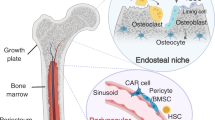
Abstract
The bone is a frequent metastatic site, with changes in the mineralized bone and the bone marrow milieu that can also prime other sites for metastasis by educating progenitor cells to support metastatic spread. Stromal and immune populations cooperatively maintain the organizationally complex bone niches and are dysregulated in the presence of a distant primary tumor and metastatic disease. Interrogating the bone niches that facilitate metastatic spread using innovative technologies holds the potential to aid in preventing metastasis in and mediated by the bone. Here, we review recent advances in bone niche biology and its adaptations in the context of cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Macedo, F. et al. Bone metastases: an overview. Oncol. Rev. 11, 321 (2017).
Zhang, W. et al. The bone microenvironment invigorates metastatic seeds for further dissemination. Cell 184, 2471–2486 (2021).
Huang, Y., Wang, H., Yue, X. & Li, X. Bone serves as a transfer station for secondary dissemination of breast cancer. Bone Res. 11, 21 (2023).
Engblom, C. et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils. Science 358, eaal5081 (2017).
Giles, A. J. et al. Activation of hematopoietic stem/progenitor cells promotes immunosuppression within the pre-metastatic niche. Cancer Res. 76, 1335–1347 (2016).
Kaplan, R. N., Psaila, B. & Lyden, D. Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer Metastasis Rev. 25, 521–529 (2006).
Giles, A. J. et al. The functional interplay between systemic cancer and the hematopoietic stem cell niche. Pharmacol. Ther. 168, 53–60 (2016).
Zacharia, B., Joy, J., Subramaniam, D. & Pai, P. K. Factors affecting life expectancy after bone metastasis in adults — results of a 5-year prospective study. Indian J. Surg. Oncol. 12, 759–769 (2021).
LaMarche, N. M. et al. An IL-4 signalling axis in bone marrow drives pro-tumorigenic myelopoiesis. Nature 625, 166–174 (2024).
Akkiraju, H. & Nohe, A. Current challenges in bone biology. Adv. Tech. Biol. Med. 3, 132 (2015).
Safi, S. et al. Bone marrow expands the repertoire of functional T cells targeting tumor-associated antigens in patients with resectable non-small-cell lung cancer. Oncoimmunology 8, e1671762 (2019).
Chen, J. Z. & Alt, F. W. Gene rearrangement and B-cell development. Curr. Opin. Immunol. 5, 194–200 (1993).
Geerman, S., Hickson, S., Brasser, G., Pascutti, M. F. & Nolte, M. A. Quantitative and qualitative analysis of bone marrow CD8+ T cells from different bones uncovers a major contribution of the bone marrow in the vertebrae. Front. Immunol. 6, 660 (2015).
Fornetti, J., Welm, A. L. & Stewart, S. A. Understanding the bone in cancer metastasis. J. Bone Miner. Res. 33, 2099–2113 (2018).
Lazic, D. et al. Landscape of bone marrow metastasis in human neuroblastoma unraveled by transcriptomics and deep multiplex imaging. Cancers 13, 4311 (2021).
Lim, W. et al. Real-time in vivo imaging of metastatic bone tumors with a targeted near-infrared fluorophore. Oncotarget 8, 65770–65777 (2017).
Lecomte, J. et al. Bone marrow-derived myofibroblasts are the providers of pro-invasive matrix metalloproteinase 13 in primary tumor. Neoplasia 14, 943–951 (2012).
Pittenger, M. F. et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen. Med. 4, 22 (2019).
Pinho, S. & Frenette, P. S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 20, 303–320 (2019).
Sivaraj, K. K. & Adams, R. H. Blood vessel formation and function in bone. Development 143, 2706–2715 (2016).
Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328 (2014).
Kusumbe, A. P., Ramasamy, S. K., Starsichova, A. & Adams, R. H. Sample preparation for high-resolution 3D confocal imaging of mouse skeletal tissue. Nat. Protoc. 10, 1904–1914 (2015).
Stucker, S., Chen, J., Watt, F. E. & Kusumbe, A. P. Bone angiogenesis and vascular niche remodeling in stress, aging, and diseases. Front. Cell Dev. Biol. 8, 602269 (2020).
Peng, Y., Wu, S., Li, Y. & Crane, J. L. Type H blood vessels in bone modeling and remodeling. Theranostics 10, 426–436 (2020).
Chen, M. et al. Skeleton–vasculature chain reaction: a novel insight into the mystery of homeostasis. Bone Res. 9, 21 (2021).
Biswas, L. et al. Lymphatic vessels in bone support regeneration after injury. Cell 186, 382–397 (2023).
Marrella, A. et al. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today 21, 362–376 (2018).
Qin, Q. et al. Neurovascular coupling in bone regeneration. Exp. Mol. Med. 54, 1844–1849 (2022).
Brazill, J. M., Beeve, A. T., Craft, C. S., Ivanusic, J. J. & Scheller, E. L. Nerves in bone: evolving concepts in pain and anabolism. J. Bone Miner. Res. 34, 1393–1406 (2019).
Wolock, S. L. et al. Mapping distinct bone marrow niche populations and their differentiation paths. Cell Rep. 28, 302–311 (2019).
Tikhonova, A. N. et al. The bone marrow microenvironment at single-cell resolution. Nature 569, 222–228 (2019).
Baccin, C. et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 22, 38–48 (2020).
Gomariz, A. et al. Quantitative spatial analysis of haematopoiesis-regulating stromal cells in the bone marrow microenvironment by 3D microscopy. Nat. Commun. 9, 2532 (2018).
Zhao, E. et al. Bone marrow and the control of immunity. Cell. Mol. Immunol. 9, 11–19 (2012).
Leitao, L. et al. Bone marrow cell response after injury and during early stage of regeneration is independent of the tissue-of-injury in 2 injury models. FASEB J. 33, 857–872 (2019).
Comazzetto, S., Shen, B. & Morrison, S. J. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev. Cell 56, 1848–1860 (2021).
Shiozawa, Y. et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Invest. 121, 1298–1312 (2011).
Carsetti, R. The development of B cells in the bone marrow is controlled by the balance between cell-autonomous mechanisms and signals from the microenvironment. J. Exp. Med. 191, 5–8 (2000).
Zehentmeier, S. & Pereira, J. P. Cell circuits and niches controlling B cell development. Immunol. Rev. 289, 142–157 (2019).
Zanna, M. Y. et al. Review of dendritic cells, their role in clinical immunology, and distribution in various animal species. Int. J. Mol. Sci. 22, 8044 (2021).
Soltan, M., Rohrer, M. D. & Prasad, H. S. Monocytes: super cells for bone regeneration. Implant Dent. 21, 13–20 (2012).
Kaur, S. et al. Role of bone marrow macrophages in controlling homeostasis and repair in bone and bone marrow niches. Semin. Cell Dev. Biol. 61, 12–21 (2017).
Kraus, R. F. & Gruber, M. A. Neutrophils—from bone marrow to first-line defense of the innate immune system. Front. Immunol. 12, 767175 (2021).
Bonomo, A. et al. A T cell view of the bone marrow. Front. Immunol. 7, 184 (2016).
Guder, C., Gravius, S., Burger, C., Wirtz, D. C. & Schildberg, F. A. Osteoimmunology: a current update of the interplay between bone and the immune system. Front. Immunol. 11, 58 (2020).
Sun, Y. et al. Macrophage–osteoclast associations: origin, polarization, and subgroups. Front. Immunol. 12, 778078 (2021).
Sender, R. et al. The total mass, number, and distribution of immune cells in the human body. Proc. Natl Acad. Sci. USA 120, e2308511120 (2023).
Fichtel, P. et al. Mesenchymal stromal cell-derived extracellular vesicles modulate hematopoietic stem and progenitor cell viability and the expression of cell cycle regulators in an age-dependent manner. Front. Bioeng. Biotechnol. 10, 892661 (2022).
Krebsbach, P. H., Kuznetsov, S. A., Bianco, P. & Robey, P. G. Bone marrow stromal cells: characterization and clinical application. Crit. Rev. Oral Biol. Med. 10, 165–181 (1999).
Jiang, W. & Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 53, e12712 (2020).
Crippa, S. & Bernardo, M. E. Mesenchymal stromal cells: role in the BM niche and in the support of hematopoietic stem cell transplantation. Hemasphere 2, e151 (2018).
Wu, J. Y., Scadden, D. T. & Kronenberg, H. M. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J. Bone Miner. Res. 24, 759–764 (2009).
Mangialardi, G., Cordaro, A. & Madeddu, P. The bone marrow pericyte: an orchestrator of vascular niche. Regen. Med. 11, 883–895 (2016).
Sato, S. et al. Bone marrow adipocytes induce cancer-associated fibroblasts and immune evasion, enhancing invasion and drug resistance. Cancer Sci. 114, 2674–2688 (2023).
Yamazaki, S. & Nakauchi, H. Bone marrow Schwann cells induce hematopoietic stem cell hibernation. Int. J. Hematol. 99, 695–698 (2014).
Wei, Q. & Frenette, P. S. Niches for hematopoietic stem cells and their progeny. Immunity 48, 632–648 (2018).
Bandyopadhyay, S. et al. Mapping the cellular biogeography of human bone marrow niches using single-cell transcriptomics and proteomic imaging. Cell 187, 3120–3140 (2024).
Gao, X. et al. Leptin receptor+ cells promote bone marrow innervation and regeneration by synthesizing nerve growth factor. Nat. Cell Biol. 25, 1746–1757 (2023).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Kaczanowska, S. et al. Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell 184, 2033–2052 (2021).
O’Donnell, R. K. et al. VEGF-A/VEGFR inhibition restores hematopoietic homeostasis in the bone marrow and attenuates tumor growth. Cancer Res. 76, 517–524 (2016).
Li, X. et al. Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFκB–TLR signaling pathway. J. Hematol. Oncol. 9, 42 (2016).
Meng, D. et al. Effects of VEGFR1+ hematopoietic progenitor cells on pre-metastatic niche formation and in vivo metastasis of breast cancer cells. J. Cancer Res. Clin. Oncol. 145, 411–427 (2019).
Kusmartsev, S. et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J. Immunol. 181, 346–353 (2008).
Anastasiadou, D. P., Quesnel, A., Duran, C. L., Filippou, P. S. & Karagiannis, G. S. An emerging paradigm of CXCL12 involvement in the metastatic cascade. Cytokine Growth Factor Rev. 75, 12–30 (2024).
Wang, Z. et al. Periostin promotes immunosuppressive premetastatic niche formation to facilitate breast tumour metastasis. J. Pathol. 239, 484–495 (2016).
Wang, Y. G., Ding, Y. X., Guo, N. Z. & Wang, S. J. MDSCs: key criminals of tumor pre-metastatic niche formation. Front. Immunol. 10, 172 (2019).
Liu, Y. & Cao, X. Characteristics and significance of the pre-metastatic niche. Cancer Cell 30, 668–681 (2016).
Veglia, F., Sanseviero, E. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 21, 485–498 (2021).
Marvel, D. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J. Clin. Invest. 125, 3356–3364 (2015).
Gerber-Ferder, Y. et al. Breast cancer remotely imposes a myeloid bias on haematopoietic stem cells by reprogramming the bone marrow niche. Nat. Cell Biol. 25, 1736–1745 (2023).
Sanmartin, M. C. et al. Bone marrow/bone pre-metastatic niche for breast cancer cells colonization: the role of mesenchymal stromal cells. Crit. Rev. Oncol. Hematol. 164, 103416 (2021).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Peinado, H., Lavotshkin, S. & Lyden, D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin. Cancer Biol. 21, 139–146 (2011).
Li, X. Q., Zhang, R., Lu, H., Yue, X. M. & Huang, Y. F. Extracellular vesicle-packaged CDH11 and ITGA5 induce the premetastatic niche for bone colonization of breast cancer cells. Cancer Res. 82, 1560–1574 (2022).
Maroni, P., Gomarasca, M. & Lombardi, G. Long non-coding RNAs in bone metastasis: progresses and perspectives as potential diagnostic and prognostic biomarkers. Front. Endocrinol. 14, 1156494 (2023).
Cheng, J., Zhang, K., Qu, C., Peng, J. & Yang, L. Non-coding RNAs derived from extracellular vesicles promote pre-metastatic niche formation and tumor distant metastasis. Cancers 15, 2158 (2023).
Lu, X. & Kang, Y. Chemokine (C–C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J. Biol. Chem. 284, 29087–29096 (2009).
Seubert, B. et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 61, 238–248 (2015).
Jing, B. et al. IL6/STAT3 signaling orchestrates premetastatic niche formation and immunosuppressive traits in lung. Cancer Res. 80, 784–797 (2020).
Cai, R. et al. Primary breast tumor induced extracellular matrix remodeling in premetastatic lungs. Sci. Rep. 13, 18566 (2023).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015).
Sun, Y. X. et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J. Cell. Biochem. 89, 462–473 (2003).
Kai, F., Drain, A. P. & Weaver, V. M. The extracellular matrix modulates the metastatic journey. Dev. Cell 49, 332–346 (2019).
Si, J., Wang, C., Zhang, D., Wang, B. & Zhou, Y. Osteopontin in bone metabolism and bone diseases. Med. Sci. Monit. 26, e919159 (2020).
Shen, X. & Falzon, M. Parathyroid hormone-related protein upregulates integrin expression via an intracrine pathway in PC-3 prostate cancer cells. Regul. Pept. 113, 17–29 (2003).
Guise, T. A. et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Invest. 98, 1544–1549 (1996).
Isali, I. et al. Growth factors involve in cellular proliferation, differentiation and migration during prostate cancer metastasis. Int. J. Cell Biol. Physiol. 2, 1–13 (2019).
Dougherty, K. M. et al. Parathyroid hormone-related protein as a growth regulator of prostate carcinoma. Cancer Res. 59, 6015–6022 (1999).
Swami, S. et al. Parathyroid hormone 1 receptor signaling mediates breast cancer metastasis to bone in mice. JCI Insight 8, e157390 (2023).
Paiva, A. E. et al. Pericytes in the premetastatic niche. Cancer Res. 78, 2779–2786 (2018).
Murgai, M. et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat. Med. 23, 1176–1190 (2017).
Shankman, L. S. et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 21, 628–637 (2015).
Singh, A. et al. Angiocrine signals regulate quiescence and therapy resistance in bone metastasis. JCI Insight 4, e125679 (2019).
Wang, Z. M. et al. Metastasis-associated fibroblasts: an emerging target for metastatic cancer. Biomark. Res. 9, 47 (2021).
Deborde, S. & Wong, R. J. The role of Schwann cells in cancer. Adv. Biol. 6, e2200089 (2022).
Sun, C. C. et al. Tumor-associated nonmyelinating Schwann cell-expressed PVT1 promotes pancreatic cancer kynurenine pathway and tumor immune exclusion. Sci. Adv. 9, eadd6995 (2023).
Kitamura, T. et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 212, 1043–1059 (2015).
Lin, Y. X., Xu, J. X. & Lan, H. Y. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J. Hematol. Oncol. 12, 76 (2019).
Monteiro, A. C. et al. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS ONE 8, e68171 (2013).
Zhang, R., Peng, S. & Zhu, G. The role of secreted osteoclastogenic factor of activated T cells in bone remodeling. Jpn. Dent. Sci. Rev. 58, 227–232 (2022).
Clements, M. E. & Johnson, R. W. Breast cancer dormancy in bone. Curr. Osteoporos. Rep. 17, 353–361 (2019).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Yip, R. K. H. et al. Mammary tumour cells remodel the bone marrow vascular microenvironment to support metastasis. Nat. Commun. 12, 6920 (2021).
Kunisaki, Y. et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643 (2013).
Nobre, A. R. et al. Bone marrow NG2+/nestin+ mesenchymal stem cells drive DTC dormancy via TGFβ2. Nat. Cancer 2, 327–339 (2021).
Sandiford, O. A. et al. Mesenchymal stem cell-secreted extracellular vesicles instruct stepwise dedifferentiation of breast cancer cells into dormancy at the bone marrow perivascular region. Cancer Res. 81, 1567–1582 (2021).
Zhao, L. et al. The relationship between mesenchymal stem cells and tumor dormancy. Front. Cell Dev. Biol. 9, 731393 (2021).
Gasson, J. C. Molecular physiology of granulocyte–macrophage colony-stimulating factor. Blood 77, 1131–1145 (1991).
Sethi, N., Dai, X., Winter, C. G. & Kang, Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging Notch signaling in bone cells. Cancer Cell 19, 192–205 (2011).
Bidwell, B. N. et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 18, 1224–1231 (2012).
Albrengues, J. et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227 (2018).
Mayhew, V., Omokehinde, T. & Johnson, R. W. Tumor dormancy in bone. Cancer Rep. 3, e1156 (2020).
Monteran, L. et al. Bone metastasis is associated with acquisition of mesenchymal phenotype and immune suppression in a model of spontaneous breast cancer metastasis. Sci. Rep. 10, 13838 (2020).
Yao, K. et al. Multidimensional analysis to elucidate the possible mechanism of bone metastasis in breast cancer. BMC Cancer 23, 1213 (2023).
Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 (2005).
Wang, M., Xia, F., Wei, Y. & Wei, X. Molecular mechanisms and clinical management of cancer bone metastasis. Bone Res. 8, 30 (2020).
Kozlow, W. & Guise, T. A. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J. Mammary Gland Biol. Neoplasia 10, 169–180 (2005).
Ibrahim, T. et al. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer 116, 1406–1418 (2010).
Coleman, R. E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 12, 6243s–6249s (2006).
Zhang, L., Zhang, J., Li, Z., Wu, Y. & Tong, Z. Comparison of the clinicopathological characteristics and prognosis between Chinese patients with breast cancer with bone-only and non-bone-only metastasis. Oncol. Lett. 20, 92 (2020).
Hartkopf, A. D. et al. Prognostic relevance of disseminated tumour cells from the bone marrow of early stage breast cancer patients — results from a large single-centre analysis. Eur. J. Cancer 50, 2550–2559 (2014).
Braun, S., Auer, D. & Marth, C. The prognostic impact of bone marrow micrometastases in women with breast cancer. Cancer Invest. 27, 598–603 (2009).
Chin, H. & Kim, J. Bone metastasis: concise overview. Fed. Pract. 32, 24–30 (2015).
Liu, C. et al. Immune checkpoint inhibitor therapy for bone metastases: specific microenvironment and current situation. J. Immunol. Res. 2021, 8970173 (2021).
Kfoury, Y. et al. Human prostate cancer bone metastases have an actionable immunosuppressive microenvironment. Cancer Cell 39, 1464–1478 (2021).
Reinstein, Z. Z. et al. Overcoming immunosuppression in bone metastases. Crit. Rev. Oncol. Hematol. 117, 114–127 (2017).
Edwards, S. C., Hoevenaar, W. H. M. & Coffelt, S. B. Emerging immunotherapies for metastasis. Br. J. Cancer 124, 37–48 (2021).
Jiao, S. et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 179, 1177–1190 (2019).
Le Rochais, M., Hemon, P., Pers, J. O. & Uguen, A. Application of high-throughput imaging mass cytometry Hyperion in cancer research. Front. Immunol. 13, 859414 (2022).
Cheng, S., Nethi, S. K., Rathi, S., Layek, B. & Prabha, S. Engineered mesenchymal stem cells for targeting solid tumors: therapeutic potential beyond regenerative therapy. J. Pharmacol. Exp. Ther. 370, 231–241 (2019).
Richardson, D. S. & Lichtman, J. W. Clarifying tissue clearing. Cell 162, 246–257 (2015).
Schrijver, W. A. et al. Influence of decalcification procedures on immunohistochemistry and molecular pathology in breast cancer. Mod. Pathol. 29, 1460–1470 (2016).
Sadozai, H. et al. Distinct stromal and immune features collectively contribute to long-term survival in pancreatic cancer. Front. Immunol. 12, 643529 (2021).
Sowder, M. E. & Johnson, R. W. Enrichment and detection of bone disseminated tumor cells in models of low tumor burden. Sci. Rep. 8, 14299 (2018).
Black, S. et al. CODEX multiplexed tissue imaging with DNA-conjugated antibodies. Nat. Protoc. 16, 3802–3835 (2021).
Hipps, D. et al. Detecting respiratory chain defects in osteoblasts from osteoarthritic patients using imaging mass cytometry. Bone 158, 116371 (2022).
Qiao, H. & Tang, T. Engineering 3D approaches to model the dynamic microenvironments of cancer bone metastasis. Bone Res. 6, 3 (2018).
Salamanna, F., Contartese, D., Maglio, M. & Fini, M. A systematic review on in vitro 3D bone metastases models: a new horizon to recapitulate the native clinical scenario? Oncotarget 7, 44803–44820 (2016).
Coutu, D. L., Kokkaliaris, K. D., Kunz, L. & Schroeder, T. Three-dimensional map of nonhematopoietic bone and bone-marrow cells and molecules. Nat. Biotechnol. 35, 1202–1210 (2017).
Kokkaliaris, K. D. et al. Adult blood stem cell localization reflects the abundance of reported bone marrow niche cell types and their combinations. Blood 136, 2296–2307 (2020).
Agarwal, P. et al. Mesenchymal niche-specific expression of Cxcl12 controls quiescence of treatment-resistant leukemia stem cells. Cell Stem Cell 26, 123 (2020).
Gruneboom, A. et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat. Metab. 1, 236–250 (2019).
Mai, H. et al. Whole-body cellular mapping in mouse using standard IgG antibodies. Nat. Biotechnol. 42, 617–627 (2024).
Renier, N. et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014).
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013).
Li, W., Germain, R. N. & Gerner, M. Y. High-dimensional cell-level analysis of tissues with Ce3D multiplex volume imaging. Nat. Protoc. 14, 1708–1733 (2019).
Molino, G., Montalbano, G., Pontremoli, C., Fiorilli, S. & Vitale-Brovarone, C. Imaging techniques for the assessment of the bone osteoporosis-induced variations with particular focus on micro-CT potential. Appl. Sci. 10, 8939 (2020).
Jovic, D. et al. Single-cell RNA sequencing technologies and applications: a brief overview. Clin. Transl. Med. 12, e694 (2022).
Ombrato, L. et al. Metastatic-niche labelling reveals parenchymal cells with stem features. Nature 572, 603–608 (2019).
Marx, V. Method of the year: spatially resolved transcriptomics. Nat. Methods 18, 9–14 (2021).
Du, J. et al. Advances in spatial transcriptomics and related data analysis strategies. J. Transl. Med. 21, 330 (2023).
Godet, I. et al. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat. Commun. 10, 4862 (2019).
Anderson, M., Moshnikova, A., Engelman, D. M., Reshetnyak, Y. K. & Andreev, O. A. Probe for the measurement of cell surface pH in vivo and ex vivo. Proc. Natl Acad. Sci. USA 113, 8177–8181 (2016).
Hasegawa, T., Kikuta, J. & Ishii, M. Imaging the bone–immune cell interaction in bone destruction. Front. Immunol. 10, 596 (2019).
Dondossola, E. et al. Intravital microscopy of osteolytic progression and therapy response of cancer lesions in the bone. Sci. Transl. Med. 10, eaao5726 (2018).
Lo Celso, C. et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457, 92–96 (2009).
Christodoulou, C. et al. Live-animal imaging of native haematopoietic stem and progenitor cells. Nature 578, 278–283 (2020).
Junt, T. et al. Dynamic visualization of thrombopoiesis within bone marrow. Science 317, 1767–1770 (2007).
Upadhaya, S. et al. Intravital imaging reveals motility of adult hematopoietic stem cells in the bone marrow niche. Cell Stem Cell 27, 336–345 (2020).
Bixel, M. G. et al. Flow dynamics and HSPC homing in bone marrow microvessels. Cell Rep. 18, 1804–1816 (2017).
Hawkins, E. D. et al. T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature 538, 518–522 (2016).
Tang, R. et al. A versatile system to record cell–cell interactions. eLife 9, e61080 (2020).
Graham, N. & Qian, B. Z. Mesenchymal stromal cells: emerging roles in bone metastasis. Int. J. Mol. Sci. 19, 1121 (2018).
Ara, T. & Declerck, Y. A. Interleukin-6 in bone metastasis and cancer progression. Eur. J. Cancer 46, 1223–1231 (2010).
Mukaida, N., Zhang, D. & Sasaki, S. I. Emergence of cancer-associated fibroblasts as an indispensable cellular player in bone metastasis process. Cancers 12, 2896 (2020).
Delprat, V., Huart, C., Feron, O., Soncin, F. & Michiels, C. The impact of macrophages on endothelial cells is potentiated by cycling hypoxia: enhanced tumor inflammation and metastasis. Front. Oncol. 12, 961753 (2022).
Paiva, A. E. et al. Endothelial cells as precursors for osteoblasts in the metastatic prostate cancer bone. Neoplasia 19, 928–931 (2017).
Raymaekers, K., Stegen, S., van Gastel, N. & Carmeliet, G. The vasculature: a vessel for bone metastasis. BoneKEy Rep. 4, 742 (2015).
Luo, G., He, Y. & Yu, X. Bone marrow adipocyte: an intimate partner with tumor cells in bone metastasis. Front. Endocrinol. 9, 339 (2018).
Dai, R., Liu, M., Xiang, X., Xi, Z. & Xu, H. Osteoblasts and osteoclasts: an important switch of tumour cell dormancy during bone metastasis. J. Exp. Clin. Cancer Res. 41, 316 (2022).
Ottewell, P. D. The role of osteoblasts in bone metastasis. J. Bone Oncol. 5, 124–127 (2016).
Lawson, M. A. et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 6, 8983 (2015).
Riquelme, M. A., Cardenas, E. R. & Jiang, J. X. Osteocytes and bone metastasis. Front. Endocrinol. 11, 567844 (2020).
Anloague, A. & Delgado-Calle, J. Osteocytes: new kids on the block for cancer in bone therapy. Cancers 15, 2645 (2023).
Navarro, R., Compte, M., Alvarez-Vallina, L. & Sanz, L. Immune regulation by pericytes: modulating innate and adaptive immunity. Front. Immunol. 7, 480 (2016).
Er, E. E. et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat. Cell Biol. 20, 966–978 (2018).
Sun, L., Chen, S. & Chen, M. Schwann cells in the tumor microenvironment: need more attention. J. Oncol. 2022, 1058667 (2022).
Deborde, S. et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Invest. 126, 1538–1554 (2016).
Zhang, S. H. et al. Immunomodulation by Schwann cells in disease. Cancer Immunol. Immunother. 69, 245–253 (2020).
Elefteriou, F. Role of sympathetic nerves in the establishment of metastatic breast cancer cells in bone. J. Bone Oncol. 5, 132–134 (2016).
Yoneda, T., Hiasa, M., Okui, T. & Hata, K. Sensory nerves: a driver of the vicious cycle in bone metastasis? J. Bone Oncol. 30, 100387 (2021).
Shurin, M. R., Shurin, G. V., Zlotnikov, S. B. & Bunimovich, Y. L. The neuroimmune axis in the tumor microenvironment. J. Immunol. 204, 280–285 (2020).
Leblanc, R. et al. Interaction of platelet-derived autotaxin with tumor integrin αVβ3 controls metastasis of breast cancer cells to bone. Blood 124, 3141–3150 (2014).
Anvari, S., Osei, E. & Maftoon, N. Interactions of platelets with circulating tumor cells contribute to cancer metastasis. Sci. Rep. 11, 15477 (2021).
Schwartz, M., Zhang, Y. & Rosenblatt, J. D. B cell regulation of the anti-tumor response and role in carcinogenesis. J. Immunother. Cancer 4, 40 (2016).
Xiang, L. & Gilkes, D. M. The contribution of the immune system in bone metastasis pathogenesis. Int. J. Mol. Sci. 20, 999 (2019).
Chan, I. S. & Ewald, A. J. The changing role of natural killer cells in cancer metastasis. J. Clin. Invest. 132, e143762 (2022).
Hu, W. et al. Neutrophil extracellular traps facilitate cancer metastasis: cellular mechanisms and therapeutic strategies. J. Cancer Res. Clin. Oncol. 149, 2191–2210 (2023).
Liu, Y. F. et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell 30, 243–256 (2016).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Wang, Y. et al. Targeting myeloid-derived suppressor cells in cancer immunotherapy. Cancers 12, 2626 (2020).
Xiang, H. et al. Cancer-associated fibroblasts promote immunosuppression by inducing ROS-generating monocytic MDSCs in lung squamous cell carcinoma. Cancer Immunol. Res. 8, 436–450 (2020).
Hotchkiss, K. A. et al. Mechanisms by which tumor cells and monocytes expressing the angiogenic factor thymidine phosphorylase mediate human endothelial cell migration. Cancer Res. 63, 527–533 (2003).
Robinson, A., Han, C. Z., Glass, C. K. & Pollard, J. W. Monocyte regulation in homeostasis and malignancy. Trends Immunol. 42, 104–119 (2021).
Ma, R. Y. et al. Monocyte-derived macrophages promote breast cancer bone metastasis outgrowth. J. Exp. Med. 217, e20191820 (2020).
Mendoza-Reinoso, V., McCauley, L. K. & Fournier, P. G. J. Contribution of macrophages and T cells in skeletal metastasis. Cancers 12, 1014 (2020).
Radtke, A. J. et al. IBEX: an iterative immunolabeling and chemical bleaching method for high-content imaging of diverse tissues. Nat. Protoc. 17, 378–401 (2022).
Alberti, D., Guarniero, M., Maciola, A. K., Dotta, E. & Pasqual, G. Engineering ligand and receptor pairs with LIPSTIC to track cell–cell interactions. Curr. Protoc. 1, e311 (2021).
Acknowledgements
The authors thank S. Kaczanowska, C. Contreras and all members of the Kaplan laboratory for their editing of this Review; and thank S. Morrison for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jackett, K.N., Browne, A.T., Aber, E.R. et al. How the bone microenvironment shapes the pre-metastatic niche and metastasis. Nat Cancer 5, 1800–1814 (2024). https://doi.org/10.1038/s43018-024-00854-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-024-00854-6
This article is cited by
-
Engineered bone matrix models for understanding breast cancer skeletal metastasis
Cancer and Metastasis Reviews (2026)
-
Modeling the effects of radiation on the bone tumor microenvironment: opportunities for exploring combination therapies in microphysiologic systems
Cellular & Molecular Biology Letters (2025)
-
Delivery of miR-29a/29c-3p by serum exosomes promotes osteogenesis through TET3-dependent Sox9 demethylation and PI3K/Akt activation
Journal of Nanobiotechnology (2025)
-
A versatile platform for chemical engineering of exosomes empowered by ADP-ribosyl cyclases
Nature Communications (2025)