Abstract
Individuals with non-small-cell lung cancer (NSCLC) with metastases to the ipsilateral mediastinum or subcarinal lymph nodes (N2 disease) have poor long-term survival. This exploratory analysis from the randomized phase 3 CheckMate 77T study assessed clinical outcomes by nodal status in individuals with stage III NSCLC who received neoadjuvant nivolumab plus chemotherapy followed by surgery and adjuvant nivolumab (nivolumab) versus neoadjuvant chemotherapy followed by surgery and adjuvant placebo (placebo). Here we show that among patients with N2 disease, nivolumab versus placebo improved event-free survival (1-year rate, 70% versus 45%; hazard ratio, 0.46 (95% confidence interval, 0.30–0.70)) and pathological complete response rate (22.0% versus 5.6%); 77% versus 73% had definitive surgery, of whom 84% versus 74% received a simple lobectomy. Furthermore, nivolumab improved outcomes versus placebo in patients with multistation N2 NSCLC (1-year event-free survival rate: 71% versus 46%; hazard ratio, 0.43 (0.21–0.88); pathological complete response rate, 29.0% versus 2.7%). In the N2 subgroup with definitive surgery, 67% and 59% of patients had nodal downstaging after surgery (57% versus 44% downstaged to node-negative disease). Median EFS in randomized patients with stage III non-N2 NSCLC was not reached with nivolumab and 17.0 months with placebo (1-year EFS rate, 74% versus 62%; hazard ratio, 0.60 (0.33–1.08)). No new safety signals were identified. These findings support perioperative nivolumab plus neoadjuvant chemotherapy as an efficacious treatment for stage III N2 disease and suggest that N2 status may not predict poor prognosis in resectable NSCLC treated with perioperative immunotherapy. ClinicalTrials.gov identifier: NCT04025879.
Similar content being viewed by others
Main
The current standard neoadjuvant treatment for eligible patients with resectable non-small-cell lung cancer (NSCLC) is a combination of immunotherapy plus chemotherapy1. In the phase 3 CheckMate 816 trial, neoadjuvant nivolumab plus chemotherapy demonstrated statistically significant improvements in event-free survival (EFS; hazard ratio (HR), 0.63; 97.38% confidence interval (CI), 0.43–0.91; P = 0.005) and rate of pathological complete response (pCR; 24.0% versus 2.2%; odds ratio (OR), 13.94 (99% CI, 3.49–55.75; P < 0.001)) versus chemotherapy alone in patients with resectable stage IB–IIIA NSCLC1. Neoadjuvant nivolumab plus chemotherapy also showed a statistically significant improvement in overall survival (OS) versus chemotherapy (HR, 0.72 (95% CI, 0.523–0.998); P = 0.048) at the 5-year analysis2. Neoadjuvant nivolumab plus chemotherapy is now approved by several regulatory bodies for eligible patients with resectable NSCLC3,4,5,6,7.
Perioperative immunotherapy-based treatment regimens for resectable NSCLC have also demonstrated superior survival and pathological response outcomes versus chemotherapy in patients with resectable NSCLC8,9,10,11,12,13,14. The phase 3 CheckMate 77T trial built on the positive findings for neoadjuvant nivolumab plus chemotherapy and demonstrated statistically significant EFS benefit (HR, 0.58 (97.36% CI, 0.42–0.81); P < 0.001) with perioperative nivolumab (neoadjuvant nivolumab plus chemotherapy followed by surgery and adjuvant nivolumab) versus perioperative placebo (neoadjuvant placebo plus chemotherapy followed by surgery and adjuvant placebo) in patients with resectable stage II–IIIB NSCLC11. These findings from CheckMate 77T led to the approval of this regimen in the United States and European Union3,4. National Comprehensive Cancer Network guidelines include treatment with neoadjuvant nivolumab plus chemotherapy and optional adjuvant nivolumab as a category 1 recommendation for eligible patients with NSCLC15. Perioperative durvalumab and pembrolizumab are also standard-of-care regimens, having demonstrated improvements in survival outcomes versus perioperative placebo in patients with resectable stage IIA–IIIB NSCLC in the phase 3 AEGEAN and KEYNOTE-671 trials, respectively8,12.
Stage IIIA–IIIB NSCLC, particularly with multistation disease, is historically associated with poor survival16,17. Treatment with concurrent chemoradiotherapy followed by consolidation immunotherapy for stage IIIA–IIIB NSCLC resulted in a 5-year survival rate of 42.9% (ref. 18). Although surgical resection is an option in select patients with stage III NSCLC19, disease recurrence is common after surgery: the 5-year recurrence-free survival rate in those with stage III NSCLC is 34% (ref. 20). Patients with stage III NSCLC with metastases to the ipsilateral mediastinum or subcarinal lymph nodes (N2 disease) have particularly limited treatment options. Two randomized trials of patients with completely resected stage IIIA N2 NSCLC reported no improvement in disease-free survival for patients with versus without postoperative radiotherapy21,22, highlighting a high unmet need in this population.
The clinical benefit of perioperative immunotherapy in patients with resectable stage III NSCLC, including subpopulations with N2 (single- and multistation N2) and non-N2 disease, is not fully understood. To further characterize outcomes in these patient populations, we report exploratory efficacy and safety outcomes from CheckMate 77T in patients with stage III resectable NSCLC with or without N2 disease.
Results
Patients, treatment and surgical summary
Of the 461 patients randomized to nivolumab (n = 229) or placebo (n = 232), as previously described11, 91 and 90 in the respective treatment arms had stage III N2 NSCLC at baseline, and 55 and 57 had stage III non-N2 NSCLC at baseline (Fig. 1). Two patients in each arm had stage III N3 NSCLC and were not included in this analysis because they were deemed unresectable. Among all patients analyzed, 157 of 181 (87%) with N2 NSCLC and 74 of 112 (66%) with non-N2 NSCLC had mediastinal staging confirmation. Patients had a median follow-up of 25.4 months (range, 15.7–44.2) as of the 6 September 2023 database lock.
Patients were enrolled between November 2019 and April 2022 and had a median follow-up of 25.4 months (range, 15.7–44.2) as of the 6 September 2023 database lock for this analysis. The denominators used to calculate percentages were based on the number of patients in each previous cohort. The superscript ‘a’ indicates that the enrollment of patients with stage III N3 NSCLC was considered a protocol deviation.
Baseline characteristics were generally similar between patients with N2 and patients with non-N2 NSCLC and between treatment arms (Table 1). In the nivolumab and placebo arms, respectively, 48 (53%) and 57 (63%) patients in the N2 subgroup had stage IIIA NSCLC and 43 (47%) and 33 (37%) had stage IIIB NSCLC; all patients in the non-N2 subgroup had stage IIIA NSCLC. The N2 subgroup had higher proportions of patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 and nonsquamous tumor histology in both treatment arms than the non-N2 subgroup, and the non-N2 subgroup had a higher proportion of patients with a tumor programmed cell death ligand 1 (PD-L1) of ≥50% in the nivolumab arm.
Among patients with N2 NSCLC, 77 (85%) and 81 (90%) completed neoadjuvant treatment in the nivolumab and placebo arms, respectively (Extended Data Fig. 1). Seventy (77%) patients in the nivolumab arm and 66 (73%) in the placebo arm underwent definitive surgery, of whom 59 (84%) and 49 (74%) had a lobectomy, and 1 (1%) and 9 (14%) had a pneumonectomy. Complete (R0) resection was achieved in 60 (86%) and 57 (86%) patients in the respective treatment arms. In the non-N2 NSCLC subgroup, 50 (91%) and 52 (91%) patients completed neoadjuvant treatment in the nivolumab and placebo arms, respectively. Forty-five (82%) patients in the nivolumab arm and 45 (79%) in the placebo arm underwent definitive surgery, of whom 36 (80%) and 32 (71%) had a lobectomy, and 6 (13%) and 4 (9%) had a pneumonectomy, respectively. R0 resection was achieved in 38 (84%) and 39 (87%) patients in the respective treatment arms. Additional surgical characteristics and outcomes are described in Supplementary Table 1.
In patients with N2 NSCLC, 56 (62%) in the nivolumab arm and 53 (59%) in the placebo arm received adjuvant treatment following surgery. By the data cutoff date, 28 (31%) and 25 (28%) patients had completed adjuvant treatment, respectively, and 25 (27%) and 25 (28%) had discontinued treatment, respectively; 3 (3%) and 3 (3%) were continuing treatment, respectively (Extended Data Fig. 1). In patients with non-N2 NSCLC, 33 (60%) in the nivolumab arm and 39 (68%) in the placebo arm received adjuvant treatment following surgery. Twenty-two (40%) in the nivolumab arm and 25 (44%) in the placebo arm had completed adjuvant treatment, and 10 (18%) in the nivolumab arm and 13 (23%) in the placebo arm had discontinued treatment; 1 (2%) in the nivolumab arm and 1 (2%) in the placebo arm were continuing treatment. In both the nivolumab and placebo arms, patients received a median of 12 (range, 1–13) and 13 (range, 1–13) doses of adjuvant treatment in the N2 and non-N2 subgroups, respectively.
The baseline characteristics of patients who completed adjuvant treatment but did not receive surgery are shown in Supplementary Table 2, and their subsequent therapies are described in the Supplementary Results.
Efficacy
Pathological response
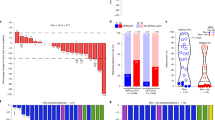
In randomized patients with stage III NSCLC, a higher proportion of patients with N2 NSCLC had a pCR with nivolumab (20/91 (22.0%)) than placebo (5/90 (5.6%); difference, 16.4%; Fig. 2a). Similarly, the pCR rate was higher among patients with non-N2 NSCLC in the nivolumab arm (14/55 (25.5%) versus 3/57 (5.3%); difference, 20.2%). Among patients who received definitive surgery, pCR rates in the N2 subgroup were higher with nivolumab (20 patients with pCR of 70 patients with definitive surgery (28.6%)) than placebo (5/66 (7.6%); difference, 21.0%); similar findings were observed in patients with definitive surgery in the non-N2 subgroup (14/45 (31.1%) versus 3/45 (6.7%); difference, 24.4%). pCR rates were also higher with nivolumab than placebo in patients with definitive surgery for both single-station N2 (11 patients with pCR of 45 patients with definitive surgery (24.4%) versus 4/37 (10.8%); difference, 13.6%) and multistation N2 subgroups (9/24 (37.5%) versus 1/29 (3.4%); difference, 34.1%; Fig. 2b).
a,b, pCR in patients with stage III NSCLC by N2 or non-N2 status (a) or single-station or multistation N2 status (b). The superscript letters (‘a’–‘l’) indicate the following 95% CIs: a, 6.5–26.5; b, 14.0–31.9; c, 1.8–12.5; d, 8.1–33.3; e, 18.4–40.6; f, 2.5–16.8; g, 6.9–33.5; h, 14.7–39.0; i, 1.1–14.6; j, 8.3–39.6; k, 18.2–46.6; l, 1.4–18.3. The superscript ‘m’ indicates that the N2 subcategory was not reported in one patient in the nivolumab arm. Superscript letters ‘n’–‘y’ indicate the following 95% CIs: n, −1.9 to 23.7; o, 9.7–30.9; p, 2.1–18.2; q, −3.6 to 29.3; r, 12.9–39.5; s, 3.0–25.4; t, 9.3–44.0; u, 14.2–48.0; v, 0.1–14.2; w, 12.7–54.0; x, 18.8–59.4; y, 0.1–17.8. The 95% CIs were determined for each treatment arm using the Clopper–Pearson method. An estimate of the unstratified difference in pCR rates and the corresponding 95% CIs were calculated using the Newcombe method.
Among all randomized patients with stage III NSCLC, a higher proportion in the nivolumab arm had a major pathological response (MPR) than in the placebo arm for both the N2 (27/91 (29.7%) versus 10/90 (11.1%); difference, 18.6%) and non-N2 subgroups (23/55 (41.8%) versus 7/57 (12.3%); difference, 29.5%; Extended Data Fig. 2a). Higher MPR rates in the nivolumab arm than in the placebo arm were also observed among patients with definitive surgery in the N2 and non-N2 subgroups and in patients with single-station and multistation N2 NSCLC (Extended Data Fig. 2a,b).
Consistent with the overall population, a higher proportion of patients with N2 NSCLC and a tumor PD-L1 of ≥1% had a pCR with nivolumab than with placebo (16 patients with pCR of 48 patients with a tumor PD-L1 of ≥1% (33.3%) versus 4/51 (7.8%); difference, 25.5%; Supplementary Table 3). The pCR rate was also higher in patients with N2 NSCLC and a tumor PD-L1 of <1% in the nivolumab versus placebo arms, although the difference between treatment arms was smaller (4/41 (9.8%) versus 1/35 (2.9%); difference, 6.9%). Similar results were observed in patients with non-N2 NSCLC with a tumor PD-L1 of ≥1% (10/30 (33.3%) in the nivolumab arm versus 1/28 (3.6%) in the placebo arm; difference, 29.8%) or a tumor PD-L1 of <1% (4/24 (16.7%) versus 2/28 (7.1%); difference, 9.5%).
EFS
Among all randomized patients with stage III N2 NSCLC, EFS was improved with perioperative nivolumab versus placebo (median EFS, 30.2 versus 10.0 months; 1-year EFS rate, 70% versus 45%; HR, 0.46 (95% CI, 0.30–0.70); Fig. 3a). Median EFS in randomized patients with stage III non-N2 NSCLC was not reached (NR) with nivolumab and 17.0 months with placebo (1-year EFS rate, 74% versus 62%; HR, 0.60 (95% CI, 0.33–1.08); Fig. 3b). Similar results were observed for nivolumab versus placebo in patients with single-station (HR, 0.49 (95% CI, 0.29–0.84)) or multistation (HR, 0.43 (95% CI, 0.21–0.88)) N2 NSCLC (Extended Data Fig. 3a,b). Nivolumab also numerically improved EFS versus placebo in the N2 and non-N2 subgroups whether patients had a tumor PD-L1 of ≥1% or <1% (Supplementary Table 4). In an exploratory analysis evaluating EFS landmarked from definitive surgery, outcomes appeared to favor nivolumab over placebo in patients with N2 (median EFS, NR versus 8.9 months; HR, 0.32 (95% CI, 0.19–0.54)) or non-N2 NSCLC (median EFS, NR versus 25.0 months; HR, 0.61 (95% CI, 0.30–1.24); Fig. 3c,d) and in those with single-station (HR, 0.40 (95% CI, 0.20–0.78)) or multistation (HR, 0.23 (95% CI, 0.09–0.58)) N2 NSCLC (Extended Data Fig. 3c,d).
a–d, EFS from randomization in patients with N2 NSCLC (a), randomization in patients with non-N2 NSCLC (b), definitive surgery in patients with N2 NSCLC (c) and definitive surgery in patients with non-N2 NSCLC (d). Superscript letters ‘a’–‘d’ indicate the following 95% CIs: a, 58–78; b, 34–55; c, 60–84; d, 48–74. The HRs and the two-sided 95% CIs for comparisons of EFS between the treatment arms were estimated using an unstratified Cox proportional hazards model using the randomized arm as a single covariate. EFS rates at 1 year were estimated using Kaplan–Meier estimates on the EFS curve for each randomized arm.
In an exploratory analysis of EFS landmarked from definitive surgery by pCR status, nivolumab improved EFS versus placebo in patients without pCR in both the N2 and non-N2 subgroups (N2 HR, 0.48 (95% CI, 0.27–0.86); non-N2 HR, 0.86 (95% CI, 0.41–1.84); Fig. 4a,b). The sample sizes for the subgroups of patients with pCR were too limited to compute HRs. Similar trends were observed for EFS from randomization (Extended Data Fig. 4a,b) and in patients with single-station or multistation N2 NSCLC (Extended Data Fig. 4c–f).
a–d, EFS from surgery in patients with N2 NSCLC by pCR status (a), patients with non-N2 NSCLC by pCR status (b), patients with N2 NSCLC without pCR who received at least one dose of adjuvant treatment (c) and patients with non-N2 NSCLC without pCR who received at least one dose of adjuvant treatment (d). The superscript letter ‘a’ indicates that EFS from surgery included only patients with definitive surgery and with pCR status available. The ‘Without pCR’ subgroup included patients with pCR negatively assessed. The superscript letter ‘b’ indicates that HRs were not calculated for subgroups with less than ten responders in either treatment arm. The HRs and the two-sided 95% CIs for comparisons of EFS between the treatment arms were estimated using an unstratified Cox proportional hazards model using the randomized arm as a single covariate; NC, not calculated.
An exploratory analysis of EFS from randomization by adjuvant treatment status suggested a numerical improvement in 1-year EFS rates with nivolumab versus placebo in patients with adjuvant therapy in both the N2 (88% versus 64%, respectively) and non-N2 subgroups (91% versus 79%; Extended Data Fig. 5a,b). Among patients without adjuvant treatment, 1-year EFS rates were numerically higher with nivolumab than with placebo in both the N2 (64% versus 0%, respectively) and non-N2 subgroups (56% versus 40%, respectively); however, these subgroups were too small to draw definitive conclusions (Extended Data Fig. 5c,d). One-year EFS rates from randomization or from surgery were numerically higher than placebo in the N2 subgroup whether patients received six or fewer doses or more than six doses of adjuvant treatment (Fig. 5 and Extended Data Fig. 6). Among patients with surgery but without pCR who received adjuvant treatment, EFS landmarked from surgery in the N2 and non-N2 subgroups also appeared longer with nivolumab than with placebo (Fig. 4c,d). In the N2 subgroup, 11 patients in the nivolumab arm and 13 in the placebo arm had surgery without pCR and no adjuvant treatment versus 10 and 6 patients, respectively, in the non-N2 subgroup. Most patients who received surgery and no adjuvant treatment did so because of study drug toxicity or disease progression (Extended Data Fig. 1).
a–d, EFS from randomization in patients with N2 NSCLC with six or fewer doses of adjuvant treatment (a), patients with N2 NSCLC with more than six doses of adjuvant treatment (b), patients with non-N2 NSCLC with six or fewer doses of adjuvant treatment (c) and patients with non-N2 NSCLC with more than six doses of adjuvant treatment (d). The superscript letter ‘a’ indicates that HRs were not calculated for subgroups with less than ten patients with events. Superscript letters ‘b’ and ‘c’ indicate the following 95% CIs: b, 44–89; c, 10–59. Superscript letter ‘d’ indicates that in the nivolumab arm, 28 patients received the maximum 13 doses of adjuvant treatment, and 13 patients received 7–12 doses; in the placebo arm, 23 patients received 13 doses of adjuvant treatment and 18 patients received 7–12 doses. Superscript letters ‘e’–‘h’ indicate the following 95% CIs: e, 79–98; f, 57–84; g, 33–98; h, NR–NR. Superscript letter ‘i’ indicates that in the nivolumab arm, 22 patients received the maximum 13 doses of adjuvant treatment, and 4 patients received 7–12 doses; in the placebo arm, 24 patients received 13 doses of adjuvant treatment, and 9 patients received 7–12 doses. Superscript letters ‘j’ and ‘k’ indicate the following 95% CIs: j, 73–98; k, 74–97. The HRs and two-sided 95% CIs for comparisons of EFS between the treatment arms were estimated using an unstratified Cox proportional hazards model using the randomized arm as a single covariate. EFS rates at 1 year were estimated using Kaplan–Meier estimates on the EFS curve for each randomized arm.
Node and tumor downstaging after surgery
Among randomized patients with baseline stage III NSCLC, 115 in the nivolumab arm and 111 in the placebo arm received definitive surgery. Postsurgical nodal downstaging was reported in 60 (52%) and 50 (45%) patients, respectively, with 53 (46%) and 40 (36%) downstaging to ypN0 (node-negative disease by pathological assessment) (Fig. 6). Seventy-three (64%) versus 62 (56%) patients had ypN0 after surgery with nivolumab versus placebo, respectively. Among patients with baseline cN1 (ipsilateral peribronchial or hilar lymph node involvement by clinical assessment), 14 of 18 (78%) versus 12 of 21 (57%) had disease downstaging to ypN0 after surgery with nivolumab and placebo, respectively; in patients with cN2 (ipsilateral mediastinum or subcarinal lymph node involvement by clinical assessment), disease downstaging was reported in 46 of 69 (67%) and 38 of 64 (59%) patients, including 39 of 69 (57%) and 28 of 64 (44%) with downstaging to ypN0. Seven (6%) patients in the nivolumab arm and five (4%) in the placebo arm experienced postsurgical nodal upstaging.
Among randomized patients with stage III NSCLC, 115 in the nivolumab arm and 111 in the placebo arm had received definitive surgery. The percentages in individual ribbons represent patients within each baseline N stage group (left) who had the indicated postsurgical N stage (right) among all patients with definitive surgery in that treatment arm. The superscript letter ‘a’ indicates that one (1%) patient in the nivolumab arm with unknown nodal stage at baseline was excluded, and postsurgical N stage was missing for three (3%) additional patients (one with cN1 at baseline and two with cN0 (no regional lymph node involvement by clinical assessment) at baseline). The superscript letter ‘b’ indicates that one (1%) patient in the placebo arm with not reported or cNX stage at baseline was excluded, and postsurgical N stage was missing for four (4%) additional patients (three with cN2 at baseline and one with cN0 at baseline).
In patients with stage III N2 NSCLC and definitive surgery, postsurgical tumor downstaging was reported in 43 of 70 (61%) in the nivolumab arm and 33 of 66 (50%) in the placebo arm; tumor downstaging to ypT0 (no evidence of tumor in the primary lesion by pathological assessment) was observed in 23 (33%) and 9 (14%) patients, respectively (Extended Data Fig. 7a). Among patients with baseline cT4, cT3, cT2 and cT1 per the American Joint Committee on Cancer Staging Manual 8th edition criteria for tumor size and location by clinical assessment, 2 of 7 (29%), 7 of 24 (29%), 9 of 25 (36%) and 5 of 14 (36%), respectively, in the nivolumab arm and 1 of 8 (12%), 3 of 17 (18%), 2 of 25 (8%) and 3 of 16 (19%), respectively, in the placebo arm had their tumor downstaged to ypT0. A total of 4 (6%) patients in the nivolumab arm and 11 (17%) in the placebo arm experienced postsurgical tumor upstaging. In patients with stage III non-N2 NSCLC and definitive surgery, postsurgical tumor downstaging was reported in 39 of 45 (87%) patients in the nivolumab arm and 34 of 45 (76%) patients in the placebo arm; tumor downstaging to ypT0 was observed in 12 (27%) and 5 (11%) patients, respectively (Extended Data Fig. 7b). One (2%) patient in the nivolumab arm and three (7%) in the placebo arm experienced postsurgical tumor upstaging.
Safety
Safety outcomes with nivolumab were similar between the N2 and non-N2 subgroups and consistent with all treated patients11. Adverse events (AEs) of any cause were observed in 90 of 91 (99%) patients in the nivolumab arm and in 87 of 90 (97%) patients in the placebo arm in the N2 subgroup and in 52 of 55 (94%) and 55 of 57 (96%) patients, respectively, in the non-N2 subgroup (Table 2). Any-grade treatment-related AEs (TRAEs) occurred in 82 (90%; 31 (34%) grade 3–4) patients in the nivolumab arm and 78 (87%; 23 (26%) grade 3–4) in the placebo arm of the N2 subgroup and in 48 (87%; 16 (29%) grade 3–4) and 48 (84%; 12 (21%) grade 3–4) patients in the non-N2 subgroup, respectively (Supplementary Table 5). Discontinuation due to AEs occurred in 28 (31%) patients in the nivolumab arm and 8 (9%) in the placebo arm in the N2 subgroup (23 (25%) and 6 (7%) due to TRAEs, respectively) and in 11 (20%) and 6 (10%) patients in the non-N2 subgroup, respectively (8 (14%) and 4 (7%) due to TRAEs). Serious AEs (SAEs) were observed in 40 (44%) and 24 (27%) patients in the N2 subgroup (24 (26%) and 10 (11%) with treatment-related SAEs) and in 21 (38%) and 20 (35%) in the non-N2 subgroup, respectively (5 (9%) and 6 (10%) with treatment-related SAEs). Treatment-related deaths occurred in two patients in the N2 subgroup in the nivolumab arm (grade 4 and 5 pneumonitis).
Surgery-related AEs occurred in 30 of 70 (43%) patients with surgery in the nivolumab arm and in 24 of 66 (36%) patients in the placebo arm in the N2 subgroup (6 (9%) and 7 (11%) with grade 3–4 events, respectively) and in 22 (49%) and 16 (36%) patients in the non-N2 subgroup (8 (18%) and 3 (7%) with grade 3–4 events). The most common surgery-related AEs are shown in Supplementary Table 6.
Discussion
This exploratory analysis from CheckMate 77T demonstrated that treatment with perioperative nivolumab improved clinical outcomes versus placebo in patients with resectable stage III NSCLC, a population with high unmet need. Among all patients with stage III NSCLC, those with N2 disease had 1-year EFS rates of 70% with nivolumab versus 45% with placebo (HR, 0.46 (95% CI, 0.30–0.70)), pCR rates of 22.0% versus 5.6%, respectively, and postsurgery nodal downstaging rates of 67% versus 59% (57% versus 44% downstaged to ypN0). Efficacy improvements were observed with nivolumab versus placebo in patients with both single-station and multistation N2 NSCLC (62% and 38% of all patients with N2 disease in this study, respectively) and were consistent with those seen in the non-N2 population. No new safety signals were reported.
The benefit of neoadjuvant or perioperative treatments with chemoimmunotherapy in patients with stage III NSCLC and nodal involvement is determined by the contribution of several important factors. First, pathological response rates, particularly pCR rates, represent one of the clearest prognostic elements; recurrence rates in patients with pCR after neoadjuvant nivolumab plus chemotherapy are low2,23,24. In CheckMate 816, median EFS with neoadjuvant nivolumab plus chemotherapy was not reached in patients with pCR versus 27.8 months in those without pCR (HR, 0.14 (95% CI 0.06–0.33))2. In the phase 2 NADIM and NADIM II trials, which evaluated neoadjuvant nivolumab plus chemotherapy followed by surgery and adjuvant nivolumab in patients with stage III resectable NSCLC, patients with pCR had a 5-year progression-free survival of 65.0% in NADIM, and all patients with pCR were alive without progression or recurrence at a median follow-up of 26.1 months in NADIM II10,25. In CheckMate 77T, pCR rates were similar for patients in the nivolumab arm with stage III N2 (22.0% (95% CI, 14.0–31.9)) and non-N2 disease (25.5% (95% CI, 14.7–39.0)). Among patients with pCR, 1-year EFS rates from randomization were 100% and 92%, respectively. The second factor is surgical outcomes. Complete (R0) resection was observed in similar proportions of patients in the nivolumab arm who had N2 (86%) or non-N2 NSCLC (84%); most patients did not need more radical surgery beyond lobectomy in either group. In particular, only 1% of patients with N2 disease in the nivolumab arm had a pneumonectomy versus 14% in the placebo arm, a notable reduction given low historical long-term survival in patients with pneumonectomy26. Furthermore, there was no increase in associated postoperative toxicity, surgery time or delays to surgery between the N2 and non-N2 groups. The third element is the impact of treatment on survival, which was again similar with nivolumab regardless of N2 status. The 1-year EFS rate was 70% for patients with N2 NSCLC in the nivolumab arm, comparable to the rate of 74% observed for patients with non-N2 NSCLC. Moreover, no differences were found whether patients had single- or multistation N2 NSCLC. In clinical practice, the extent of N2 involvement is sometimes considered a potential limiting element, along with other factors such as operability, in the decision-making process that ultimately assigns patients to surgery versus a definitive concurrent chemoradiotherapy-based approach, both of which are of curative intent. However, data from CheckMate 77T suggest that N2 status, regardless of the extent of nodal involvement, does not appear to condition a different prognosis in the context of perioperative chemoimmunotherapy.
The introduction of chemoimmunotherapy compels a shift in the global therapeutic approach for locally advanced NSCLC. Recent studies indicate that neoadjuvant chemoimmunotherapy can achieve unprecedented responses and much greater EFS rates than traditional treatments1,2, providing new opportunities for surgery to achieve R0 resections with a favorable long-term prognosis27. Studies for chemoimmunotherapy specifically in the N2 population are limited; however, the data available thus far show promise in this population, prompting thoughtful consideration of a surgical approach with perioperative immunotherapy for these patients. Neoadjuvant nivolumab plus chemotherapy improved EFS versus chemotherapy alone (HR, 0.69) in patients with nodal involvement in the CheckMate 816 trial24. The current analysis from CheckMate 77T, which is a comprehensive analysis of perioperative immunotherapy for patients with N2 NSCLC, showed clinically meaningful improvement for EFS and pCR with perioperative nivolumab in patients with N2 NSCLC. An exploratory subgroup analysis of patients with resectable stage III N2 NSCLC from AEGEAN also showed that perioperative durvalumab versus neoadjuvant chemotherapy alone improved EFS (2-year EFS rates of 66.3% versus 43.7%; HR, 0.63) and pCR rates (16.6% versus 4.9%)28. R0 resection was achieved in 94.7% versus 91.7% of patients in the durvalumab versus placebo arms, respectively. These results were similar to those observed in the overall population of patients with stage IIA–IIIB NSCLC in AEGEAN8,28. In KEYNOTE-671, treatment with perioperative pembrolizumab plus neoadjuvant chemotherapy versus perioperative placebo plus neoadjuvant chemotherapy in patients with stage III N2 NSCLC improved OS (HR, 0.74) and EFS (HR, 0.63) to a similar extent as the stage III non-N2 population (HR, 0.71 and 0.52, respectively)12. However, results are not directly comparable among the CheckMate 77T, AEGEAN and KEYNOTE-671 studies owing to the limitations of cross-trial comparisons.
The treatment of stage III NSCLC has been a subject of deep debate for years. In particular, there is a lack of consensus regarding the treatment of patients with N2 nodal involvement in the absence of reliable prognostic factors, effective treatments and clear distinction between resectable and nonresectable tumors29,30. One issue is operability conditioned by technical, anatomical or functional reserve factors to achieve definitive surgery, and another is whether initial N2 nodal involvement necessitates a different therapeutic approach. In the preimmunotherapy era, tumor downstaging after induction chemotherapy was considered an indicator of good prognosis in patients with N2 NSCLC. Yet, the overall prognosis was still poor, particularly in those with clinically visible involvement or multinodal involvement; even in those with resection, 5-year OS rates were just 21% before 2016 (ref. 31). For patients with N2 involvement without disease downstaging, definitive surgery was generally not considered a feasible treatment option32,33. The current standard of care for patients with stage III unresectable NSCLC (without genomic drivers for which targeted therapy is a standard regimen in the consolidation setting) is concurrent chemoradiotherapy, followed by consolidation therapy with durvalumab, based on the findings from the phase 3 PACIFIC trial34,35. A post hoc analysis from PACIFIC reported 18-month progression-free survival rates of 50.6% for durvalumab versus 26.0% for placebo in patients with unresectable stage IIIA N2 disease (HR, 0.46), compared to 39.7% versus 27.6% for patients without N2 NSCLC (HR, 0.62)36. Although cross-trial comparisons are complex due to differences in study design and patient populations, and caution should be applied when assessing data across studies in an exploratory fashion, results from CheckMate 77T show that clinical outcomes with perioperative nivolumab treatment were comparable regardless of whether patients had N2 or non-N2 NSCLC. These data suggest that patients with N2 NSCLC should be thoroughly discussed in a multidisciplinary setting for potential resectability in the context of a perioperative chemoimmunotherapy approach. To this end, it is important to note that ~40% of the patients randomized in PACIFIC had stage IIIA N2 disease, although PACIFIC included patients with unresectable NSCLC whereas CheckMate 77T enrolled patients with resectable NSCLC, resulting in notable clinical differences between patients in these two studies. Nodal involvement should not be the sole criterion for treatment selection in this patient population, given that patients with N2 NSCLC had similar or better outcomes for pCR, surgery rates and EFS than patients with non-N2 NSCLC in these studies.
Limitations of our analysis include its post hoc, exploratory nature, potentially leading to differences in baseline characteristics between the treatment groups and the N2 and non-N2 subgroups. The small sample sizes of some subgroups limited the interpretation of data from those populations. Additionally, the initial diagnosis of N2 or non-N2 disease and the extent of nodal involvement (for example, single versus multistation), as well as residual tumor classifications following surgery, were investigator assessed. Although most patients in this analysis had mediastinal staging confirmation at screening, a subset of patients lacked such confirmation based on the investigators’ determinations of feasibility. Finally, the analysis had a relatively short follow-up time, although results from the CheckMate 77T intent-to-treat population suggest that the EFS benefit with perioperative nivolumab is maintained with longer follow-up37.
In conclusion, this exploratory analysis from CheckMate 77T demonstrated that patients with stage III N2 NSCLC treated with neoadjuvant nivolumab plus chemotherapy followed by definitive surgery and adjuvant nivolumab have clinical outcomes similar to those in patients without N2 involvement. These results suggest that the presence of stage III N2 NSCLC may not represent a barrier for treatment with perioperative nivolumab-based therapy and that similar therapeutic approaches could be considered whether patients have single- or multistation N2 involvement, depending on other clinical characteristics. Overall, these results lend support to a paradigm shift toward chemoimmunotherapy-containing treatment, including perioperative immunotherapy, for some patients with N2 NSCLC, potentially including difficult-to-treat patients such as those with multistation nodal involvement. The most appropriate decision-making process for this population should include an expert multidisciplinary discussion in which benefits and risks, including potential patient- and disease-related factors, provider expertise and resources, are taken into account together with a thoughtful patient–provider discussion that is centered on evidence tailored to patient-specific needs.
Methods
Patients
As reported previously11, patients aged ≥18 years with treatment-naive, resectable stage IIA (>4 cm) to IIIB (T3N2) NSCLC (per the American Joint Committee on Cancer (AJCC) Staging Manual, 8th edition) who had no brain metastases and an ECOG performance status of 0 or 1 were eligible for enrollment. Patients with EGFR mutations or ALK alterations were excluded. Additional information is available in the Supplementary Methods.
Per the American Joint Committee on Cancer 8th edition criteria, clinical N2 was defined as metastasis in ipsilateral mediastinal or subcarinal nodes38. Patients in this analysis had stage III N2 (corresponding to T1/T2 stage IIIA or T3/T4 stage IIIB) or stage III non-N2 NSCLC (corresponding to T3/T4 stage IIIA) at baseline; patients with stage III N3 (corresponding to T1/T2 stage IIIB) NSCLC were excluded because they were not considered to be surgical candidates. At screening, all patients underwent mandatory positron emission tomography/computed tomography (PET/CT) scans as well as mediastinal lymph node evaluation when clinically feasible. Acceptable methods of mediastinal lymph node evaluation included mediastinoscopy, mediastinotomy, endobronchial ultrasound (EBUS), endoscopic ultrasound or CT-guided biopsy. Per protocol, patients with an EBUS-guided transbronchial needle aspiration that was negative for malignancy in a clinically positive mediastinum (PET or CT-positive mediastinum) were expected to undergo subsequent mediastinoscopy, although such confirmation was not required to be captured in the case report form. Patients with enlarged PET-positive lymph nodes visualized at subaortic lymph nodes (station 5) or the para-aortic lymph nodes (station 6) were permitted to enroll without further invasive mediastinal lymph node evaluation only if the lymph node stations could not be biopsied by routine mediastinoscopy due to a difficult approach. Preoperative PET/CT scans with contrast were also acquired at least 14 days after the last neoadjuvant dose and before surgery; patients with positive scans were recommended to undergo mediastinal evaluation by bronchoscopy/EBUS or by mediastinoscopy with pathological assessment of sites concerning for progression prior to surgery.
Trial design and treatments
CheckMate 77T (NCT04025879) is a phase 3, double-blind trial in which patients were randomized 1:1 (via an interactive response technology system) to receive perioperative nivolumab or placebo11. Randomization was stratified by tumor histology (squamous versus nonsquamous), NSCLC stage II versus III and PD-L1 status (≥1% versus <1% versus not evaluable/indeterminate). Treatment was to be administered within 3 days of randomization, with choice of chemotherapy regimen based on NSCLC histology. Patients received nivolumab (360 mg) plus chemotherapy or placebo plus chemotherapy every 3 weeks for up to four cycles during the neoadjuvant period. Afterward, patients were assessed to undergo definitive surgery within 6 weeks of the last neoadjuvant treatment. Patients were to begin adjuvant therapy (nivolumab (480 mg) or placebo every 4 weeks for up to 13 cycles) within 90 days of surgery, after undergoing radiologic restaging. Additional treatment details are provided in the Supplementary Methods.
End points and assessments
Primary and key secondary end points along with results from an interim analysis have been described previously11, and additional details are provided in the Supplementary Methods. Briefly, the primary end point was EFS, defined as the time from randomization to disease progression precluding or preventing completion of surgery, abandoned surgery due to unresectability, disease progression or recurrence after surgery, disease progression in patients without surgery or death from any cause. For this post hoc exploratory analysis, patients with N2 and non-N2 disease were assessed for EFS by blinded independent central review, pCR and MPR by blinded independent pathological review, nodal and tumor downstaging by investigators and safety. EFS landmarked from definitive surgery was defined as the time from surgery to disease progression or recurrence after surgery or death from any cause. Progression/recurrence was assessed by blinded independent central review per Response Evaluation Criteria in Solid Tumors (version 1.1). pCR was defined as 0% residual viable tumor cells after surgery in both the primary tumor (lung) and sampled lymph nodes. MPR was defined as ≤10% residual viable tumor cells after surgery in both the primary tumor (lung) and sampled lymph nodes. Completeness of resection was investigator assessed and defined as R0 (complete resection with no residual tumor), R1 (microscopic residual tumor), R2 (macroscopic residual tumor) or R(un) (uncertain resection)27. Downstaging was assessed based on the change from baseline clinical nodal and tumor stages to postsurgical pathological nodal and tumor stages. Safety was evaluated based on reports of all AEs collected within 100 days of the last study dose and categorized based on the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) and Medical Dictionary for Regulatory Activities (version 26.0).
Ethical oversight
The trial steering committee and sponsor (Bristol Myers Squibb) for CheckMate 77T designed the study. Data were collected by investigators and analyzed in collaboration with the sponsor, with an independent data monitoring committee providing oversight. The trial was performed in accordance with the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines. Independent ethics committees or institutional review boards at each trial site approved the protocol, consent form and any other patient-facing written materials. Written informed consent was provided by all patients before participation in any study procedures. This manuscript was written based on direction from the authors with sponsor-funded medical writing support. The authors confirmed the accuracy and completeness of the data in this report and approved the final draft for submission for publication. CONSORT guidelines were followed in the development of this manuscript39.
Statistics and reproducibility
Sample size was based on the primary end point (EFS) in the total population; further details on determination and justification have been reported previously11. Post hoc exploratory efficacy analyses were performed in all randomized patients with stage III N2 or non-N2 NSCLC and safety analyses in all treated patients (that is, those who received at least one dose of study medication) with stage III N2 or non-N2 NSCLC. Landmark EFS from surgery was calculated in patients with definitive surgery. The HRs and the two-sided 95% CIs for comparisons of EFS between the treatment arms were estimated using an unstratified Cox proportional hazards model using the randomized arm as a single covariate. EFS rates at 1 year were estimated using Kaplan–Meier estimates on the EFS curve for each randomized arm. The 95% CIs for pCR and MPR rates were determined for each treatment arm using the Clopper–Pearson method. An estimate of the unstratified difference in pCR and MPR rates and the corresponding 95% CI were calculated using the Newcombe method.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Qualified researchers with a clearly defined scientific objective may submit requests for deidentified and anonymized datasets to Bristol Myers Squibb. Criteria for data requests are available at https://vivli.org/ourmember/bristol-myers-squibb/, and additional information on Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html. Data considered for sharing may include nonidentifiable patient-level and study-level clinical trial data and full clinical study reports. The study protocol for CheckMate 77T is included in the Supplementary Information. Source data are provided with this paper.
Code availability
No custom code was used for statistical analyses in CheckMate 77T. All analyses were performed using SAS software (version 9.04.01M7P080620).
References
Forde, P. M. et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 386, 1973–1985 (2022).
Forde P. M. et al. Overall survival with neoadjuvant nivolumab plus chemotherapy in lung cancer. N. Engl. J. Med. 393, 741–752 (2025).
United States Food and Drug Administration. Opdivo (nivolumab) https://packageinserts.bms.com/pi/pi_opdivo.pdf (2025).
European Medicines Agency. Opdivo (nivolumab): summary of product characteristics https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf (2025).
China National Drug Administration. Instructions for Opdivo (nivolumab) injection https://www.bms.com/assets/bms/china/restricted/pi/OPDIVO_20230727.pdf (2023).
Health Canada. Opdivo: nivolumab for injection https://www.bms.com/assets/bms/ca/documents/productmonograph/OPDIVO_EN_PM.pdf (2024).
Pharmaceuticals and Medical Devices Agency. Opdivo (nivolumab) review report https://www.pmda.go.jp/files/000246571.pdf (2021).
Heymach, J. V. et al. Perioperative durvalumab for resectable non–small-cell lung cancer. N. Engl. J. Med. 389, 1672–1684 (2023).
Wakelee, H. et al. Perioperative pembrolizumab for early-stage non–small-cell lung cancer. N. Eng. J. Med. 389, 491–503 (2023).
Provencio, M. et al. Perioperative nivolumab and chemotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 389, 504–513 (2023).
Cascone, T. et al. Perioperative nivolumab in resectable lung cancer. N. Engl. J. Med. 390, 1756–1769 (2024).
Spicer, J. D. et al. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 404, 1240–1252 (2024).
Lu, S. et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the Neotorch randomized clinical trial. JAMA 331, 201–211 (2024).
Yue, D. et al. Perioperative tislelizumab plus neoadjuvant chemotherapy for patients with resectable non-small-cell lung cancer (RATIONALE-315): an interim analysis of a randomised clinical trial. Lancet Respir. Med. 13, 119–129 (2025).
Riely, G. J. et al. NCCN Guidelines insights: non–small cell lung cancer, version 7.2025: featured updates to the NCCN Guidelines. J. Natl Compr. Canc. Netw. https://doi.org/10.6004/jnccn.2025.0043 (2025).
Rami-Porta, R. et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: proposals for the revision of the TNM stage groups in the forthcoming (ninth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 19, 1007–1027 (2024).
Lee, J. G. et al. The prognostic significance of multiple station N2 in patients with surgically resected stage IIIA N2 non-small cell lung cancer. J. Korean Med. Sci. 23, 604–608 (2008).
Spigel, D. R. et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J. Clin. Oncol. 40, 1301–1311 (2022).
Casal-Mouriño, A. et al. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl. Lung Cancer Res. 10, 506–518 (2021).
Rajaram, R. et al. Recurrence-free survival in patients with surgically resected non-small cell lung cancer a systematic literature review and meta-analysis. Chest 165, 1260–1270 (2024).
Hui, Z. et al. Effect of postoperative radiotherapy for patients with pIIIA-N2 non-small cell lung cancer after complete resection and adjuvant chemotherapy: the phase 3 PORT-C randomized clinical trial. JAMA Oncol. 7, 1178–1185 (2021).
Le Pechoux, C. et al. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): an open-label, randomised, phase 3 trial. Lancet Oncol. 23, 104–114 (2022).
Provencio, M. et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 21, 1413–1422 (2020).
Deutsch, J. S. et al. Association between pathologic response and survival after neoadjuvant therapy in lung cancer. Nat. Med. 30, 218–228 (2024).
Provencio, M. et al. Perioperative chemotherapy and nivolumab in non-small-cell lung cancer (NADIM): 5-year clinical outcomes from a multicentre, single-arm, phase 2 trial. Lancet Oncol. 25, 1453–1464 (2024).
Riquet, M. et al. A review of 250 ten-year survivors after pneumonectomy for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 45, 876–881 (2014).
Edwards, J. G. et al. The IASLC lung cancer staging project: analysis of resection margin status and proposals for residual tumor descriptors for non-small cell lung cancer. J. Thorac. Oncol. 15, 344–359 (2020).
Heymach, J. V. et al. Outcomes with perioperative durvalumab (D) in pts with resectable NSCLC and baseline N2 lymph node involvement (N2 R-NSCLC): An exploratory subgroup analysis of AEGEAN. J. Clin. Oncol. 42, 8011 (2024).
Donington, J. S. & Pass, H. I. Surgical resection of non-small cell lung cancer with N2 disease. Thorac. Surg. Clin. 24, 449–456 (2014).
McElnay, P. J., Choong, A., Jordan, E., Song, F. & Lim, E. Outcome of surgery versus radiotherapy after induction treatment in patients with N2 disease: systematic review and meta-analysis of randomised trials. Thorax 70, 764–768 (2015).
Łochowski, M. et al. Five-year survival analysis and prognostic factors in patients operated on for non-small cell lung cancer with N2 disease. J. Thorac. Dis. 10, 3180–3186 (2018).
Adizie, J. B. et al. Stage III non-small cell lung cancer management in England. Clin. Oncol. 31, 688–696 (2019).
Provencio, M. et al. Real-world treatment patterns and survival outcomes for patients with stage III non-small cell lung cancer in Spain: a nationwide cohort study. Transl. Lung Cancer Res. 12, 2113–2128 (2023).
Antonia, S. J. et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 377, 1919–1929 (2017).
Antonia, S. J. et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 379, 2342–2350 (2018).
Senan, S. et al. Outcomes with durvalumab after chemoradiotherapy in stage IIIA-N2 non-small-cell lung cancer: an exploratory analysis from the PACIFIC trial. ESMO Open 7, 100410 (2022).
Cascone, T. et al. Perioperative nivolumab (NIVO) vs placebo (PBO) in patients (pts) with resectable NSCLC: updated survival and biomarker analyses from CheckMate 77T. J. Clin. Oncol. 43, LBA8010 (2025).
Detterbeck, F. C. The eighth edition TNM stage classification for lung cancer: what does it mean on main street?. J. Thorac. Cardiovasc. Surg. 155, 356–359 (2018).
Schulz, K. F., Altman, D. G., Moher, D. & CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Open Med. 4, e60–e68 (2010).
Acknowledgements
We thank the patients and families who made this study possible; the investigators and clinical study teams who participated in the trial; Dako, an Agilent Technologies company, for collaborative development of the PD-L1 IHC 28-8 pharmDx assay; Ono Pharmaceutical Company and L. Tracy for biostatistics support. Findings from this exploratory analysis of CheckMate 77T were reported, in part, at the 2024 American Society of Clinical Oncology Annual Meeting. Medical writing and editorial support for the development of this manuscript, under the direction of the authors, was provided by K. M. Fahrbach, S. Hom, C. Nelson and M. Salernitano of Ashfield MedComms, an Inizio company, funded by Bristol Myers Squibb. Bristol Myers Squibb sponsored the study and participated in the study design, data collection and analysis and writing of the manuscript.
Author information
Authors and Affiliations
Contributions
M.P., M.M.A., J.D.S., Y.G., J.H., F.T., S.L. and T.C. participated in the study conception/design, data acquisition and data interpretation for this manuscript. A.J., F.M., Y.W., A.A., F.G., N.F., F.F. and T.J.N.H. participated in data acquisition and data interpretation. C.C.E., P.S. and P.T. participated in the study conception/design, data analysis and data interpretation. V.D. participated in the study conception/design, data analysis and data interpretation. M.P. and T.C. provided major contributions to the writing of the first draft and revision of the manuscript. All authors contributed to the drafting of the manuscript, approved the final version and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
M.P. reports receiving grants or contracts from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, European Commission, F. Hoffmann-La Roche, Instituto de Salud Carlos III, Merck, Pierre Fabre Pharmaceuticals and Takeda Oncology; participating as a consultant for AstraZeneca, Bristol Myers Squibb, Eli Lilly, F. Hoffmann-La Roche, Janssen Global Services, Laboratorios Pfizer, Merck and Takeda Oncology and receiving travel reimbursement from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, F. Hoffmann-La Roche, Merck, Pierre Fabre Pharmaceuticals and Takeda Oncology. M.M.A. reports receiving grants or contracts to his institution from Amgen, AstraZeneca, Bristol Myers Squibb, Genentech and Lilly; participating as a consultant for Affini-T, AstraZeneca, Blueprint Medicines Corporation, D3Bio, EMD Serono, Gritstone, Iovance, Merck, Merus, Mirati, Novartis, Pfizer and Synthekine and participating on data safety monitoring/advisory boards for Apollomics and Bristol Myers Squibb. J.D.S. reports receiving grants or contracts to his institution from CLS Therapeutics, F. Hoffmann-La Roche, Merck and Protalix BioTherapeutics and participating as a consultant for AstraZeneca, Bristol Myers Squibb, ChemoCentryx, Daiichi Sankyo, Eisai, F. Hoffmann-La Roche, Merck, Pfizer Canada, Protalix BioTherapeutics and Regeneron Pharmaceuticals. F.M. reports participation as a speaker or lecturer for Amgen, AstraZeneca, F. Hoffmann-La Roche, Novartis, Pfizer and R-Pharm US; participating on a scientific advisory board for AstraZeneca and Pfizer; participating as an expert witness for AstraZeneca and receiving compensation for editorial work from AstraZeneca and travel reimbursement from AstraZeneca, Merck and Novartis. A.A. reports travel reimbursement from Johnson and Johnson, Merck and Servier Affaires Medicales and other competing interests from Servier Affaires Medicales. F.G. reports research grants to his institution from Pfizer, Roche and Takeda and consulting or lecturing fees from Amgen, AstraZeneca, Bristol Myers Squibb, Janssen, MSD, Pfizer, Roche, Sanofi, Takeda and Viatris. N.F. reports a research grant to himself from Roche and participation as a consultant or advisor for AbbVie, Amgen, AstraZeneca, BeiGene, BerlinChemie, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Janssen Oncology, Lilly, Merck, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, Roche, Sanofi and Takeda. T.J.N.H. reports research grants to his institution from AZD, Bristol Myers Squibb, Gilead, GSK and Roche and participation as a consultant or advisor for Bristol Myers Squibb. F.T. reports grants or contracts to his institution from Boehringer Ingelheim Japan, Chugai Pharmaceutical, Eli Lilly Japan, Ono Pharmaceutical and Taiho Pharmaceutical and participation as a speaker or lecturer for AstraZeneca, Boehringer Ingelheim Japan, Bristol Myers Squibb, Chugai Pharmaceutical, Covidien Japan, Eli Lilly Japan, Johnson and Johnson, MSD, Ono Pharmaceutical and Taiho Pharmaceutical. S.L. reports receiving grants or contracts to his institution from AstraZeneca, BeiGene, Bristol Myers Squibb, Hansoh Pharmaceutical, Heng Rui, Hutchison and Lilly Suzhou Pharmaceutical; research support from AstraZeneca, BeiGene, Bristol Myers Squibb, Hanson Pharma, Hutchison Whampoa, Jiangsu Hengrui and Roche; participation as a speaker or lecturer for AstraZeneca, Hanson Pharma, Heng Rui, Jiangsu Hengrui and Roche and participation as a consultant for AstraZeneca, Boehringer Ingelheim, GenomiCare, Hutchison MediPharma, Hutchison Whampoa, InventisBio, Menarini Silicon Biosystems, Pfizer, Pfizer Canada, Roche, Yuhan and Zai Lab. C.C.E., P.S., P.T. and V.D. are employees and shareholders of Bristol Myers Squibb. T.C. reports receiving speaker fees/honoraria from ASCO Post, AstraZeneca, Bio Ascend, Bristol Myers Squibb, IDEOlogy Health, Medical Educator Consortium, Medscape, OncLive, Physicians’ Education Resources, PeerView and Targeted Oncology; participation fees as a consultant and/or advisor from AstraZeneca, BioNTech, Bristol Myers Squibb, Caris Life Sciences, Daiichi Sankyo, Genentech, Johnson & Johnson, Merck, Moderna, Nuvalent, oNKo-Innate, Pfizer, RAPT Therapeutics, Regeneron and Summit Therapeutics; and institutional research funding to her institution from AstraZeneca, Bristol Myers Squibb and Merck. A.J., Y.G., Y.W., F.F. and J.H. declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Clemens Aigner, Jared Foster, Masato Karayama, Cecile Le Pechoux and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Treatment and surgery summary in patients with stage III N2 and non-N2 NSCLC.
Neoadjuvant treatment, surgery, and adjuvant treatment by treatment arm and N2 status in all randomized patients. The denominators used to calculate percentages were based on patients in each N2 or non-N2 subgroup. aOne patient in the nivolumab N2 subgroup, 1 patient in the nivolumab non-N2 subgroup, 2 patients in the placebo N2 subgroup, and 1 patient in the placebo non-N2 subgroup did not undergo definitive surgery but did receive adjuvant treatment. AE, adverse event.
Extended Data Fig. 2 MPR in patients with stage III N2 and non-N2 NSCLC.
Patients with (a) N2 or non-N2 NSCLC and (b) single-station or multi-station N2 status. a–l95% CI: a6.9–29.8; b20.5–40.2; c5.5–19.5; d8.5–36.9; e27.2–51.0; f7.5–26.1; g13.2–44.1; h28.7–55.9; i5.1–23.7; j16.2–51.5; k35.8–66.3; l6.5–29.5. mThe N2 subcategory was not reported in 1 patient in the nivolumab arm. n − 2.0–26.1; o13.6–36.6; p4.3–23.0; q − 4.0–31.8; r18.2–46.6; s6.2–32.0; t10.3–49.6; u24.5–60.9; v3.0–25.4; w15.0–60.1; x32.8–74.4; y3.9–31.7. The 95% CIs were determined for each treatment arm using the Clopper-Pearson method. An estimate of the unstratified difference in MPR rates and the corresponding 95% CI were calculated using the Newcombe method. CI, confidence interval; MPR, major pathological response; NSCLC, non-small cell lung cancer.
Extended Data Fig. 3 EFS in patients with stage III N2 NSCLC by N2 station.
EFS from (a) randomization in patients with single-station N2 NSCLC, (b) randomization in patients with multi-station N2 NSCLC, (c) definitive surgery in patients with single-station N2 NSCLC, and (d) definitive surgery in patients with multi-station N2 NSCLC. aThe N2 subcategory was not reported in 1 patient in the nivolumab arm. b–e95% CI: b54–79; c30–58; d50–84; e28–62. The HRs and the two-sided 95% CIs for comparisons of EFS between the treatment arms were estimated using an unstratified Cox proportional hazards model using the randomized arm as a single covariate. EFS rates at 1 year were estimated using Kaplan-Meier estimates on the EFS curve for each randomized arm. CI, confidence interval; EFS, event-free survival; HR, hazard ratio; NR, not reached; NSCLC, non-small cell lung cancer.
Extended Data Fig. 4 EFS in patients with stage III N2 and non-N2 NSCLC with or without pCR.
EFS from (a) randomization in patients with N2 NSCLC, (b) randomization in patients with non-N2 NSCLC, (c) randomization in patients with single-station N2 NSCLC, (d) randomization in patients with multi-station N2 NSCLC, (e) definitive surgery in patients with single-station N2 NSCLC, and (f) definitive surgery in patients with multi-station N2 NSCLC. aEFS from randomization included all randomized patients. The “Without pCR” subgroup included patients with pCR negatively assessed, patients without definitive surgery, and patients with definitive surgery but without pCR status available. bHRs were NC for subgroups with <10 responders in either treatment arm. c–j95% CI: c100–100; d100–100; e47–71; f30–52; g100–100; h57–99; i50–80; j45–72. kThe N2 subcategory was not reported in 1 patient in the nivolumab arm. l–s95% CI: l100–100; m100–100; n45–73; o24–53; p100–100; q100–100; r32–76; s27–60. tEFS from surgery included only patients with definitive surgery and with pCR status available. The “Without pCR” subgroup included patients with pCR negatively assessed. The HRs and the two-sided 95% CIs for comparisons of EFS between the treatment arms were estimated using an unstratified Cox proportional hazards model using the randomized arm as a single covariate. EFS rates at 1 year were estimated using Kaplan-Meier estimates on the EFS curve for each randomized arm. CI, confidence interval; EFS, event-free survival; HR, hazard ratio; NC, not calculated; NR, not reached; NSCLC, non-small cell lung cancer; pCR, pathological complete response.
Extended Data Fig. 5 EFS in patients with stage III N2 or non-N2 NSCLC by adjuvant treatment status.
EFS from randomization in (a) patients with N2 NSCLC with at least one dose of adjuvant treatment, (b) patients with non-N2 NSCLC with at least one dose of adjuvant treatment, (c) patients with N2 NSCLC without adjuvant treatment, and (d) patients with non-N2 NSCLC without adjuvant treatment. a,b95% CI: a76–94; b50–75. cHRs were NC for subgroups with <10 patients with events. d–i95% CI: d74–97; e63–89; f30–84; gNR–NR; h21–81; i5–75. The HRs and the two-sided 95% CIs for comparisons of EFS between the treatment arms were estimated using an unstratified Cox proportional hazards model using the randomized arm as a single covariate. EFS rates at 1 year were estimated using Kaplan-Meier estimates on the EFS curve for each randomized arm. CI, confidence interval; EFS, event-free survival; HR, hazard ratio; NC, not calculated; NR, not reached; NSCLC, non-small cell lung cancer.
Extended Data Fig. 6 EFS from definitive surgery in patients with stage III N2 or non-N2 NSCLC by number of adjuvant treatment doses.
EFS from surgery in (a) patients with N2 NSCLC with ≤6 doses of adjuvant treatment, (b) patients with N2 NSCLC with >6 doses of adjuvant treatment, (c) patients with non-N2 NSCLC with ≤6 doses of adjuvant treatment, and (d) patients with non-N2 NSCLC with >6 doses of adjuvant treatment. aHRs were NC for subgroups with <10 responders in either treatment arm. bIn the nivolumab arm, 28 patients received the maximum 13 doses of adjuvant treatment and 13 patients received 7–12 doses; in the placebo arm, 23 patients received 13 doses of adjuvant treatment and 18 patients received 7–12 doses. cIn the nivolumab arm, 22 patients received the maximum 13 doses of adjuvant treatment and 4 patients received 7–12 doses; in the placebo arm, 24 patients received 13 doses of adjuvant treatment and 9 patients received 7–12 doses. The HRs and the two-sided 95% CIs for comparisons of EFS between the treatment arms were estimated using an unstratified Cox proportional hazards model using the randomized arm as a single covariate. CI, confidence interval; EFS, event-free survival; HR, hazard ratio; NC, not calculated; NR, not reached; NSCLC, non-small cell lung cancer.
Extended Data Fig. 7 Change in T stage from baseline to post-definitive surgery in patients with stage III N2 and non-N2 NSCLC and definitive surgery.
Patients with (a) stage III N2 NSCLC and definitive surgery or (b) non-N2 NSCLC and definitive surgery. Among randomized patients with stage III N2 NSCLC, 70 in the nivolumab arm and 66 in the placebo arm had received definitive surgery. Among randomized patients with stage III N2 NSCLC, 45 in each arm had received definitive surgery. Percentages in individual ribbons represent patients within each baseline T stage group (left) who had the indicated post-surgical T stage (right) among all patients with definitive surgery in that treatment arm and N2 status subgroup. aOne (2%) patient with N2 NSCLC in the placebo arm with not reported or cTX stage at baseline was excluded. bPost-surgical T stage was missing for 3 (7%) patients with non-N2 NSCLC in the nivolumab arm. cOne (1%) patient with non-N2 NSCLC in the placebo arm with not reported or cTX stage at baseline was excluded, and post-surgical T stage was missing for 1 (2%) additional patient with cT4 at baseline.
Supplementary information
Supplementary Information
Supplementary Results, Methods, Tables 1–6, Clinical trial protocol and Statistical analysis plan.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Provencio, M., Awad, M.M., Spicer, J.D. et al. Clinical outcomes with perioperative nivolumab by nodal status in patients with stage III resectable NSCLC: phase 3 CheckMate 77T exploratory analysis. Nat Cancer 7, 169–181 (2026). https://doi.org/10.1038/s43018-025-01104-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-025-01104-z