Abstract
Wearable sensors have evolved significantly, making personalized medicine and real-time disease management possible. However, current digital healthcare is limited to only certain diseases, such as diabetes, due to the lack of mature technologies that can detect small biomolecules. In particular, despite the early detection of chronic kidney disease (CKD) being significant in preventing life-threatening end-stage kidney disease, the development of wearable sensors for CKD monitoring is still in the early stages. In this Perspective, we propose a wearable digital healthcare concept for non-invasive, continuous CKD monitoring, discuss optimal biofluids, biomarkers and bioreceptors to create a wearable CKD sensing platform, and provide insight into potential challenges faced by the technology as well as opportunities.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a major global health burden that affects ~10–15% of the world’s adult population1. Considering the increasing prevalence of lifestyle-associated diseases such as hypertension, diabetes and obesity, the prevalence of CKD is also expected to increase continuously2. As CKD can be developed to life-threatening cardiovascular diseases, end-stage kidney disease (ESKD), requiring lifelong kidney replacement therapy, and even death, considerable efforts have been made to identify the causes and risk factors of CKD3. Given that CKD is a gradually progressive disease that leads to irreversible impairment of the kidney function, it is important to enable the early detection of CKD, before onset, as well as early-stage management4. Furthermore, CKD is asymptomatic in the early stages and the appearance of symptoms is typically uncommon until stages 4 or 5. For this reason, when the individual notices a symptom, the kidneys are likely to be quite damaged, which leads to a radical change in patient’s life. For example, the patients need to visit the hospital every two days for dialysis or, in a severe case, they would need a kidney transplant. In this regard, the importance of the early diagnosis of CKD cannot be overemphasised.
CKD can be identified with simple tests such as blood-based creatinine and/or urine-based tests5. Both tests are commonly prescribed in routine clinical practice, and the collected samples are analysed using laboratory techniques such as liquid chromatography. However, such testing methods are costly, time-consuming and require non-portable instruments and trained professionals (Fig. 1a). Furthermore, not only do the patients require visits to the clinic, but they also suffer from invasive blood tests. At-home kidney test kits using blood and urine are also available in the market, as convenient self-monitoring tools, but they have some limitations. For example, while blood-based test kits can measure levels of several biomarkers such as an estimated glomerular filtration rate (eGFR), creatinine and blood urea nitrogen (BUN), repeated blood sampling via a finger-prick method is moderate invasive and there may be risk of infection. Furthermore, as the blood samples need to be transported to a lab for analysis, a turnaround is slow, ranging from 2 days to 3 weeks, depending on the product. On the other hand, urine-based test kits like dipsticks normally measure a urine albumin-to-creatinine ratio (UACR) and albumin levels. Such a test allows a quick colour change in the presence of biomarkers, which delivers rapid results in the comfort of your own home. However, as the results are interpreted by the visual comparison with the enclosed colour chart, the tests only provide semiquantitative data, which is not suitable to make clinical decisions. The urinalysis tests have additional limitations: susceptibility to urine discolouration, sample contamination and differences in sensitivity and selectivity depending on the products of reagent strip used6. Furthermore, these test kits do not allow real-time monitoring of the kidney function, and thus fail in early detection of CKD as well as its efficient management.
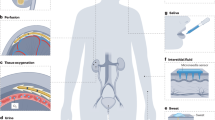
a current CKD monitoring in clinic: non-real-time blood and urine tests. b Future wearable healthcare for CKD. Wearable patch will remotely and continuously monitor a range of biomarkers for CKD through either sweat or ISF. Collected sensor readings can be classified and analysed with big data techniques. This system will provide not only an early clinical intervention but also an efficient management of CKD.
Dramatic advances in wearable sensors7,8 and soft microfluidics9,10 provide attractive sets of capabilities to build wearable CKD healthcare, in which the biomarkers are possible to be detected in a non-invasive, simultaneous and continuous way. In the envisioned landscape of wearable CKD monitoring, chemical sensors integrated into wristbands or patches will simultaneously and selectively measure a range of CKD biomarkers (e.g., creatinine, urea and cystatin C) through sweat or interstitial fluid (ISF) (Fig. 1b). The wearable sensor can be used as not only a non-invasive, rapid diagnostic tool for outpatients but also a remote tool for real-time CKD monitoring. To be specific, the sensors attached on the skin will continuously monitor concentrations of the biomarkers, and deviations from the baseline levels can be fingerprinted to allow autonomous and early diagnosis of CKD as well as its efficient management. In particular, given that serum creatinine is normally measured at an annual checkup, continuous CKD monitoring allows for early diagnosis without having to wait for this annual checkup. Furthermore, as CKD patients can experience a dramatic decline in kidney function at any time, the continuous monitoring of CKD biomarkers can improve treatment outcomes by timey interventions. The goal of this Perspective article is to discuss optimal biofluids, biomarkers and sensing technologies to build a wearable platform that allows CKD monitoring, provide an insight into several considerations, when the wearables are designed, and discuss expected impacts of digital CKD healthcare on society, clinics and economics.
Selection of target biofluids
There are several candidate biofluids for CKD monitoring: blood, ISF, tears, urine, saliva and sweat, but most of them are not suitable for the wearable platform that allows continuous monitoring of biomarkers. For example, blood cannot be monitored continuously and noninvasively using a wearable format. Tears can be also used as a target biofluid, but the contact lens-type sensor may cause eye irritation. This stimulates tear glands and leads to reflex tears, which may change the analyte concentration and thus make false readings11. Urine and saliva are more suitable for point-of-care (POC) testing, in which collected samples are applied to disposable sensors, rather than using a wearable format. ISF and sweat are known to be a convenient platform for continuous monitoring of biomarkers12,13, but also have their own challenges, for instance, difficulty in access to ISF and in continuous sweat collection. Despite of these challenges, ISF and sweat seem to be the optimal choices as they can be accessible with wearable patch at convenient locations on the body through either microneedles or chemical stimulation in a continuous way.
Selection of biomarkers
According to the KDIGO CKD Guideline 2024, CKD is defined as abnormalities of kidney structure or function lasting for a minimum of 3 months. eGFR (based on the level of creatinine), BUN and UACR are normally used as biomarkers for CKD diagnosis in clinic. The normal level of GFR is ~130 mL/min/1.73 m2 for men and 120 mL/min/1.73 m2 for women, with a considerable variation among individuals according to age, sex and body size. Although the classic method for GFR measurements is the urinary clearance of inulin and remains the gold standard, this technique is cumbersome in practice, and therefore the most conventional method is to estimate GFR based on the level of creatinine measured in blood6. The normal range of BUN is known to vary 2.1–7.1 mM by age and gender14, and UACR is less than 30 mg/g15. In clinic, CKD is generally diagnosed when an eGFR is lower than 60 mL/min/1.73 m2, or markers of kidney damage are observed (e.g., UACR ≥ 30 mg/g, persistent haematuria, structural abnormalities detected by histology or imaging.
The CKD biomarkers such as creatinine and urea are also present in ISF and sweat. For example, creatinine is known to have a concentration of 45–110 μM in ISF (that is a similar level to that of serum), and a lower value of 9.4–18 μM in sweat16. More abundant urea can be found in biofluids: 3.9–4.9 mM in ISF17 and around 22.2 mM in sweat18. As the concentration ranges are normally detectable using the electrochemical sensing technology, creatinine and urea can be selected as biomarkers in both ISF and sweat for wearable CKD monitoring. It is worth mentioning that while creatinine levels highly rely on the muscle mass of the individual, urea levels can be affected by several factors like dietary intake, ageing as well as liver function, which may lead to false reading and thus dangerous misdiagnosis. Simultaneous monitoring of various biomarkers like creatinine, urea and their ratio can help with reliable clinical decision-making for CKD. In addition, cystatin C has been identified as an endogenous biomarker for eGFR, given that its concentration is not dependent on other factors including muscle mass. As for the concentration in biofluids, the level of serum cystatin C ranges around 60–90 nM19, and around 5 times more cystatin C can be found in ISF20. The sweat concentration has not been reported yet, but the sweat level of cystatin C with a high molecular weight (13 kg/mol) is anticipated to be significantly lower than that in serum and ISF. This is because the filtration of extracellular matrix tight junctions hinders such a large molecule from passing through the skin21. For the development of wearable cystatin C sensors, therefore, ISF will be the optimal choice as the target biofluid considering its relative abundance. Given that the biomarker concentrations in blood tend to decrease in sweat and ISF, it is required to confirm that the analyte levels in the biofluids for wearables correlate well with blood levels, then extract the conversion factors for each biomarker. This will provide an opportunity to push wearable sensors for CKD monitoring into the clinical market.
State-of-the-art sensing technology
As creatinine biosensing has been most extensively investigated for CKD monitoring, here we provide an overview of current state-of-the-art sensing technology with a focus on creatinine sensors. Creatinine has a low molecular weight (113.12 g/mol) and moderate water solubility. This molecular property is similar to other abundant biomolecules in the body, which makes it challenging to detect, and thus no mature technology exists to detect such biomolecules. Recent studies on creatinine sensors can be divided into two detection methods: indirect and direct detection of creatinine. There are two types of techniques for indirect detection of creatinine in terms of the readout format: optical and electrochemical methods. In the former case, the current gold standard technique is the Jaffe method that involves a colorimetric readout of the samples when picric acid interacts with creatinine to form an orange-red compound (Janovsky complex) readily detected by UV-vis spectrometers22. A recent report shows that the Jaffe-based optical detection can be performed by a palm-sized immunosensing device with creatinine antibodies immobilised23. To be specific, the immobilised antibody captures creatinine, and the addition of picric acid gives a colour change to the device, which is then analysed by image RGB processing. This hand-held and miniaturised device opens the possibility of PoC-type diagnosis for renal disease. However, the Jaffe reaction is affected by various factors like temperature and pH16, and the use of picric acid gives toxicity and safety issues, which is holding back its use as a wearable diagnostic device. For this reason, electrochemical methods have been attracting more attention to build the wearable platform for CKD monitoring. The most common electrochemical method is based on enzymatic reactions, and there are two types of enzymatic sensors for creatinine detection: a single enzyme or multiple enzyme system24. In the single enzyme system, creatinine deiminase is commonly used to catalyse hydrolysis of creatinine, which generates ammonia that can be detected by either a pH or ammonium-selective sensor25. This type of sensor provides the level of creatinine in an indirect way by measuring the concentration of such byproduct molecules like NH4+ ions. Although using a single enzyme is relatively simple, this approach can suffer from interference from endogenous ammonia in biofluids. This issue can be overcome by using the three-enzyme method: creatininase, creatinase and sarcosine oxidase (SOx)26. For example, creatininase catalyses the hydrolysis of creatinine to creatine, followed by subsequent hydrolysis to urea and sarcosine by creatinase. Subsequently, sarcosine gives an electron to a redox centre of SOx, and O2 takes out the electron from the redox centre, then is converted into H2O2. Finally, a highly catalytic electrode like platinum makes electrochemical oxidation of H2O2 (i.e., giving electrons to the electrode), which leads to changes in sensing signals. Although this system can avoid interference from ammonia, this approach is susceptible to interference from endogenous creatine at the second step of the catalytic cycle. To resolve this problem, it is recommended to use a dual-sensor approach, in which separate sensors are used to measure both creatinine and creatine and then subtract the measured signal of creatine from that of creatinine27. As an alternative to avoid such interfering effects, a recent study proposed another novel sensor configuration consisting of the following layers (in this order): an ammonium-selective electrode (as the sensing part), a single enzyme layer (i.e., creatinine deiminase as a bioreceptor) and an anion-exchange membrane (as a physical barrier against cationic interferences)28. In this sensor configuration, creatine is not produced by the enzymatic reaction due to the absence of creatininase, and the anion-exchange membrane can effectively block the endogenous NH4+ ions, which can minimise the effect of such interfering species. In the case of indirect detection of creatinine, this sensor configuration seems to be most promising given the relatively simple design and effective blocking of the interfering species. Furthermore, the same design can be used to create urea sensors by changing creatinine deiminase to urease (that makes hydrolysis of urea to NH4+ ions).
Creatinine molecules can be detected directly without such chemical transformation. This sensing method is based on affinity detection by antibodies29 or molecularly imprinted polymers (MIPs)30,31,32. In particular, MIPs have been attracting a great deal of attention due to their high selectivity to target molecules and freedom of molecular design of binding sites. The MIPs can be prepared by copolymerising a functional monomer and crosslinker in the presence of the target molecules (in this case, creatinine) as molecular templates, followed by removal of target molecules from the polymer product, leaving behind binding sites with affinity for the target molecule (Fig. 2a)33. These binding sites are known to have a binding affinity similar to that of antibody-antigen systems. Furthermore, MIPs have a definite advantage over antibodies, such as better stability and facile integration with an electronic device, which makes them ideal bioreceptors for wearable sensors. For these reasons, the molecularly imprinting technique has become the forefront of the development of creatinine sensors34,35. Another key technology for direct detection of creatinine is to use Cu-creatinine interactions36. For example, Liu et al.37 reported a disposable screen-printed flexible electrode array which allows simultaneous detection of creatinine and albumin based on the Cu-creatinine interaction and albumin antibodies, respectively (Fig. 2b). A combination of the two techniques (i.e., MIP and Cu-creatinine interaction) has also been performed by using an MIP coated with CuO nanoparticles (NPs)38, providing a detection limit of 0.083 μM that is 100 times lower than the expected concentration range (9.4–18 μM) in sweat, which makes this sensor architecture a promising candidate for wearable creatinine sensors. Furthermore, this sensing structure can also be used as a gate electrode in organic electrochemical transistors (OECTs), in which a faradaic current induced by the electrochemical reaction between CuO and creatinine at the gate electrode is amplified and measured in the form of the channel current (Fig. 2c). Given the fact that OECTs provide considerable signal amplification33, the limit of detection and sensitivity are expected to improve further.
a A fabrication process of a molecularly imprinted polymer. Reproduced with permission from ref. 33, copyright (AAAS, 2018). b A flexible and disposable electrode array that allows simultaneous detection of creatinine and albumin. Reproduced with permission from ref. 37, copyright (Elsevier, 2022). c Illustration of our proposed sensor architecture for creatinine detection. The selective layer consists of an MIP and conductive polymer with CuO nanoparticles, and this layer is coated on a gate electrode of an OECT.
In addition, urea can be detected by an indirect or direct way. The former is to use an enzyme (urease) as a bioreceptor. Urease catalyses the hydrolysis of urea generating the NH4+ byproduct. The ammonium ion can be detected by an ion-selective membrane (urease-coupled NH4+ ion-selective electrodes)39 or doping/de-doping of conductive polymers such as PANI:PSS40. The latter is to employ an MIP layer. For example, a recent paper reported reduced graphene oxide(rGO)/polydopamine-based MIPs41. Here, rGO allows an increase in both electrical conductivity and surface area of the polymer, which facilitates not only the efficient electron transfer but also high sensitivity for target biomolecules. As for detecting cystatin C, due to its high molecular weight of 13 kg/mol, it is typically detected by immunoassay techniques42 or electrochemical methods such as immunosensors using antibodies as bioreceptors43,44.
Several CKD sensor technologies reported within the last five years are listed in Table 1 to provide a comprehensive comparison of the sensing performance. Considering the fact that the creatinine level of patients with renal diseases increases to >200 μM or even >700 μM from normal levels (9.4−18 μM in sweat, 45–110 μM in ISF)45, the two techniques (Cu2O NPs+PAA gel46 and rGO/PDA-MIP41) exhibiting a wide detection range seem to be more practical in the clinical settings. The other sensing techniques for creatinine monitoring need to be optimised further to detect the creatinine level present in body fluids both in physiological and pathological conditions. As for urea detection, the rGO/PDA-MIP technique also shows the best performance in terms of sensitivity, detection range, and limit of detection (LOD)41. For cystatin C sensors, an interdigitated electrode coated with PPy/CNT shows the best sensing performance as the structure allows large charge storage capacity and high effective surface area44.
Considerations for developing wearable sensors
There have been two remarkable papers on wearable sensors for detection of CKD biomarkers. One of which is a colorimetric wearable patch, integrated with a microfluidic system, that allows simultaneous measurements of creatinine and urea along with the sweat rate (Fig. 3a)47. While this work provides a proof-of-concept that CKD biomarkers can be monitored noninvasively through sweat, this wearable patch does not provide continuous monitoring since certain activities are required to collect sweat. Furthermore, considering the fact that the colorimetric sensor requires subsequent analysis by image RGB processing to provide quantitative readout for measured concentrations, the electrochemical sensor seems to be a better choice as it provides quantitative values of biomarker concentrations directly. The other one is an ISF-based cystatin C sensor for POC testing, in which the ISF is collected by a microneedle patch, and then CKD diagnosis is performed by a cystatin C immunoassay (Fig. 3b)48. While this prototype allows for POC-type detection of CKD, the cystatin C sensor was not designed in a wearable format and so does not provide continuous detection of cystatin C in ISF. Continuous monitoring of cystatin C could be achievable by coating certain bioreceptors (that selectively react with cystatin C) onto microneedles and then inserting them into the dermis for continuous access to ISF (Fig. 3c, d) as in the continuous glucose monitoring (CGM) system like the Freestyle Libre sensor. As an alternative, the ISF can be extracted to the skin surface using a process called reverse iontophoresis. A topical current is applied between the two electrodes, which electro-osmotically extracts ISF with biomolecules to the skin surface (Fig. 3e). However, repeated electrical stimulation may cause skin irritation and, in the extreme case, skin burning, and thus it is necessary to make efforts to lower current density required for the electrical stimulation. Abundant biomarkers in sweat like creatinine and urea could be noninvasively monitored using a wearable patch. Note that the composition of sweat relies on the method of sweat stimulation (e.g., exercise, heat or chemical induction)12. For instance, while sweat produced during physical activities like exercise provides dynamic analyte profiles (suitable for fitness monitoring), sweat secreted at rest allows the detection of equilibrium analyte levels, which may reflect underlying health conditions. For this reason, at-rest sweat will be more suitable for CKD diagnosis. The most common method for generating sweat at rest is local chemical stimulation called iontophoresis. A sweat-inducing drug (e.g., pilocarpine8, carbachol49) entrapped in hydrogels is released under the skin by the current applied between the two electrodes, which stimulates nearby sweat glands to secrete sweat (Fig. 3f). Both extraction methods for sweat and ISF could be integrated with the wearable patch for simultaneous measurements of various CKD biomarkers. To be specific, as shown in Fig. 3g, the levels of creatinine and urea can be measured by sensors in the vicinity of the anode, in which sweat is generated by the sweat-inducing drug. At the same time, cystatin C can be detected by the sensor close by the cathode, where ISF is extracted by reverse iontophoresis. For on-body CKD monitoring through sweat, in particular, general considerations for sweat sensing should be taken: sweat evaporation and collection. Small volumes of secreted sweat evaporate rapidly, thereby changing the biomarker concentration. Furthermore, as new sweat is secreted, it mixes with previously secreted sweat, leading to sensor readings which are a rolling average of analyte concentrations rather than real-time measurements. In order to overcome these issues, sweat sensors need to be integrated with a microfluidic system that controls the flow of sweat (Fig. 3h)9. For example, the microfluidic channels direct old sweat away from the sensors as new sweat is secreted, which enables real-time and continuous measurements of analyte concentration while encasing the sweat to minimise evaporation. In addition, natural sweat collection at rest can be considered as an ideal method that can replace chemical stimulation. However, as resting thermoregulatory sweat has a very low secretion rate, it is generally thought to be difficult to collect sufficient quantities of sweat for analysis during sedentary activities without the chemical stimulation. A recent paper has reported that the use of a microfluidic system equipped with a hydrophilic filler makes it possible to achieve rapid sweat uptake at rest (Fig. 3i)10. This shows great promise for continuous, on-body CKD monitoring through sweat. In addition, the effect of interfering species should be considered. There are various interfering species (e.g., ascorbic acid, uric acid) present in body fluids. For example, ascorbic acid (AA), also known as vitamin C, exists in sweat with a concentration of 10–50 μM50, and the level of AA continuously changes. The AA is an electroactive molecule that is easy to be oxidised (i.e., give electrons) with an applied voltage, which leads to a change in an output signal of the sensor and thus misdiagnosis. This issue can be minimised by the use of a permselective membrane based on charge exclusion51 and size exclusion52. Other challenges to wearable sensors such as sensor integration, user acceptance and power consumption are well described on the recent Perspective paper53.
a Colorimetric wearable patch that allows the detection of CKD biomarkers (creatinine and urea). Reproduced with permission from ref. 47, copyright (Royal Society of Chemistry, 2019). b An ISF-based cystatin C sensor for POC tests. Reproduced with permission from ref. 48, copyright (Elsevier, 2022). c Schematic illustration of a microneedle-type sensor that can detect biomarkers through an ISF. d An example of the microneedle-based ISF sensors. Reproduced under the terms of CC-BY 4.0 from ref. 59, copyright (Elsevier, 2017). e An ISF extraction method using reverse iontophoresis. Applied current enables analytes to be secreted electro-osmotically to the skin surface along with ISF. f A sweat induction method based on iontophoresis. A topical current is applied between hydrogels containing a sweat-stimulating drug. This allows the drug to be injected beneath the skin surface, which makes glands secrete sweat. g An example of a wearable patch capable of extracting both the ISF and sweat. h All-laser-engraved microfluidic channels allow accurate real-time sweat analysis. Reproduced with permission from ref. 9, copyright (Springer Nature, 2020). i Inclusion of a hydrophilic filler in the microfluidic channel enables a natural sweat collection without exercise, thermal or chemically induced methods. Reproduced under the terms of CC-BY 4.0 from ref. 10, copyright (Springer Nature, 2021). j The use of OECTs as a transducer allows significant signal amplification, facilitating a highly sensitive biosensor. Reproduced with permission from ref. 33, copyright (AAAS, 2018).
There are special considerations for the detection of CKD-related biomarkers. Cystatin C is known to be an ideal CKD biomarker as its concentration does not depend on muscle mass. However, a trace amount (60–90 nM) of cystatin C exists in serum19, and even it is expected to be a much lower concentration (a few nM) in sweat. This means that, if we want to measure such a biomarker through sweat, the sensor is required to be highly sensitive. Otherwise, the sensors would fail in providing reliable decision-making on the diagnosis of CKD. This issue can be solved by using a transistor as an amplifying transducer. Among various transistor architectures, an OECT is well-known to be the best option in terms of sensitivity due to its superior signal amplification property54. Given that an OECT-based cortisol sensor is reported to exhibit a limit-of-detection of a few nM (Fig. 3j)33, it is expected that a trace amount of cystatin C in ISF/sweat could be measurable using this technology. There would be two more issues hindering accurate diagnosis of CKD: sweat rate and pH effects. The concentrations of some analytes depend on the secretion rate of sweat. For example, the levels of creatinine and urea are reported to decrease with an increase in sweat rate55. Since changes in analyte concentrations can originate from changes in sweat rate, the sweat rate is required to be measured simultaneously, which can provide accurate interpretation of the concentration changes. Lastly, most creatinine/urea sensors have been developed using enzymes, as bioreceptors, whose activity changes with biofluid pH12. Therefore, a change in measured concentrations may result from a change in pH rather than actual variations in concentration. To resolve both issues, in addition to sensors for the CKD biomarkers, sweat rate and pH sensors also need to be integrated into the wearable platform.
Outlook
The advance in wearable bioelectronics for kidney health monitoring will give rise to substantial impacts on the medical and industrial sectors. For instance, this breakthrough holds significant potential for personalised, predictive, and ultimately preventive healthcare for individuals suffered from chronic renal disorders or those susceptible to kidney failure. Furthermore, this wearable platform could minimise the need for invasive procedures, such as biopsies, and improve treatment outcomes by timely interventions. This would not only improve the overall well-being of patients but also reduce the need for acute treatments and hospitalisations, which can mitigate the burden on the healthcare system. The wearable technology for CKD monitoring is also expected to have significant market potential given the increasing prevalence of CKD worldwide and a high demand for its personalised treatment. Moreover, given that the deployment of this wearable technology can be expanded to other diseases like liver dysfunction by simply changing target biomarkers, the wearable medical device market is anticipated to grow further.
To transfer the wearable technology to a business, it is crucial to cheaply manufacture wearable sensors. They can be fabricated inexpensively with scalable additive manufacturing techniques56,57 (e.g., inkjet/screen printing or roll-to-roll printing) and the laser-engraving technology9. In addition, as sweat and ISF are rarely used in clinical settings, there is no readily available database to refer to when analysing patient data from wearable sensors. Therefore, gathering such database must come before the clinical use of wearable sensors. Furthermore, to achieve preventive healthcare for CKD, it is necessary for interdisciplinary collaboration among medical doctors, healthcare providers and experts on bioelectronics and big data/machine learning. Lastly, while developing the platform, issues regarding ethics and data privacy must be considered, which will help establishing the legal environment of digital healthcare.
References
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 395, 709–733 (2020).
McCullough, K. et al. Measuring the population burden of chronic kidney disease: a systematic literature review of the estimated prevalence of impaired kidney function. Nephrol. Dial. Transplant. 27, 1812–1821 (2012).
Coresh, J. Update on the burden of CKD. J. Am. Soc. Nephrol. 28, 1020–1022 (2017).
Chen, T. K., Knicely, D. H. & Grams, M. E. Chronic kidney disease diagnosis and management. JAMA 322, 1294–1304 (2019).
Xie, Y. et al. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 94, 567–581 (2018).
Feehally, J., Floege, J., Tonelli, M. & Johnson, R. J. Comprehensive clinical nephrology (Elsevier, 2018).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Sempionatto, J. R. et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 5, 737–748 (2021).
Yang, Y. et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 38, 217–224 (2020).
Nyein, H. Y. Y. et al. A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat. Commun. 12, 1823 (2021).
Bandodkar, A. J. & Wang, J. Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol. 32, 363–371 (2014).
Bariya, M., Nyein, H. Y. Y. & Javey, A. Wearable sweat sensors. Nat. Electron. 1, 160–171 (2018).
Goud, K. Y. et al. Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: toward Parkinson management. ACS Sens. 4, 2196–2204 (2019).
Walker, H. K., Hall, W. D. & Hurst, J. W. Clinical methods: the history, physical, and laboratory examinations (Butterworths, 1990).
Ren, F., Li, M., Xu, H., Qin, X. & Teng, Y. Urine albumin‐to‐creatinine ratio within the normal range and risk of hypertension in the general population: a meta‐analysis. J. Clin. Hypertens. 23, 1284–1290 (2021).
Cánovas, R., Cuartero, M. & Crespo, G. A. Modern creatinine (Bio)sensing: challenges of point-of-care platforms. Biosens. Bioelectron. 130, 110–124 (2019).
Ebah, L., Brenchley, P., Coupes, B. & Mitra, S. A modified in vivo flow variation technique of microdialysis for sampling uremic toxins in the subcutaneous interstitial compartment. Blood Purif. 32, 96–103 (2011).
Huang, C.-T., Chen, M.-L., Huang, L.-L. & Mao, I.-F. Uric acid and urea in human sweat. Chin. J. Physiol. 45, 109–115 (2002).
Mussap, M. & Plebani, M. Biochemistry and clinical role of human cystatin C. Crit. Rev. Clin. Lab. Sci. 41, 467–550 (2004).
Tran, B. Q. et al. Proteomic characterization of dermal interstitial fluid extracted using a novel microneedle-assisted technique. J. Proteome Res. 17, 479–485 (2018).
Xu, J., Fang, Y. & Chen, J. Wearable biosensors for non-invasive sweat diagnostics. Biosensors 11, 245 (2021).
Butler, A. R. The Jaffé reaction. Part II. A kinetic study of the Janovsky complexes formed from creatinine (2-imino-1-methylimazolidin-4-one) and acetone. J. Chem. Soc. Perkin Trans. 2, 853–857 (1975).
Divya, Mahapatra, S. & Chandra, P. Design and engineering of a palm-sized optical immunosensing device for the detection of a kidney dysfunction biomarker. Biosensors 12, 1118 (2022).
Lad, U., Khokhar, S. & Kale, G. M. Electrochemical creatinine biosensors. Anal. Chem. 80, 7910–7917 (2008).
Thompson, H. & Rechnitz, G. A. Ion electrode based enzymic analysis of creatinine. Anal. Chem. 46, 246–249 (1974).
Tsuchida, T. & Yoda, K. Multi-enzyme membrane electrodes for determination of creatinine and creatine in serum. Clin. Chem. 29, 51–55 (1983).
Berberich, J. A., Chan, A., Boden, M. & Russell, A. J. A stable three-enzyme creatinine biosensor. 3. Immobilization of creatinine amidohydrolase and sensor development. Acta Biomater. 1, 193–199 (2005).
Liu, Y., Cánovas, R., Crespo, G. A. & Cuartero, M. Thin-layer potentiometry for creatinine detection in undiluted human urine using ion-exchange membranes as barriers for charged interferences. Anal. Chem. 92, 3315–3323 (2020).
Wei, F. et al. Serum creatinine detection by a conducting-polymer-based electrochemical sensor to identify allograft dysfunction. Anal. Chem. 84, 7933–7937 (2012).
Pan, J., Chen, W., Ma, Y. & Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 47, 5574–5587 (2018).
Gonzalez-Gallardo, C. L., Arjona, N., Álvarez-Contreras, L. & Guerra-Balcázar, M. Electrochemical creatinine detection for advanced point-of-care sensing devices: a review. RSC Adv. 12, 30785–30802 (2022).
Song, Z. et al. Molecularly imprinted polymers based materials and their applications in chromatographic and electrophoretic separations. TrAC Trends Anal. Chem. 146, 116504 (2022).
Parlak, O., Keene, S. T., Marais, A., Curto, V. F. & Salleo, A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci. Adv. 4, eaar2904 (2018).
Prabhu, S. N., Mukhopadhyay, S. C., Gooneratne, C. P., Davidson, A. S. & Liu, G. Molecularly imprinted polymer-based detection of creatinine towards smart sensing. Med. Devices Sens. 3, e10133 (2020).
Cho, M. G., Hyeong, S., Park, K. K. & Chough, S. H. In situ preparation of fine particles and characterization of molecularly imprinted polymer for creatinine prepared from polymer anion and Al3+. Polymer 221, 123587 (2021).
Chen, C. H. & Lin, M. S. A novel structural specific creatinine sensing scheme for the determination of the urine creatinine. Biosens. Bioelectron. 31, 90–94 (2012).
Jia, Y. et al. Battery-free and wireless tag for in situ sensing of urinary albumin/creatinine ratio (ACR) for the assessment of albuminuria. Sens. Actuators B Chem. 367, 132050 (2022).
Nontawong, N. et al. Novel amperometric flow-injection analysis of creatinine using a molecularly-imprinted polymer coated copper oxide nanoparticle-modified carbon-paste-electrode. J. Pharm. Biomed. Anal. 175, 112770 (2019).
Eggenstein, C. et al. A disposable biosensor for urea determination in blood based on an ammonium-sensitive transducer. Biosens. Bioelectron. 14, 33–41 (1999).
Uzunçar, S., Meng, L., Turner, A. P. F. & Mak, W. C. Processable and nanofibrous polyaniline:polystyrene-sulphonate (nano-PANI:PSS) for the fabrication of catalyst-free ammonium sensors and enzyme-coupled urea biosensors. Biosens. Bioelectron. 171, 112725 (2021).
Li, Y. et al. A point-of-care sensing platform for multiplexed detection of chronic kidney disease biomarkers using molecularly imprinted polymers. Adv. Funct. Mater. 34, 2316865 (2024). This work demonstrates highly sensitive creatinine/urea sensors with a limit-of-detection of the femtomolar level, facilitating a point-of-care sensing platform for simultaneous detection of the CKD biomarkers.
Finney, H., Newman, D. J., Gruber, W., Merle, P. & Price, C. P. Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the behring nephelometer systems (BNA, BN II). Clin. Chem. 43, 1016–1022 (1997).
Devi, K. S. S. & Krishnan, U. M. Microfluidic electrochemical immunosensor for the determination of cystatin C in human serum. Microchim. Acta 187, 1–12 (2020).
Ferreira, P. A. B. et al. An ultrasensitive Cystatin C renal failure immunosensor based on a PPy/CNT electrochemical capacitor grafted on interdigitated electrode. Colloids Surf. B Biointerfaces 189, 110834 (2020).
Dong, Y. et al. A disposable printed amperometric biosensor for clinical evaluation of creatinine in renal function detection. Talanta 248, 123592 (2022).
Kalasin, S., Sangnuang, P., Khownarumit, P., Tang, I. M. & Surareungchai, W. Salivary creatinine detection using a Cu(I)/Cu(II) catalyst layer of a supercapacitive hybrid sensor: a wireless IoT device to monitor kidney diseases for remote medical mobility. ACS Biomater. Sci. Eng. 6, 5895–5910 (2020).
Zhang, Y. et al. Passive sweat collection and colorimetric analysis of biomarkers relevant to kidney disorders using a soft microfluidic system. Lab Chip 19, 1545–1555 (2019). This work demonstrates colorimetric wearable sensors equipped with microfluidics for simultaneous detection of creatinine and urea through sweat, which shows the potential for the development of wearable CKD sensing platforms.
Chen, Y.-J. et al. Microneedle patches integrated with lateral flow cassettes for blood-free chronic kidney disease point-of-care testing during a pandemic. Biosens. Bioelectron. 208, 114234 (2022). This work shows microneedle patches that can detect cystatin C in interstitial fluids, which opens up new avenues for rapid, blood-free kidney test kits.
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022).
Wang, Z. et al. Engineering materials for electrochemical sweat sensing. Adv. Funct. Mater. 31, 2008130 (2021).
Liao, C., Zhang, M., Niu, L., Zheng, Z. & Yan, F. Highly selective and sensitive glucose sensors based on organic electrochemical transistors with graphene modified gate electrodes. J. Mater. Chem. B 1, 3820–3829 (2013).
Soldatkina, O. V. et al. Improvement of amperometric transducer selectivity using nanosized phenylenediamine films. Nanoscale Res. Lett. 12, 594 (2017).
Gutruf, P. Towards a digitally connected body for holistic and continuous health insight. Commun. Mater. 5, 2 (2024).
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 17086 (2018).
Emrich, H. M. et al. Sweat composition in relation to rate of sweating in patients with cystic fibrosis of the pancreas. Pedia. Res. 2, 464–478 (1968).
Strand, E. J. et al. Printed organic electrochemical transistors for detecting nutrients in whole plant sap. Adv. Mater. Technol. 8, 2100853 (2022).
Lee, Y. et al. Tunable organic active neural probe enabling near‐sensor signal processing. Adv. Mater. 35, 2301782 (2023).
Kumar, R. K. R., Shaikh, M. O., Kumar, A., Liu, C.-H. & Chuang, C.-H. Zwitterion-functionalized cuprous oxide nanoparticles for highly specific and enzymeless electrochemical creatinine biosensing in human serum. ACS Appl. Nano Mater. 6, 2083–2094 (2023).
Sharma, S., Saeed, A., Johnson, C., Gadegaard, N. & Cass, A. E. Rapid, low cost prototyping of transdermal devices for personal healthcare monitoring. Sens. Bio-sens. Res. 13, 104–108 (2017).
Acknowledgements
S.H. acknowledges an Incheon National University Research Grant in 2024 (2024-0029), and this work was also supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) (no. NRF-2022R1C1C1002780).
Author information
Authors and Affiliations
Contributions
S.H. and J.-S.K. conceived the idea. C.-Y.J. provided a clinical view and contributed to the ‘Introduction’ section. S.H. wrote the ‘Selection of target biofluids/biomarkers’ and ‘Considerations for developing wearable sensors’ sections and produced Fig. 3. S.Y. contributed to the ‘State-of-the-art sensing technology’ section and produced Fig. 2. J.-S.K. contributed to the ‘Outlook’ section. D.Y.J. and T.L. produced Fig. 1 and Table 1, respectively. S.H. edited the manuscript and all authors revised the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications materials thank the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Rona Chandrawati and John Plummer. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, S., Yamamoto, S., Jung, CY. et al. Wearable sensors for monitoring chronic kidney disease. Commun Mater 5, 153 (2024). https://doi.org/10.1038/s43246-024-00606-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43246-024-00606-0
This article is cited by
-
Non-invasive and user-friendly visual detection of salivary phosphate using enzymatic biosensor test strip for chronic kidney disease screening
Analytical and Bioanalytical Chemistry (2026)
-
A comprehensive review on the integration of microneedle technologies with biosensing platforms for advancements in fabrication, biomarker detection, and therapeutic monitoring in precision medicine
Discover Pharmaceutical Sciences (2025)