Abstract
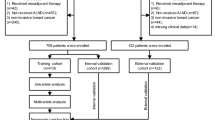
Sentinel lymph node (SLN) biopsy with ultrastaging is standard in endometrial and vulvar cancers, whereas systematic pelvic lymphadenectomy (PLND) remains recommended in cervical cancer. The SENTIX trial prospectively evaluated the safety of SLN biopsy without PLND in early-stage cervical cancer. Female patients, International Federation of Gynaecology and Obstetrics 2018 stage IA1/LVSI+ to IB2 disease, were enrolled between 2016 and 2020 across 47 sites in 18 countries. All underwent SLN biopsy followed by hysterectomy/trachelectomy. Patients with undetected, unilateral or intraoperatively metastatic SLNs were excluded from the intention-to-treat cohort. SLNs were assessed by pathological ultrastaging. Of 731 patients enrolled, 594 formed the intention-to-treat cohort. SLN metastases were identified in 82 patients (12%), 56.1% intraoperatively and 43.9% by ultrastaging. At 2 years, the recurrence rate was 6.1% (one-sided 95% CI 7.9%), confirming noninferiority to the 7% reference rate. Two-year disease-free and overall survival rates were 93.3% (95% CI 94.9–91.6) and 97.9% (95% CI 98.9–97.0), respectively. Here we show that SLN biopsy without systematic PLND did not increase the risk of recurrence in patients with early-stage cervical cancer. Pathological ultrastaging of SLNs detected about 44% of N1 cases, which would be missed by a standard lymph node assessment. Trial registration: ClinicalTrials.gov (NCT02494063).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are provided within the article and its supplementary materials. Patient-sensitive data that are subject to privacy regulations are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Oonk, M. H. et al. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: results from GROINSS-V, a multicentre observational study. Lancet Oncol. 11, 646–652 (2010).
Ballester, M. et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO). Lancet Oncol. 12, 469–476 (2011).
Cusimano, M. C. et al. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 156, 157–164 (2021).
Rossi, E. C. et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 18, 384–392 (2017).
Soliman, P. T. et al. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecol. Oncol. 146, 234–239 (2017).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Cervical Cancer. Version 2.2024. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (2024).
Cibula, D. et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Int. J. Gynecol. Cancer 28, 641–655 (2018).
Cibula, D. et al. ESGO/ESTRO/ESP guidelines for the management of patients with cervical cancer - update 2023. Int. J. Gynecol. Cancer 33, 649–666 (2023).
Margioula-Siarkou, C. et al. Sentinel lymph node staging in early-stage cervical cancer: a comprehensive review. J. Clin. Med. https://doi.org/10.3390/jcm13010027 (2023).
Ronsini, C. et al. The oncological implication of sentinel lymph node in early cervical cancer: a meta-analysis of oncological outcomes and type of recurrences. Medicina https://doi.org/10.3390/medicina58111539 (2022).
Dundr, P. et al. Pathologic protocols for sentinel lymph nodes ultrastaging in cervical cancer. Arch. Pathol. Lab. Med. https://doi.org/10.5858/arpa.2019-0249-RA (2019).
Mauro, J. et al. Survival after sentinel node biopsy alone in early-stage cervical cancer: a systematic review. Int. J. Gynecol. Cancer 33, 1370–1375 (2023).
Cibula, D. et al. The annual recurrence risk model for tailored surveillance strategy in patients with cervical cancer. Eur. J. Cancer 158, 111–122 (2021).
Chiva, L. SUCCOR study: an international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int. J. Gynecol. Cancer 30, 449–450 (2020).
Mathevet, P. et al. Sentinel lymph node biopsy and morbidity outcomes in early cervical cancer: results of a multicentre randomised trial (SENTICOL-2). Eur. J. Cancer 148, 307–315 (2021).
Ramirez, P. T. et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. New Engl. J. Med. 379, 1895–1904 (2018).
Plante, M. et al. Simple versus radical hysterectomy in women with low-risk cervical cancer. New Engl. J. Med. 390, 819–829 (2024).
Schmeler, K. M. et al. ConCerv: a prospective trial of conservative surgery for low-risk early-stage cervical cancer. Int. J. Gynecol. Cancer 31, 1317–1325 (2021).
Kocian, R. et al. Sentinel lymph node pathological ultrastaging: final outcome of the SENTIX prospective international study in patients with early-stage cervical cancer. Gynecol. Oncol. 188, 83–89 (2024).
Lecuru, F. R. et al. SENTICOL III: an international validation study of sentinel node biopsy in early cervical cancer. A GINECO, ENGOT, GCIG and multicenter study. Int. J. Gynecol. Cancer 29, 829–834 (2019).
Tu, H. et al. Sentinel lymph node biopsy versus pelvic lymphadenectomy in early-stage cervical cancer: a multi-center randomized trial (PHENIX/CSEM 010). Int. J. Gynecol. Cancer 30, 1829–1833 (2020).
Vergote, I. et al. European Network of Gynaecological Oncological Trial Groups’ requirements for trials between academic groups and pharmaceutical companies. Int. J. Gynecol. Cancer 20, 476–478 (2010).
Cibula, D. et al. A prospective multicenter trial on sentinel lymph node biopsy in patients with early-stage cervical cancer (SENTIX). Int. J. Gynecol. Cancer 29, 212–215 (2019).
Cibula, D. et al. Sentinel lymph node mapping and intraoperative assessment in a prospective, international, multicentre, observational trial of patients with cervical cancer: the SENTIX trial. Eur. J. Cancer 137, 69–80 (2020).
Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 105, 103–104 (2009).
Querleu, D., Cibula, D. & Abu-Rustum, N. R. 2017 update on the Querleu-Morrow classification of radical hysterectomy. Ann. Surg. Oncol. 24, 3406–3412 (2017).
Nemejcova, K. et al. Central pathology review in SENTIX, a prospective observational international study on sentinel lymph node biopsy in patients with early-stage cervical cancer (ENGOT-CX2). Cancers https://doi.org/10.3390/cancers12051115 (2020).
Borčinová, M. et al. Challenges in lower limb lymphoedema assessment based on limb volume change: lessons learnt from the SENTIX prospective multicentre study. Gynecol. Oncol. 164, 76–84 (2022).
Zapardiel, I. et al. Voiding recovery after radical parametrectomy in cervical cancer patients: an international prospective multicentre trial - SENTIX. Gynecol. Oncol. 160, 729–734 (2021).
Cibula, D. et al. Bilateral ultrastaging of sentinel lymph node in cervical cancer: lowering the false-negative rate and improving the detection of micrometastasis. Gynecol. Oncol. 127, 462–466 (2012).
Cibula, D. et al. Prognostic significance of low volume sentinel lymph node disease in early-stage cervical cancer. Gynecol. Oncol. 124, 496–501 (2012).
Ayhan, A. et al. Prognostic factors in FIGO stage IB cervical cancer without lymph node metastasis and the role of adjuvant radiotherapy after radical hysterectomy. Int. J. Gynecol. Cancer 14, 286–292 (2004).
Bodurka-Bevers, D. et al. Posttherapy surveillance of women with cervical cancer: an outcomes analysis. Gynecol. Oncol. 78, 187–193 (2000).
Chittithaworn, S., Hanprasertpong, J., Tungsinmunkong, K. & Geater, A. Association between prognostic factors and disease-free survival of cervical cancer stage IB1 patients undergoing radical hysterectomy. Asian Pac. J. Cancer Prev. 8, 530–534 (2007).
Hoogendam, J. P., Verheijen, R. H., Wegner, I. & Zweemer, R. P. Oncological outcome and long-term complications in robot-assisted radical surgery for early stage cervical cancer: an observational cohort study. BJOG 121, 1538–1545 (2014).
Landoni, F. et al. Class II versus class III radical hysterectomy in stage IB-IIA cervical cancer: a prospective randomized study. Gynecol. Oncol. 80, 3–12 (2001).
Laterza, R. M. et al. Recurrence of early stage cervical cancer after laparoscopic versus open radical surgery. Int. J. Gynecol. Cancer 26, 547–552 (2016).
Lee, C. L., Wu, K. Y., Huang, K. G., Lee, P. S. & Yen, C. F. Long-term survival outcomes of laparoscopically assisted radical hysterectomy in treating early-stage cervical cancer. Am. J. Obstet. Gynecol. 203, 165.e161–167 (2010).
Lim, K. C., Howells, R. E. & Evans, A. S. The role of clinical follow up in early stage cervical cancer in South Wales. BJOG 111, 1444–1448 (2004).
Park, J. Y. et al. Outcomes after radical hysterectomy in patients with early-stage adenocarcinoma of uterine cervix. Br. J. Cancer 102, 1692–1698 (2010).
Park, N. Y. et al. Oncologic results and surgical morbidity of laparoscopic nerve-sparing radical hysterectomy in the treatment of FIGO stage IB cervical cancer: long-term follow-up. Int. J. Gynecol. Cancer 21, 355–362 (2011).
Sartori, E. et al. Early stage cervical cancer: adjuvant treatment in negative lymph node cases. Gynecol. Oncol. 107, S170–S174 (2007).
Togami, S., Kamio, M., Yanazume, S., Yoshinaga, M. & Douchi, T. Can pelvic lymphadenectomy be omitted in stage IA2 to IIB uterine cervical cancer? Int. J. Gynecol. Cancer 24, 1072–1076 (2014).
Zanagnolo, V. et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int. J. Gynecol. Cancer 26, 568–574 (2016).
Acknowledgements
This work was supported by grants from the Czech Health Research Council (NV19-03-00023; no. 16-31643A), Charles University in Prague (UNCE/24/MED/018, UNCE/MED/008 and PROGRES Q28/LF1) and the Charles University Research program (Cooperatio – Maternal and Childhood Care, Neonatology) and by an institutional grant from the General University Hospital in Prague (CZ-DRO-VFN64165). The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. We acknowledge the investigators from all 47 sites participating in the SENTIX trial. We also thank all the medical specialists, data and case managers, secretaries, study coordinators and other people involved in the SENTIX trial. We also acknowledge the support of N. D. Smith (Freelance Medical Writer, Auckland, New Zealand) for providing English-language editorial support in the preparation of this article under guidance from the authors.
Author information
Authors and Affiliations
Contributions
Conception: D.C. and R.K. Data curation: D.C. and R.K. Formal analysis: J.J. and D.C. Funding acquisition: D.C. Investigation: D.C., S.M., R.K., P.D., J.K., I.Z., O.A., F.L., J.P., F.R., M.Z., L.R.C.W.v.L., A.T., J.S., L.M., M.O., R.P., A.F.P., A.P., A.B., K.N., D.F. and C.K. Methodology: D.C., R.K., P.D., A.B. and D.F. Project administration: D.C. and R.K. Validation: D.C., R.K., J.J., P.D. and K.N. Writing – original draft: D.C. Writing – review and editing: All authors. Accountable for all aspects of the work: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Stephanie Daignault-Newton, Catherine Uzan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–6.
Source data
Source Data Figs. 1–5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cibula, D., Marnitz, S., Jarkovský, J. et al. Sentinel lymph node biopsy without systematic pelvic lymphadenectomy in females with early-stage cervical cancer: final outcome of the SENTIX prospective, single-arm, noninferiority, international trial. Nat Cancer 6, 1585–1594 (2025). https://doi.org/10.1038/s43018-025-01016-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-025-01016-y
This article is cited by
-
Laparoskopisches Operieren beim frühen Zervixkarzinom
gynäkologie + geburtshilfe (2025)