removal of per- and polyfluoroalkyl substances (PFAS) during pyrolysis treatments of biosolids. J. Hazard. Mater. Lett. 4, 100079 (2023).
Appleman, T. D., Dickenson, E. R. V., Bellona, C. & Higgins, C. P. Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. J. Hazard. Mater. 260, 740–746 (2013).
Ochoa-Herrera, V. & Sierra-Alvarez, R. Removal of perfluorinated surfactants by sorption onto granular activated carbon, zeolite and sludge. Chemosphere 72, 1588–1593 (2008).
Zeng, C. et al. Removing per- and polyfluoroalkyl substances from groundwaters using activated carbon and ion exchange resin packed columns. AWWA Water Sci. 2, e1172 (2020).
Kothawala, D. N., Köhler, S. J., Östlund, A., Wiberg, K. & Ahrens, L. Influence of dissolved organic matter concentration and composition on the removal efficiency of perfluoroalkyl substances (PFASs) during drinking water treatment. Water Res. 121, 320–328 (2017).
Tang, C. Y., Fu, Q. S., Robertson, A. P., Criddle, C. S. & Leckie, J. O. Use of reverse osmosis membranes to remove perfluorooctane sulfonate (PFOS) from semiconductor wastewater. Environ. Sci. Technol. 40, 7343–7349 (2006).
Smith, S. J., Wiberg, K., McCleaf, P. & Ahrens, L. Pilot-scale continuous foam fractionation for the removal of per- and polyfluoroalkyl substances (PFAS) from landfill leachate. ACS ES&T Water 2, 841–851 (2022).
Vecitis, C. D., Park, H., Cheng, J., Mader, B. T. & Hoffmann, M. R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Front. Environ. Sci. Eng. China 3, 129–151 (2009).
Burns, D. J., Stevenson, P. & Murphy, P. J. C. PFAS removal from groundwaters using surface-active foam fractionation. Remediation J. 31, 19–33 (2021).
McCleaf, P. et al. Removal efficiency of multiple poly- and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Res. 120, 77–87 (2017).
Saeidi, N., Harnisch, F., Presser, V., Kopinke, F.-D. & Georgi, A. Electrosorption of organic compounds: state of the art, challenges, performance, and perspectives. Chem. Eng. J. 471, 144354 (2023).
Vu, C. T. & Wu, T. Recent progress in adsorptive removal of per- and poly-fluoroalkyl substances (PFAS) from water/wastewater. Crit. Rev. Environ. Sci. Technol. 52, 90–129 (2022).
Lin, Z.-W., Wang, J., Dyakiv, Y., Helbling, D. E. & Dichtel, W. R. Structural features of styrene-functionalized cyclodextrin polymers that promote the adsorption of perfluoroalkyl acids in water. ACS Appl. Mater. Interfaces 16, 28409–28422 (2024).
Hale, S. E. et al. Sorbent amendment as a remediation strategy to reduce PFAS mobility and leaching in a contaminated sandy soil from a Norwegian firefighting training facility. Chemosphere 171, 9–18 (2017).
Divine, C. et al. Field demonstration of in situ stabilization (ISS) of per- and polyfluoroalkyl substances in soil with remBind®. J. Hazard. Mater. 499, 140127 (2025).
Grimison, C. et al. The efficacy of soil washing for the remediation of per- and poly-fluoroalkyl substances (PFASs) in the field. J. Hazard. Mater. 445, 130441 (2023).
Høisæter, Å et al. Excavated vs novel in situ soil washing as a remediation strategy for sandy soils impacted with per- and polyfluoroalkyl substances from aqueous film forming foams. Sci. Total. Environ. 794, 148763 (2021).
Khan, M. Y., So, S. & da Silva, G. Decomposition kinetics of perfluorinated sulfonic acids. Chemosphere 238, 124615 (2020).
Ram, H., DePompa, C. M. & Westmoreland, P. R. Thermochemistry of gas-phase thermal oxidation of C2 to C8 perfluorinated sulfonic acids with extrapolation to C16. J. Phys. Chem. A 128, 3387–3395 (2024).
Ram, H., Sadej, T. P., Murphy, C. C., Mallo, T. J. & Westmoreland, P. R. Thermochemistry of species in gas-phase thermal oxidation of C2 to C8 perfluorinated carboxylic acids. J. Phys. Chem. A 128, 1313–1326 (2024).
Altarawneh, M., Almatarneh, M. H. & Dlugogorski, B. Z. Thermal decomposition of perfluorinated carboxylic acids: kinetic model and theoretical requirements for PFAS incineration. Chemosphere 286, 131685 (2022).
Blotevogel, J., Giraud, R. J. & Rappé, A. K. Incinerability of PFOA and HFPO-DA: mechanisms, kinetics, and thermal stability ranking. Chem. Eng. J. 457, 141235 (2023).
Bentel, M. J. et al. Defluorination of per- and polyfluoroalkyl substances (PFASs) with hydrated electrons: structural dependence and implications to PFAS remediation and management. Environ. Sci. Technol. 53, 3718–3728 (2019).
Blotevogel, J., Giraud, R. J. & Borch, T. Reductive defluorination of perfluorooctanoic acid by zero-valent iron and zinc: a DFT-based kinetic model. Chem. Eng. J. 335, 248–254 (2018).
Blotevogel, J., Joyce, J. P., Hill, O. L. & Rappé, A. K. Headgroup dependence and kinetic bottlenecks of gas-phase thermal PFAS destruction. ACS ES&T Eng. https://doi.org/10.1021/acsestengg.4c00726 (2025).
Weber, N. H. et al. Thermal decomposition of PFOA: influence of reactor and reaction conditions on product formation. Chem. Eng. Sci. 278, 118924 (2023).
Trang, B. et al. Low-temperature mineralization of perfluorocarboxylic acids. Science 377, 839–845 (2022).
LaZerte, J. D., Hals, L. J., Reid, T. S. & Smith, G. H. Pyrolyses of the salts of the perfluoro carboxylic acids. J. Am. Chem. Soc. 75, 4525–4528 (1953).
Weber, N. H. et al. Modeling and experimental study on the thermal decomposition of perfluorooctanesulfonic acid (PFOS) in an α-alumina reactor. Ind. Eng. Chem. Res. 61, 5453–5463 (2022).
Weber, N. H. et al. Influence of reactor composition on the thermal decomposition of perfluorooctanesulfonic acid (PFOS). J. Hazard. Mater. 461, 132665 (2024).
Yao, B. et al. The first quantitative investigation of compounds generated from PFAS, PFAS-containing aqueous film-forming foams and commercial fluorosurfactants in pyrolytic processes. J. Hazard. Mater. 436, 129313 (2022).
Xu, M.-G. et al. Direct measurement of fluorocarbon radicals in the thermal destruction of perfluorohexanoic acid using photoionization mass spectrometry. Sci. Adv. 11, 3363 (2025).
Wang, F., Shih, K., Lu, X. & Liu, C. Mineralization behavior of fluorine in perfluorooctanesulfonate (PFOS) during thermal treatment of lime-conditioned sludge. Environ. Sci. Technol. 47, 2621–2627 (2013).
Wang, F., Lu, X., Li, X. & Shih, K. Effectiveness and mechanisms of defluorination of perfluorinated alkyl substances by calcium compounds during waste thermal treatment. Environ. Sci. Technol. 49, 5672–5680 (2015).
Abou-Khalil, C. et al. Enhancing the thermal mineralization of perfluorooctanesulfonate on granular activated carbon using alkali and alkaline-earth metal additives. Environ. Sci. Technol. 58, 11162–11174 (2024).
Wang, F., Lu, X., Shih, K. & Liu, C. Influence of calcium hydroxide on the fate of perfluorooctanesulfonate under thermal conditions. J. Hazard. Mater. 192, 1067–1071 (2011).
Knappe, D. R. U., Dagois, G. & DeWolfe, J. R. Effect of calcium on thermal regeneration of GAC. J. AWWA 84, 73–80 (1992).
40 CFR § 63.1206 – When and How Must You Comply with the Standards and Operating Requirements? Title 40, Chapter I, Subchapter C, Part 63, Subpart EEE (USEFA, 2025).
40 CFR § 264.343 – Incinerators: Performance Standards. Title 40, Chapter I, Subchapter I, Part 264, Subpart O (USEPA, 2025).
Interim Guidance on the Destruction and Disposal of Perfluoroalkyl and Polyfluoroalkyl Substances and Materials Containing Perfluoroalkyl and Polyfluoroalkyl Substances—Version 2 (2024) (USEPA, 2024).
Troxler, W. et al. PFAS Destruction by a Hazardous Waste Incinerator: Testing Results (USEPA, 2025).
Weber, N. H. et al. Kinetics of decomposition of PFOS relevant to thermal desorption remediation of soils. Ind. Eng. Chem. Res. 60, 9080–9087 (2021).
Air Pollution Control Technology Fact Sheet. EPA-CICA Fact Sheet EPA-452/F-03-022 (USEPA, 2020).
Jordens, A., Makoudi, M., Saadaoui, H. & Haemers, J. Remediation of dioxin-contaminated soils through thermal desorption and vapor management via thermal oxidizer at Bien Hoa airbase, Vietnam. Environ. Eng. Manag. J. 22, 1735–1744 (2023).
Shields, E. P., Roberson, W. R., Ryan, J. V. & Jackson, S. R. The use of air pollution controls to reduce the gas-phase emissions of per- and polyfluoroalkyl substances from a fluoropolymer manufacturing facility. Environ. Sci. Technol. Lett. 12, 768–773 (2025).
Huber, S., Moe, M. K., Schmidbauer, N., Hansen, G. H. & Herzke, D. Emissions from Incineration of Fluoropolymer Materials. A Literature Survey (Norwegian Institute for Air Research, 2009); https://nilu.com/wp-content/uploads/dnn/12-2009-dhe-nyutgave4.pdf.
Bamdad, H., Papari, S., Moreside, E. & Berruti, F. High-temperature pyrolysis for elimination of per- and polyfluoroalkyl substances (PFAS) from biosolids. Processes 10, 2187 (2022).
Sørmo, E. et al. The decomposition and emission factors of a wide range of PFAS in diverse, contaminated organic waste fractions undergoing dry pyrolysis. J. Hazard. Mater. 454, 131447 (2023).
McNamara, P. et al. Pyrolysis transports, and transforms, PFAS from biosolids to py-liquid. Environ. Sci. 9, 386–395 (2023).
Mayerberger, E. et al. Destruction of PFAS during thermal reactivation of granular activated carbon used in potable water treatment. Remediation J. 35, 70030 (2025).
Watanabe, N., Takata, M., Takemine, S. & Yamamoto, K. Thermal mineralization behavior of PFOA, PFHxA, and PFOS during reactivation of granular activated carbon (GAC) in nitrogen atmosphere. Environ. Sci. Pollut. Res. 25, 7200–7205 (2015).
Xiao, F. et al. Thermal stability and decomposition of perfluoroalkyl substances on spent granular activated carbon. Environ. Sci. Technol. Lett. 7, 343–350 (2020).
Hao, S. et al. Hydrothermal alkaline treatment for destruction of per- and polyfluoroalkyl substances in aqueous film-forming foam. Environ. Sci. Technol. 55, 3283–3295 (2021).
Wen, J., Neha, S., Biller, P., Kristensen, K. & Vergeynst, L. Detection of volatile hydroperfluoroalkanes during hydrothermal liquefaction of perfluoroalkyl carboxylic acids at circumneutral pH. J. Hazard. Mater. 476, 134955 (2024).
Rosansky, S. et al. Field demonstration of PFAS destruction in various alcohol-resistant AFFFs using supercritical water oxidation (SCWO). ACS ES&T Water 4, 4486–4496 (2024).
Ross, I. et al. A review of emerging technologies for remediation of PFASs. Remediation J. 28, 101–126 (2018).
Hori, H. et al. Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant. Environ. Sci. Technol. 39, 2383–2388 (2005).
Zhang, C., Hopkins, Z. R., McCord, J., Strynar, M. J. & Knappe, D. R. U. Fate of per- and polyfluoroalkyl ether acids in the total oxidizable precursor assay and implications for the analysis of impacted water. Environ. Sci. Technol. Lett. 6, 662–668 (2019).
Qian, L., Kopinke, F.-D., Scherzer, T., Griebel, J. & Georgi, A. Enhanced degradation of perfluorooctanoic acid by heat-activated persulfate in the presence of zeolites. Chem. Eng. J. 429, 132500 (2022).
Cao, C.-S. et al. A review on the advancement in photocatalytic degradation of poly/perfluoroalkyl substances in water: insights into the mechanisms and structure-function relationship. Sci. Total Environ. 946, 174137 (2024).
Shi, L. et al. A review of electrooxidation systems treatment of poly-fluoroalkyl substances (PFAS): electrooxidation degradation mechanisms and electrode materials. Environ. Sci. Pollut. Res. 31, 42593–42613 (2024).
Park, H. et al. Reductive degradation of perfluoroalkyl compounds with aquated electrons generated from iodide photolysis at 254 nm. Photochem. Photobiol. Sci. 10, 1945–1953 (2011).
Cui, J., Gao, P. & Deng, Y. Destruction of per- and polyfluoroalkyl substances (PFAS) with advanced reduction processes (ARPs): a critical review. Environ. Sci. Technol. 54, 3752–3766 (2020).
O’Connor, N. et al. Forever no more: complete mineralization of per- and polyfluoroalkyl substances (PFAS) using an optimized UV/sulfite/iodide system. Sci. Total Environ. 888, 164137 (2023).
Blotevogel, J., Thagard, S. M. & Mahendra, S. Scaling up water treatment technologies for PFAS destruction: current status and potential for fit-for-purpose application. Curr. Opin. Chem. Eng. 41, 100944 (2023).
Singh, R. K. et al. Breakdown products from perfluorinated alkyl substances (PFAS) degradation in a plasma-based water treatment process. Environ. Sci. Technol. 53, 2731–2738 (2019).
Price, G. J., Ashokkumar, M., Hodnett, M., Zequiri, B. & Grieser, F. Acoustic emission from cavitating solutions: implications for the mechanisms of sonochemical reactions. J. Phys. Chem. B 109, 17799–17801 (2005).
Moriwaki, H. et al. Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid. Environ. Sci. Technol. 39, 3388–3392 (2005).
Zhao, Y. et al. Formation of volatile chlorinated and brominated products during low temperature thermal decomposition of the representative PFAS perfluorohexane sulfonate (PFHxS) in the presence of NaCl and NaBr. Environ. Pollut. 348, 123782 (2024).
Vargette, L. D. S. et al. Prospects of complete mineralization of per- and polyfluoroalkyl substances by thermal destruction methods. Curr. Opin. Chem. Eng. 42, 100954 (2023).
DiStefano, R., Feliciano, T., Mimna, R. A., Redding, A. M. & Matthis, J. Thermal destruction of PFAS during full-scale reactivation of PFAS-laden granular activated carbon. Remediation J. 32, 231–238 (2022).
Other Test Method 45 (OTM-45). Measurement of Per- and Polyfluorinated Alkyl Substances from Stationary Sources (USEPA, 2021).
Research on per- and polyfluoroalkyl substances (PFAS). USEPA https://www.epa.gov/chemical-research/research-and-polyfluoroalkyl-substances-pfas (2025).
McDonough, J. T. et al. Validation of supercritical water oxidation to destroy perfluoroalkyl acids. Remediation J. 32, 75–90 (2022).
Wickersham, L. C. et al. Characterization of PFAS air emissions from thermal application of fluoropolymer dispersions on fabrics. J. Air Waste Manag. Assoc. 73, 533–552 (2023).
Thoma, E. D. et al. Pyrolysis processing of PFAS-impacted biosolids, a pilot study. J. Air Waste Manag. Assoc. 72, 309–318 (2022).
Dolatabad, A. A. et al. Thermal degradation of long-chain fluorinated greenhouse gases: stability, byproducts, and remediation approaches. ACS ES&T Eng. 5, 389–401 (2025).
Dreyer, A. & Ebinghaus, R. Polyfluorinated compounds in ambient air from ship- and land-based measurements in northern Germany. Atmos. Environ. 43, 1527–1535 (2009).
Saawarn, B., Mahanty, B., Hait, S. & Hussain, S. Sources, occurrence, and treatment techniques of per- and polyfluoroalkyl substances in aqueous matrices: a comprehensive review. Environ. Res. 214, 114004 (2022).
Gillespie, A. J. R. et al. US EPA advances in PFAS air science. USEPA https://cleanairact.org/wp-content/uploads/2023/09/2_Gillespie_AAPCA-Fall-2023-PFAS-in-air.pdf (2023).
Ryan, J. V. & Gullett, B. Final test report: PFAS emissions measurement methods development and emissions characterization study at National Response Corporation Alaska, LLC AFFF Contaminated Soil Thermal Treatment Facility. USEPA https://apps.dtic.mil/sti/pdfs/AD1134823.pdf (2019).
Cheng, L. et al. Historical blood serum samples from Wilmington, North Carolina: the importance of ultrashort-chain per- and polyfluoroalkyl substances. Environ. Sci. Technol. 59, 23125–23135 (2025).
Dunder, T. A., Geyer, T. J., Kinner, L. L. & Plummer, G. M. in Optical Instrumentation for Gas Emissions Monitoring and Atmospheric Measurements Vol. 2366 (eds Leonelli, J. et al.) 174 (SPIE, 1995).
Method 320 – Measurement of Vapor Phase Organic and Inorganic Emissions by Extractive Fourier Transform Infrared (FTIR) Spectroscopy (USEPA, 2019).
ASTM D6348-12(2020) Standard Test Method for Determination of Gaseous Compounds by Extractive Direct Interface Fourier Transform Infrared (FTIR) Spectroscopy (ASTM, 2020).
Baker, T. J. et al. An infrared spectral database for gas-phase quantitation of volatile per- and polyfluoroalkyl substances (PFAS). J. Quant. Spectrosc. Radiat. Transf. 295, 108420 (2023).
Miser, C. S., Davis, W. R., McNesby, K. L., Hoke, S. H. & Leonnig, M. K. Measurement of carbonyl fluoride, hydrogen fluoride, and other combustion byproducts during fire suppression testing by Fourier transform infrared spectroscopy. In Proc. Halon Options Technical Working Conference 190–203 (NIST, 1998).
Li, S., Lyons-Hart, J., Banyasz, J. & Shafer, K. Real-time evolved gas analysis by FTIR method: an experimental study of cellulose pyrolysis. Fuel 80, 1809–1817 (2001).
Blommaerts, N. et al. Ultrafast screening of commercial sorbent materials for VOC adsorption using real-time FTIR spectroscopy. Sep. Purif. Technol. 207, 284–290 (2018).
Telegeiev, I. et al. In situ FTIR reactor for monitoring gas-phase products during a (photo)catalytic reaction in the liquid phase. Anal. Chem. 90, 14586–14592 (2018).
Hauchecorne, B., Tytgat, T., Terrens, D., Vanpachtenbeke, F. & Lenaerts, S. Validation of a newly developed FTIR in situ reactor: real time study of photocatalytic degradation of nitric oxide. Infrared Phys. Technol. 53, 469–473 (2010).
Stec, A. A. et al. Quantification of fire gases by FTIR: experimental characterisation of calibration systems. Fire Saf. J. 46, 225–233 (2011).
de Gouw, J. A., Howard, C. J., Custer, T. G., Baker, B. M. & Fall, R. Proton-transfer chemical-ionization mass spectrometry allows real-time analysis of volatile organic compounds released from cutting and drying of crops. Environ. Sci. Technol. 34, 2640–2648 (2000).
Lee, B. H. et al. An iodide-adduct high-resolution time-of-flight chemical-ionization mass spectrometer: application to atmospheric inorganic and organic compounds. Environ. Sci. Technol. 48, 6309–6317 (2014).
Zhang, W. & Zhang, H. Secondary ion chemistry mediated by ozone and acidic organic molecules in iodide-adduct chemical ionization mass spectrometry. Anal. Chem. 93, 8595–8602 (2021).
Blake, R. S., Wyche, K. P., Ellis, A. M. & Monks, P. S. Chemical ionization reaction time-of-flight mass spectrometry: multi-reagent analysis for determination of trace gas composition. Int. J. Mass Spectrom. 254, 85–93 (2006).
Yuan, B. et al. A high-resolution time-of-flight chemical ionization mass spectrometer utilizing hydronium ions (H3O+ ToF-CIMS) for measurements of volatile organic compounds in the atmosphere. Atmos. Meas. Tech. 9, 2735–2752 (2016).
Munson, M. S. B. & Field, F. H. Chemical ionization mass spectrometry. I. General introduction. J. Am. Chem. Soc. 88, 2621–2630 (1966).
Munson, B. Chemical ionization mass spectrometry: theory and applications. In Encyclopedia of Analytical Chemistry (eds Meyers, R. A. & Sparkman, O. D.) (Wiley, 2006).
Rewerts, J. N., Morré, J. T., Massey Simonich, S. L. & Field, J. A. In-vial extraction large volume gas chromatography mass spectrometry for analysis of volatile PFASs on papers and textiles. Environ. Sci. Technol. 52, 10609–10616 (2018).
Isemura, T., Kakita, R., Tamaoki, A. & Yonemori, S. Chemical ionization mass spectrometry of hydrofluorocarbons, hydrofluorocarbon ethers and perfluoroalkenes. J. Fluor. Chem. 80, 81–85 (1996).
Riedel, T. P., Lang, J. R., Strynar, M. J., Lindstrom, A. B. & Offenberg, J. H. Gas-phase detection of fluorotelomer alcohols and other oxygenated per- and polyfluoroalkyl substances by chemical ionization mass spectrometry. Environ. Sci. Technol. Lett. 6, 289–293 (2019).
Riedel, T. P. et al. Low temperature thermal treatment of gas-phase fluorotelomer alcohols by calcium oxide. Chemosphere 272, 129859 (2021).
Bowers, B. B., Thornton, J. A. & Sullivan, R. C. Evaluation of iodide chemical ionization mass spectrometry for gas and aerosol-phase per- and polyfluoroalkyl substances (PFAS) analysis. Environ. Sci. Process. Impacts 25, 277–287 (2023).
Folkerson, A. P., Schneider, S. R., Abbatt, J. P. D. & Mabury, S. A. Avoiding regrettable replacements: can the introduction of novel functional groups move pfas from recalcitrant to reactive? Environ. Sci. Technol. 57, 17032–17041 (2023).
Veres, P. et al. Development of negative-ion proton-transfer chemical-ionization mass spectrometry (NI-PT-CIMS) for the measurement of gas-phase organic acids in the atmosphere. Int. J. Mass Spectrom. 274, 48–55 (2008).
Young, C. J., Joudan, S., Tao, Y., Wentzell, J. J. B. & Liggio, J. High time resolution ambient observations of gas-phase perfluoroalkyl carboxylic acids: implications for atmospheric sources. Environ. Sci. Technol. Lett. https://doi.org/10.1021/acs.estlett.4c00897 (2024).
Huey, L. G., Hanson, D. R. & Howard, C. J. Reactions of SF6− and I− with atmospheric trace gases. J. Phys. Chem. 99, 5001–5008 (1995).
Gkatzelis, G. I. et al. Identifying volatile chemical product tracer compounds in U.S. cities. Environ. Sci. Technol. 55, 188–199 (2020).
Yang, Z. et al. Review and prospect on portable mass spectrometer for recent applications. Vacuum 199, 110889 (2022).
Snyder, D. T., Pulliam, C. J., Ouyang, Z. & Cooks, R. G. Miniature and fieldable mass spectrometers: recent advances. Anal. Chem. 88, 2–29 (2016).
Feider, C. L., Krieger, A., DeHoog, R. J. & Eberlin, L. S. Ambient ionization mass spectrometry: recent developments and applications. Anal. Chem. 91, 4266–4290 (2019).
Kuo, T.-H., Dutkiewicz, E. P., Pei, J. & Hsu, C.-C. Ambient ionization mass spectrometry today and tomorrow: embracing challenges and opportunities. Anal. Chem. 92, 2353–2363 (2020).
Chen, R. et al. Recent applications of ambient ionization mass spectrometry in environmental analysis. Trends Environ. Anal. Chem. 15, 1–11 (2017).
Brown, H. M., McDaniel, T. J., Fedick, P. W. & Mulligan, C. C. The current role of mass spectrometry in forensics and future prospects. Anal. Methods 12, 3974–3997 (2020).
Takáts, Z., Wiseman, J. M., Gologan, B. & Cooks, R. G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 306, 471–473 (2004).
Cody, R. B., Laramée, J. A. & Durst, H. D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 77, 2297–2302 (2005).
West, C. P., Brown, H. M. & Fedick, P. W. Molecular characterization of the thermal degradation of per- and polyfluoroalkyl substances in aqueous film-forming foams via temperature-programmed thermal desorption–pyrolysis–direct analysis in real time–mass spectrometry. Environ. Sci. Technol. Lett. 10, 308–315 (2023).
Heffernan, D. et al. Screening of volatile organic compounds (VOCs) from liquid fungal cultures using ambient mass spectrometry. Anal. Bioanal. Chem. 415, 4615–4627 (2023).
Dryahina, K., Polášek, M., Jašík, J., Sovová, K. & Španěl, P. Ion chemistry in dielectric barrier discharge ionization: recent advances in direct gas phase analyses. Mass Spectrom. Rev. https://doi.org/10.1002/mas.21914 (2024).
Martínez-Jarquín, S. & Winkler, R. Low-temperature plasma (LTP) jets for mass spectrometry (MS): ion processes, instrumental set-ups, and application examples. Trends Anal. Chem. 89, 133–145 (2017).
Gong, X., Shi, S. & Gamez, G. Real-time quantitative analysis of valproic acid in exhaled breath by low temperature plasma ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 28, 678–687 (2017).
Li, D., Tian, Y., Zhao, Z., Li, W. & Duan, Y. Ambient ionization and direct identification of volatile organic compounds with microwave-induced plasma mass spectrometry. J. Mass Spectrom. 50, 388–395 (2015).
Wu, C., Siems, W. F. & Hill, H. H. Secondary electrospray ionization ion mobility spectrometry/mass spectrometry of illicit drugs. Anal. Chem. 72, 396–403 (2000).
Steiner, W. E., Clowers, B. H., Haigh, P. E. & Hill, H. H. Secondary ionization of chemical warfare agent simulants: atmospheric pressure ion mobility time-of-flight mass spectrometry. Anal. Chem. 75, 6068–6076 (2003).
Tam, M. & Hill, H. H. Secondary electrospray ionization-ion mobility spectrometry for explosive vapor detection. Anal. Chem. 76, 2741–2747 (2004).
Basler, S. et al. Molecular breath profile of acute COPD exacerbations. J. Breath Res. 19, 016011 (2025).
Arnold, K. et al. Early detection of bacterial pneumonia by characteristic induced odor signatures. BMC Infect. Dis. 24, 1467 (2024).
Singh, K. D. et al. Translating secondary electrospray ionization–high-resolution mass spectrometry to the clinical environment. J. Breath Res. 12, 027113 (2018).
Martínez-Lozano, P., Rus, J., Fernández de la Mora, G., Hernández, M. & Fernández de la Mora, J. Secondary electrospray ionization (SESI) of ambient vapors for explosive detection at concentrations below parts per trillion. J. Am. Soc. Mass Spectrom. 20, 287–294 (2009).
Liu, W. et al. Tracking indoor volatile organic compounds with online mass spectrometry. Trends Anal. Chem. 171, 117514 (2024).
Liu, S. et al. Ozone oxidation of the flame retardant BDE-209: kinetics and molecular-level analysis of the gas-phase product compounds. ACS Earth Space Chem. 8, 2166–2175 (2024).
Marks, J., Kantamaneni, R., Pape, D. & Rand, S. in Essential Readings in Light Metals, 1032–1036 (Springer, 2016).
Smart, B. E. & Fernandez, R. E. Fluorinated aliphatic compounds. In Kirk-Othmer Encyclopedia of Chemical Technology (Wiley, 2000).
Ye, R. et al. A method to measure total gaseous fluorine. Environ. Sci. Technol. Lett. 11, 1062–1067 (2024).
Liang, Y. et al. Municipal sewage sludge incineration and its air pollution control. J. Clean. Prod. 295, 126456 (2021).
Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances (OECD, 2021)
Buck, R. C. et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag. 7, 513–541 (2011).
O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 37, 308–319 (2008).
Lorpaiboon, W. & Ho, J. High-level quantum chemical prediction of C–F bond dissociation energies of perfluoroalkyl substances. J. Phys. Chem. A 127, 7943–7953 (2023).
Research on per- and polyfluoroalkyl substances (PFAS). ITRC https://pfas-1.itrcweb.org/wp-content/uploads/2023/12/Full-PFAS-Guidance-12.11.2023.pdf (2023).
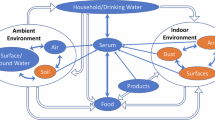
De Silva, A. O. et al. PFAS exposure pathways for humans and wildlife: a synthesis of current knowledge and key gaps in understanding. Environ. Toxicol. Chem. 40, 631–657 (2021).
Sunderland, E. M. et al. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 29, 131–147 (2018).
Lang, J. R., Allred, B. M., Field, J. A., Levis, J. W. & Barlaz, M. A. National estimate of per- and polyfluoroalkyl substance (PFAS) release to U.S. municipal landfill leachate. Environ. Sci. Technol. 51, 2197–2205 (2017).
Brusseau, M. L., Anderson, R. H. & Guo, B. PFAS concentrations in soils: background levels versus contaminated sites. Sci. Total Environ. 740, 140017 (2020).
Barton, C. A., Butler, L. E., Zarzecki, C. J., Flaherty, J. & Kaiser, M. Characterizing perfluorooctanoate in ambient air near the fence line of a manufacturing facility: comparing modeled and monitored values. J. Air Waste Manag. Assoc. 56, 48–55 (2006).
Hu, X. C. et al. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ. Sci. Technol. Lett. 3, 344–350 (2016).
Sun, M. et al. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the Cape Fear river watershed of North Carolina. Environ. Sci. Technol. Lett. 3, 415–419 (2016).
Goodrum, P. E., Anderson, J. K., Luz, A. L. & Ansell, G. K. Application of a framework for grouping and mixtures toxicity assessment of PFAS: a closer examination of dose-additivity approaches. Toxicol. Sci. 179, 262–278 (2021).
Kabadi, S. V. et al. Characterizing biopersistence potential of the metabolite 5:3 fluorotelomer carboxylic acid after repeated oral exposure to the 6:2 fluorotelomer alcohol. Toxicol. Appl. Pharmacol. 388, 114878 (2020).
Wolf, C. J., Rider, C. V., Lau, C. & Abbott, B. D. Evaluating the additivity of perfluoroalkyl acids in binary combinations on peroxisome proliferator-activated receptor-α activation. Toxicology 316, 43–54 (2014).
Shoemaker, J. et al. Method 537.1: Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography Tandem Mass Spectrometry (LC/MS/MS) (USEPA, 2020).
Wendelken, S. C., Rosenblum, L. & USEPA. Method 533: Determination of Per- and Polyfluoroalkyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (USEPA, 2019).
EPA Office of Water. Method 1633, Revision A: Analysis of Per-and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS (USEPA, 2024).
Other Test Method 50 (OTM-50) Sampling and Analysis of Volatile Fluorinated Compounds from Stationary Sources Using Passivated Stainless-Steel Canisters (USEPA, 2024).
Acknowledgements
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the US Environmental Protection Agency (EPA) or Department of Defense (DOD). Any mention of trade names, manufacturers or products does not imply an endorsement by the United States Government or the US EPA or DOD. US EPA and DOD and its employees do not endorse any commercial products, services or enterprises. This review was supported, in part, by funding from the US Army Corps of Engineers (W912HZ-23-2-0009) and various Strategic Environmental Research and Development Program (SERDP) and Environmental Security Technology Certification Program (ESTCP) projects through the US DOD.
Author information
Authors and Affiliations
Contributions
S. Silsby provided overall coordination for the Review. S. Silsby, S. Sühnholz, D.R.U.K. and C.P.H. developed the concept for the Review. Initial draft writing was conducted by S. Silsby, S. Sühnholz, M.Q., K.D., C.Y., D.R.U.K. and C.P.H. Figures were drafted by S. Silsby, S. Sühnholz, M.Q. and N.S. All authors contributed views and opinions, and reviewed the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.S.G. is an employee of Alcoa Corporation, which is involved in bauxite mining, alumina refining and aluminium smelting operations worldwide. R.S.G. is an inventor of patents US9795920B2, US9327237B2, US8894748B2, US8137649B2, US7906089B2, US7645430B2 and EU2265357 in the area of gas exhaust treatment as well as patents US8673152B2, US8206586B2, US8157995B2, US7897049B2 and US5837145 in the area of water treatment. C.P.H., T.J.S. and S.H. are inventors on patent US11577111 related to the use of hydrothermal alkaline conditions for PFAS destruction. T.J.S. is also a paid technical advisor for Aquagga, Inc., which has licensed patent US11577111. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Earth & Environment thanks Denis O’Carroll, Benjamin Fennell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
US National Toxicology Program: https://ntp.niehs.nih.gov/publications
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silsby, S., Sühnholz, S., Qanbarzadeh, M. et al. Air emissions during destruction of PFAS-containing materials. Nat Rev Earth Environ (2026). https://doi.org/10.1038/s43017-025-00755-x
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43017-025-00755-x