Abstract
Snow algal blooms lead to glacier and snow surface albedo reduction and subsequent melting. To explore snow algal distribution patterns and potential to cause blooms, we investigated snow algae in the Tibetan, Antarctic, Arctic, and Alps. Our results demonstrated that Tibetan and Alps had higher algal richness than Antarctic snow. Only 19 ASVs were shared among regions, with their relative abundance being lower in the Tibetan compared to the Arctic and Alps but similar in Antarctica. Furthermore, the algal community varied substantially across sampling sites, with 34% of the identified taxa endemic to Tibetan glacier snow. Cosmopolitan bloom-causing algae were scarce in most Tibetan Plateau snow, which was potentially limited by a low snow depth that prevented their growth. Alternatively, Hydrurus sp., which may cause golden-brown snow, was abundant (up to 54.4%) on Tibetan. Therefore, cosmopolitan and endemic species jointly enhance the potential of producing algal blooms in Tibetan glaciers.
Similar content being viewed by others
Introduction
Snow algae, a group of unicellular eukaryotes that thrive in snow on glaciers and ice sheets, on seasonal snow, and on permanent snowfields1,2, are primary producers in snow ecosystems and sustain heterotrophic organisms2,3. Snow algae exhibit remarkable adaptations to extreme environmental conditions, characterized by low temperatures, nutrient deficiencies, and intense UV radiation2,4. To withstand such challenges, snow algae have evolved to synthesize pigments including astaxanthin and other carotenoids2. Under favorable conditions, algae can bloom extensively on snow surfaces, thereby forming macroscopically visible colored snow patches, altering snow color from white to red, green, or other colors. Such snow algal blooms have been observed in Antarctica4, the Arctic5, the European Alps6, as well as in Japan7, Russia8, North America9, and Australia10. The presence of colored snow reduces snow surface albedo5,11, further accelerating snow melt rates and reducing cryosphere mass balance together with dust deposition6,12. Specifically, high dust deposition can accelerate glacier melting that inhibits algal growth, while lower dust deposition provides essential nutrients that promote algal growth. Snow algae in polar regions comprise both cosmopolitan taxa and endemic species13, yet the knowledge of snow algae on the Tibetan Plateau remains limited. Understanding the distribution patterns and environmental drivers of snow algae on a global scale is crucial for predicting their spatial impact on the cryosphere under climate change.
A diverse range of snow algal species can cause algal blooms but globally dominant are Chlorophyceae, including Sanguina, Chloromonas, Chlamydomonas, and Chlainomonas11,14. These cosmopolitan taxa are the major causes of snow algal blooms across Antarctica, the Arctic, and Alpine mountains11,13. Nevertheless, other lineages, including Hydrurus15, Prasiolales16, Rosetta17, and Stramenopiles18 (although not considered “true” snow algae), have also been reported to cause algal blooms in Antarctica, Columbia, and the Arctic.
Several factors have been predicted to influence the occurrence of snow algal blooms, including the liquid water availability (snow melting period duration and algal growth interruption by new snow cover), solar radiation, and nutrients (phosphorous and nitrogen)19,20. Soto et al. investigated the algal community dynamics during a 35-day period in an Antarctic snowfield, finding that the community assembly was predominantly driven by stochastic processes21, rather than being controlled by nutrient availability. Spatially, a comparative analysis of Arctic and Antarctic snow algae communities revealed the dominance of endemic taxa, suggesting dispersal limitations13. Similarly, Remias et al. revealed the distinct algal community between the snow/ice in the Arctic and Alps18. Thus, while a few algal taxa are distributed globally, endemic species present greater diversity.
The Tibetan Plateau is the highest plateau in the world. It has the third largest number of glaciers after the Antarctic and Arctic, with diverse landscapes and complex bedrock types22. These unique geographical features influence environmental factors such as snow cover conditions and snowmelt duration, which are important factors influencing the occurrence of algal blooms. Due to global warming, the snow-covered area on the Tibetan Plateau tended to decrease at elevations above 2000 m23. For the high elevation area, snow melting occurs between April and August, with a decreasing trend in the duration of snow melting24. This decreasing trend may influence the development of algal blooms.
Previous studies have reported the occurrence of red snow on the south slope of the Himalayas and Tianshan, which is located at the north edge of the Tibetan Plateau. Onuma et al. predicted the potential of algal blooms in glacier surface snow using a snow algae model, finding that the potential of red snow was low for the center region of the Tibetan Plateau19. However, this simulation was based on a dataset of red algal blooms with the dominant algal species being the cosmopolitan genera, including Chloromonas, Sanguina, and Chlainomonas. A lack of knowledge on the distribution of algae in the central Tibetan glacier could impair modeling accuracy. Thus, knowledge of the algal community in the glacier snow of the central Tibetan Plateau could further enhance our understanding of the snow algal biogeography and the potential for algal blooms in the region.
To address the above knowledge gap, we explored the diversity, taxonomic composition, and distribution patterns of snow algae in glaciers in the central Tibetan Plateau; identified endemic and cosmopolitan taxa by comparing them with those reported in the snowfields and/or glacier snow of the Antarctic, Arctic, and Alpine regions; and also identified the potential climate and environmental drivers of cosmopolitan taxa. We hypothesize that the algal community in the surface snow of Tibetan glaciers would also be dominated by endemic taxa. Furthermore, the climate and chemical parameters of Tibetan glaciers would be unfavorable for the occurrence of snow algal blooms. Our results showed that Tibetan and Alpine snow exhibit higher algal richness than Antarctic snow, and the relative abundance of shared ASVs was lower in Tibetan and Antarctic samples. The Tibetan Plateau has the highest number of endemic species. Although cosmopolitan bloom-causing algae were scarce on most Tibetan Plateau glaciers, Hydrurus sp. (which causes golden-brown snow) was abundant there.
Results
Regional algal diversity
Amplicon sequencing targeting the 18S rRNA gene was used to investigate the diversity, taxonomy, and community structure of microeukaryotic algae from snow samples collected in various locations (Fig. 1). The richness of algal amplicon sequencing variants (ASVs) showed a significant disparity, with a notably higher richness in the Tibetan Plateau and Alpine snow in contrast to those from the Antarctic snow (Fig. 2a; Supplementary Table 1; Wilcox test, p < 0.001). Additionally, the evenness was significantly higher in the Tibetan Plateau samples than in the Antarctic snow (Fig. 2b; Supplementary Table 1; Wilcox test, p < 0.001).
Taxonomic composition and community structure of algal communities
We then investigated the taxonomic composition and community structure variations in the algal communities. The algal communities were dominated by Chlorophyta and Ochrophyta (Fig. 3a, b). Unlike the other regions, the Tibetan Plateau snow samples had a low relative abundance of Chlorophyta but a high relative abundance of Ochrophyta. At the class level (Supplementary Fig. 2), the relative abundance of Chlorophyceae in Tibetan Plateau snow was significantly lower than in samples from the Alps and Antarctica (Kruskal-Wallis test, p = 0.00137 and 0.00967, respectively). In contrast, the relative abundance of Chrysophyceae was significantly higher in the Tibetan Plateau samples than in the Alps (Kruskal-Wallis test, the Tibetan Plateau, with p = 0.01205). At the ASV level, community structures varied significantly (PERMANOVA, p < 0.01) on a regional level (Fig. 3c).
Taxonomic composition of snow algae communities (a) and principal component analysis of algae (b) community at the phylum level. Abundance-weighted community structure variations using nonmetric multidimensional scaling (NMDS) ordination plots for all samples analyzed (c). ANT Antarctic, ARC Arctic, TP Tibetan Plateau.
Comparison of endemic and cosmopolitan algal species between regions
We compared the abundance of shared and unique ASVs among different regions, which represent cosmopolitan and endemic species, respectively. We identified a total of 633 algal ASVs across all samples, with only 19 ASVs being shared across all four regions (Fig. 4a). Remarkably, these 19 ASVs were the dominant algae in Arctic and Alpine samples, with their relative abundance accounting for 91% and 73% of their algal communities, respectively. However, the relative abundance of these 19 ASVs was significantly lower in the Antarctic and Tibetan samples, at approximately 30% and 10%, respectively (Fig. 4b). Furthermore, the Tibetan and Alpine samples comprised a substantial number of endemic ASVs, accounting for 34% and 25% of their identified ASVs, respectively, whereas endemic ASVs accounted for only ~3% and ~7% of the identified ASVs in the Arctic and Antarctic samples, respectively (Fig. 4a).
Shared ASVs between Alps, ANT, ARC, and TP (a). The relative abundance and number of shared algal ASVs (b) across the four regions. Cosmopolitan algae dominated the communities in Arctic and Alpine samples, while their contribution to Antarctic and Tibetan samples was significantly lower. ANT Antarctic, ARC Arctic, TP Tibetan Plateau.
Regional dominant snow algae
The top 20 most abundant ASVs were identified by the averaged relative abundance across all samples. Their distribution patterns were examined to assess the presence of cosmopolitan bloom-causing algae in Tibetan glaciers. Our analysis revealed three distinct clusters of communities based on their ASV compositions (Fig. 5a). Cluster I comprised most Antarctic snow samples, along with four Arctic samples and four Alpine samples; Cluster II mainly consisted of Tibetan samples, also with one Antarctic sample included; and Cluster III comprised mostly Alpine samples and one Tibetan sample.
We taxonomically annotated the dominant algae ASVs by BLAST against the NCBI database and found that the identified three clusters were dominated by distinct algae lineages (Fig. 5; supplementary Table 2). Cluster I was dominated by cosmopolitan red snow-causing taxa, Sanguina nivaloides (ASV1), Chloromonas sp. (ASV20), and Chlorominima collina (ASV30). Cluster III was also dominated by cosmopolitan algae, including Chloromonas miwae (ASV15), Chloromonas muramotoi (ASV155), Chloromonas kaweckae (ASV66), and Chloromonas sp. (ASV22). In contrast, the algal communities of Cluster II were dominated by ASV103, ASV173, ASV68, ASV237, ASV281, ASV161, and ASV14, all of which were of freshwater or terrestrial origin based on algal base records (https://www.algaebase.org). Nevertheless, cosmopolitan red snow-causing algae were identified in Tibetan glaciers, including ASV1, ASV73, ASV15, ASV155, and ASV74. Ten samples had relative abundances greater than 10%, including ANM (5), QY (1), MGGQ (2), PL (1), and ARJS (1).
Environmental factors that influence the development of snow algae blooms in Tibetan glaciers
We investigated the effect of environmental factors on cosmopolitan bloom-causing algae, specifically on Tibetan glacier snow. This analysis focused on ASV1 (Sanguina nivaloides), ASV15 (Chloromonas miwae), ASV73 (Chlainomonas sp.), ASV74 (Chloromonas sp.), and ASV155 (Chloromonas muramotoi). These ASVs are cosmopolitan taxa, which have been reported to cause red snow. Unfortunately, physicochemical parameters for the Antarctic, Arctic, and Alps were either unavailable or were quantified using different methods, so our analysis was confined to Tibetan samples.
The measured parameters in snow meltwater included nutrients (total phosphorus, total organic carbon, and total nitrogen), chemical factors (pH, conductivity, F−, Cl−, and SO42− ions), and glacier properties (summer and winter snow depth, as well as air temperature). We performed UPGMA cluster analysis of the samples based on Bray-Curtis dissimilarity, revealing three distinct groups (Fig. 6a). Group 1 was all ANM samples, which indicates that ANM exhibits a higher relative abundance of bloom-causing cosmopolitan algae and therefore presents a high potential for bloom development. Comparatively, groups 2 and 3 have very low relative abundance of bloom-causing cosmopolitan algae. The relative abundance of these genera in group 1 (91.6%) was significantly (p = 0.004) higher than in group 3 (0.3%) (Fig. 6a). Multivariant analysis (DistLM analysis) showed that winter and summer snow depth significantly influenced the cosmopolitan bloom-causing algae community (Supplementary Table 3). These two factors explained 23.58% and 23.74% of the variations, respectively (Fig. 6b; Supplementary Table 3). Additionally, the relative abundance of cosmopolitan bloom-causing algae had a significant correlation with the winter snow depth, summer snow depth, winter temperature, and Cl− (Supplementary Table 4).
Cluster analysis of the bloom-causing algae communities (Fig. 5b) on the Tibetan Plateau and the difference in their relative abundance (a). Samples from group 1 are ANM samples, which exhibits a higher relative abundance of bloom-causing cosmopolitan algae. Samples of group 2 and 3 have a very low relative abundance of bloom-causing cosmopolitan algae. DistLM analysis identified the importance of environmental variables on bloom-causing algae (b).
Discussion
Snow algae are a major cause of albedo reduction on the snow and glacier surface, contributing to accelerated melting and glacier retreat13. There are limited reports on the distribution of algae in the snow of Tibetan glaciers in the literature, limiting our understanding of the global snow algae biogeography and the capacity to estimate the potential of algal blooms. Our work revealed the unique distribution patterns of microeukaryotic snow algae on 13 glaciers of the Tibetan Plateau, evidencing the potential for the development of algal blooms in Tibetan glaciers.
Unique snow algal communities in Tibetan glaciers compared with the Antarctic, Arctic, and Alps
The algal community in the snow of Tibetan glaciers and the Alps exhibited higher richness compared with that in the snow of the Antarctic (Fig. 2a). This may be attributed to a more diverse algal source, as the Tibetan Plateau and Alps are typically located at the center of the continent, which is home to various ecosystem types, such as grasslands, forests, and lakes25,26. The cells of psychrotolerant algae from these ecosystems can be passively transported onto snow surfaces by wind, leading to a higher richness20. In addition, the Tibetan glacier snow exhibited the highest algal evenness, significantly higher than the Antarctic snow (Fig. 2b). Antarctic snow exhibited the lowest evenness index (Fig. 2b). Evenness reflects the balance and distribution of different algal species within a community. A high evenness indicates that all species are present in similar proportions, reflecting a lack of strong environmental selection27. Furthermore, the Antarctic snow samples were either green or red and thus visible blooming, which is caused by the rapid proliferation of algae, reducing the community evenness.
Tibetan glacier snow exhibited a higher abundance of Chrysophyceae than Alps snow and a higher abundance of Trebouxiophyceae than Antarctic and Alps snow (Supplementary Fig. 2). This highlights the unique snow algal composition on the Tibetan Plateau. Among the abundant Chlorophyta, Tibetan glaciers and Alpine snow exhibited a high abundance of Chloromonas miwae and Chloromonas muramotoi (Fig. 5), which are typically found in mountainous areas28,29, highlighting the distinct algal community compared with polar regions. Additionally, four samples from the Alps demonstrated microbial features similar to Arctic glaciers (Fig. 3c). This could be attributed to their unique central European location, which makes them susceptible to the dispersal of microorganisms from both Arctic and European regions30,31. Thus, the available sources of algae influence the diversity and composition of algal communities in snow.
Endemic species dominated the algae community in Tibetan glaciers
A few algal ASVs are widespread in the snow of the Antarctic, the Arctic, the Alps, and the Tibetan Plateau (Fig. 4a). This is consistent with the previous findings in the Antarctic and Arctic glaciers, where a few cosmopolitan algae species were observed13. The Tibetan Plateau algae are more similar to the Alps, with 118 ASVs shared by them, and these species are mainly Chlorophyceae, Chrysophyceae, and Trebouxiophyceae. The Tibetan Plateau showed the greatest number of endemic species, which may be attributed to its unique geographic location and habitat heterogeneity. The formation of the Tibetan Plateau created unique geographic barriers, such as the Himalayas32, which facilitated the formation of endemic species. In addition, high altitude and complex topography result in diverse microenvironments that promote species differentiation33. However, analysis based on 18S rRNA gene sequences has extremely limited resolution on speciation. Further studies employing comparative genomic analysis from cultured isolates or single-cell genomic sequencing can further enhance our understanding of the dispersal and speciation of snow algae on a global scale.
We noticed that the relative abundance of the cosmopolitan algae identified here was substantially lower in the Antarctic and Tibetan Plateau than in the Arctic and Alps (Fig. 4b). Possible explanations include barriers for algae dispersal and the influence of environmental conditions in the Antarctic and Tibetan Plateau. A previous study on the bipolar distribution of algae concluded that geographical barriers for algae dispersal exist between the Arctic and Antarctic13, resulting in a significant number of regional endemic algae species. The microbial dispersal into Antarctica is constrained by the airborne transportation34,35. For the Tibetan Plateau, the airborne transport (snowfall) is due to the Indian monsoon and westerlies36, which exhibit distinct seasonal patterns. Specifically, the snowfall in the Indian monsoon-dominated region mainly occurs in the summer months, experiencing rapid melting due to warm temperatures. In contrast, the snowfall in the westerlies-dominated region happens in winter months, and the snow cover is also more stable and persistent. However, red snow has been reported on the south slope of the Himalayas (Nepal) and Tianshan Mountain19, which are under the influence of the Indian monsoon and westerlies, respectively. Thus, it is intriguing that algal blooms are occurring on the surface of glaciers with distinct snowfall patterns but not in the center region.
Cosmopolitan and endemic species jointly enhance the potential for algal blooms development in Tibetan glacier snow
The range of algae that can cause blooms on snow expands rapidly, including both cosmopolitan algae that cause red snow globally and local species that occur sporadically. Cosmopolitan genera like Chloromonas sp. and Sanguina nivaloides have been found in the Arctic, Antarctic, and Alps regions37,38, causing red snow at a global scale. The relative abundance of these cosmopolitan lineages is generally low across the Tibetan glacier snow, while exceeding 75% in four Tibetan snow samples. All these samples were collected from the Tibetan Amne Machin glacier (ANM, Fig. 6a), located in the northeast of the Tibetan Plateau. The relative abundance variation of these cosmopolitan bloom-causing snow algae positively correlated with the depth of winter and summer snow. ANM has the highest snow depth in both winter and summer compared with other Tibetan glaciers investigated, reflecting the key roles of snow depth in mediating algal composition. The duration of snow melting6 is an essential modulator of red algal blooms, while frequent snowfall prevents algae growth by reducing photosynthesis19. The snow depth is much lower in Tibetan glaciers than in polar and Alpine glaciers. Thus, a high snow depth may extend melting duration in Tibetan glaciers, which gives sufficient time for cosmopolitan bloom-causing algae to grow on glacier surfaces. Nevertheless, the relationship between snowfall, snow melting rates, and algal blooms needs to be further investigated, as this may also be crucial to understanding the distribution of algal blooms globally.
Nutrient constraints are also critical for snow algal growth. Among the measured chemical parameters, only Cl− exhibited a significant correlation with the relative abundance of cosmopolitan bloom-causing algae (Supplementary Table 4). Cl− is an ion typically indicating precipitation sourced from saline environments (such as the ocean, saltwater lake, and soil) in ice core studies39. As the precipitation of ANM is influenced by the monsoon that originates from the Indian Ocean, the positive correlation may reflect the influence of snowfall, which is required for snow algae growth. Other nutrients, such as phosphorus and nitrogen, have been proposed to be the determinants of algal growth40,41. However, for Tibetan samples, we did not observe any significant correlation between total phosphorus and total nitrogen with the relative abundance of bloom-causing algae. The mean total phosphorus and total nitrogen of the Tibetan glacier snow were 107 μg/L and 0.28 mg/L (Supplementary Table 5), respectively. This is on a similar scale compared with the total phosphorus and total nitrogen in Antarctic, which were 50 μg/L and 0.26 mg/L42, respectively. This indicates that the snow algae on the Tibetan Plateau may not be limited by phosphorus or nitrogen.
Hydrurus foetidus-like algae dominated Tibetan glacier surface snow (Fig. 5). Early studies have reported that Hydrurus sp. are capable of causing macroscopically visible yellow colorations, which affect the albedo in various snow locations15,43. Thus, this highlights the distinctive composition of Tibetan glaciers and the presence of bloom-forming cells, despite the absence of visible blooms. Hydrurus sp. possess different pigments from Chloromonas sp. and Sanguina. It accumulates fucoxanthin, which gives them a yellowish color15. There is very limited literature on the influence of algal blooms other than red or green in color on snow albedo, so their influence on glacier melting needs to be further evaluated. Furthermore, Hydrurus sp. are identified in Arctic and Tibetan snowfields, as well as the ablation zone of Tibetan glacier snow, suggesting that they may be candidates for bloom-causing algae at a global scale. They are psychrotolerant and possess a protective polysaccharide sheath against environmental stress44, which could make them better adapted to the strong UV radiation of the Tibetan Plateau. In addition to Hydrurus sp., other Chloromonas-related species were identified in the snow of Tibetan glaciers sporadically, such as Chloromonas miwae and Chloromonas muramotoi28,29. These algae can cause blooms in snow in non-polar regions, further highlighting the potential for development of algal blooms in Tibetan glacier snow.
Conclusion
Our study reveals the unique distribution patterns of microeukaryotic algae across 13 glaciers on the Tibetan Plateau, indicating a high algal diversity dominated by endemic species. The presence of both cosmopolitan and endemic algae indicates the potential for the development of algal blooms, which is influenced by factors such as snow depth and nutrients like Cl−. Additionally, the wide distribution and high relative abundance of Hydrurus sp. across Tibetan glacier surface snow highlights the potential of golden-brown snow development. As they are less visible compared with red and green snow, further investigations are required for their implication on glacier melting under climate change. Moreover, algae abundance is another indicator of bloom formation. Further analysis considering snow algae abundance and their environmental drivers may provide additional knowledge on the control of algal blooming in Tibetan glacier snow.
Materials and methods
Snow sampling
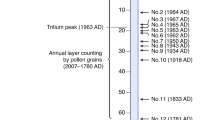
Twenty-six snow samples from 13 different glaciers on the Tibetan Plateau were collected between 2018 and 2021 (Fig. 1; Supplementary Table 6). Colored snow was not visible on the Tibetan Plateau during collection. The collected samples were transported in a frozen state to the laboratory of the Pan-Third Pole Environmental Center at Lanzhou University, China. After the snow samples were melted, they were filtered through 0.2 μm membranes that were transferred into 2 ml centrifuge tubes and then immediately refrozen and subsequently stored at −80 °C until DNA extraction procedures. The Arctic snow samples were collected from S-Greenland near the QAS-M (2 samples) and QAS-U (2 samples) weather stations. These samples were classified as ‘clean snow’ as they contained no visible particles, yet snow algae started to grow, as confirmed by microscopy observation (Olympus, Japan). Sample collection, preservation, and transport back to the home laboratory followed previously developed protocols45. Antarctic (18 samples) snow samples were collected from the Antarctic Peninsula46. Alps (16 samples) data were sourced from previously published studies47.
Environmental characterization of snow on the Tibetan Plateau
Due to the limited sample availability, the physicochemical properties were only quantified in 13 out of 26 Tibetan snow samples after they were filtered through 0.45 µm filters (Biosharp, China) (Supplementary Table 5). For the filtrate, total phosphorus (TP) was measured using ammonium molybdate spectrophotometry48; total organic carbon (TOC) was measured using a TOC analyzer (TOC-L CPH, SHIMATSU, Japan)49; the concentration of nitrate nitrogen (NO3-N) was measured using an elemental analyzer (Smartchem 200, AMS, Italy)50; F−, Cl−, and SO42− were analyzed using an ion chromatography system (ICS-900, Thermo Fisher, United States)51; pH and electrical conductivity were measured using a pH meter (PHS-3C, INESA, Shanghai). Temperature and snow depth were obtained from the National Tibetan Plateau Data Center (https://data.tpdc.ac.cn/home)52,53,54,55. No chemical data are available for samples from the rest of the regions.
DNA extraction, polymerase chain reaction (PCR), and high-throughput sequencing
Tibetan and Antarctic snow total DNA was extracted by the FastDNA® SPIN Kit for Soil (MP Biomedical, Santa Ana, CA, USA)46,56. Half of the membrane was sheared for DNA extraction, and the concentration of extracted DNA was measured using a Qubit Fluorometer-Nucleic Acid Concentration Assay and Protein Quantifier. We chose primers 1391f (5’-GTACA CACCGCCCGTC-3’) and EukBR (5’-TGATCCTTCTGCA GGTTCACCTAC-3’) to amplify the V9 hypervariable region of the algal sequence57. The PCR mixture (25 μL) contained 1 × PCR buffer, 1.5 mM of MgCl2, 0.4 μM each of deoxynucleoside triphosphate base, 1.0 μM of each primer, 0.5 U of Ex Taq (Takara), and 20 ng of DNA template. The 18S cycling program was as follows: 94 °C, 3 min; 35 cycles of 94 °C, 45 s; 57 °C, 1 min; 72 °C, 90 s; final extension 72 °C, 10 min. Target PCR products were then sequenced on an Illumina HiSeq 2500 sequencer (PE 250) at Magigene Biotechnology Co. Ltd. (Guangzhou, China). Arctic and Alps snow total DNA extraction and high-throughput sequencing followed previously developed protocols45,47, respectively.
Data processing
Due to the different sources of data, the amplicon sequence data of the 18S rRNA gene were processed separately, and then the quality-filtered reads were merged. For Antarctic and Tibetan snow samples, the forward and reverse reads were merged using USEARCH (v.11.0) with the “fastq_mergepairs” command and then quality filtered using the “fastq_filter” command58. For Arctic samples (only forward reads were used) and Alps samples (the forward and reverse reads were already merged after being downloaded), they were quality filtered using the “fastq_filter” of USEARCH (v.11.0)58. Then, the quality-filtered sequences of Antarctic, Arctic, Alps, and Tibetan samples were merged and processed together. The merged sequences were aligned using the “align.seqs” command in Mothur (v.1.40)59, which were then trimmed to the same length. Thereafter, amplicon sequence variants (ASVs) were identified using the USEARCH algorithm60. The sequences were classified using the Bayesian classifier against the Silva database (release 138), and only photosynthetic algae were retained (i.e., Chlorophyta, Diatomea, Ochrophyta, Dinoflagellata, Euglenozoa, and Cryptophyceae). Due to the high variation of reads per sample, the algae dataset was not subsampled. To ensure the diversity can be compared, we generated rarefaction curves, and any samples that did not reach a plateau were removed (Supplementary Fig. 2).
Due to a communication misunderstanding, the DNA samples from Arctic snow were sequenced on a PE150 platform, with insufficient DNA for another sequencing run. There was insufficient overlap between the forward and reverse reads; therefore, only the forward reads were used for analysis. To validate the results, we analyzed forward reads from Tibetan, Antarctic, and Arctic samples, finding consistent results (Supplementary Figs. 3–6). This indicates that the use of forward reads from the Arctic does not affect our conclusions.
Statistical analysis
The richness (number of ASVs) and evenness indices were calculated from the rarefied ASV table using the “diversity” function in the vegan package of R61. The alpha diversity indices (richness and evenness) in the different regions were compared using the Wilcox test. The community structure differences between samples were calculated based on Hellinger-transformed Bray-Curtis dissimilarity, with the results being visualized using a nonmetric multidimensional scaling ordination plot. Permutational ANOVA (PERMANOVA) was used to test the significance of snow and sampling location influences62 using the “adonis2” function in the vegan R package61. Hierarchical cluster analysis using the unweighted pair-group method with arithmetic means was performed by the “hclust” function in the vegan R package61. Heatmap was used to show differences of algae in different regions using the pheatmap R package. The top 20 most abundant ASVs were identified by the averaged relative abundance across all samples, and their taxonomy was identified by comparing against the NCBI database by BLAST. Distance-based multivariate multiple regression (DistLM) analysis was used to analyze the relationship between community composition and environmental variables63. Here, we used this approach to identify key environmental factors that best explain the changes in community composition of cosmopolitan taxa on the Tibetan Plateau using Primer 664.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The 18S rRNA sequences of the Arctic and Tibetan glacier samples have been submitted to NCBI SRA with accession number PRJNA1061834 and are available at the following URL: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1061834/.
References
Boetius, A., Anesio, A. M., Deming, J. W., Mikucki, J. A. & Rapp, J. Z. Microbial ecology of the cryosphere: sea ice and glacial habitats. Nat. Rev. Microbiol. 13, 677–690 (2015).
Anesio, A. M., Lutz, S., Chrismas, N. A. M. & Benning, L. G. The microbiome of glaciers and ice sheets. npj Biofilms Microbiomes 3, 1–11 (2017).
Hamilton, T. L. & Havig, J. Primary productivity of snow algae communities on stratovolcanoes of the Pacific Northwest. Geobiology 15, 280–295 (2017).
Fujii, M. et al. Microbial community structure, pigment composition, and nitrogen source of red snow in Antarctica. Microb. Ecol. 59, 466–475 (2010).
Lutz, S. et al. The biogeography of red snow microbiomes and their role in melting arctic glaciers. Nat. Commun. 7, 11968 (2016).
Roussel, L. et al. Snowmelt duration controls red algal blooms in the snow of the European Alps. Proc. Natl Acad. Sci. USA 121, e2400362121 (2024).
Nakashima, T. et al. Spatial and temporal variations in pigment and species compositions of snow algae on Mt. Tateyama in Toyama Prefecture, Japan. Front. Plant Sci. 12, 689119 (2021).
Tanaka, S. et al. Snow algal communities on glaciers in the Suntar-Khayata Mountain Range in eastern Siberia, Russia. Polar Sci. 10, 227–238 (2016).
Engstrom, C. B. & Quarmby, L. M. Satellite mapping of red snow on North American glaciers. Sci. Adv. 9, eadi3268 (2023).
Marchant, H. J. Snow algae from the Australian Snowy Mountains. Phycologia 21, 178–184 (1982).
Engstrom, C. B., Yakimovich, K. M. & Quarmby, L. M. Variation in snow algae blooms in the coast range of British Columbia. Front. Microbiol. 11, 569 (2020).
Chevrollier, L.-A. et al. Separating the albedo reducing effect of different light absorbing particles on snow using deep learning. Cryosphere 19, 1527–1538 (2025).
Segawa, T. et al. Bipolar dispersal of red-snow algae. Nat. Commun. 9, 3094 (2018).
Procházková, L., Remias, D., Holzinger, A., Řezanka, T. & Nedbalová, L. Ecophysiological and morphological comparison of two populations of Chlainomonas sp. (Chlorophyta) causing red snow on ice-covered lakes in the High Tatras and Austrian Alps. Eur. J. Phycol. 53, 230–243 (2018).
Remias, D., Jost, S., Boenigk, J., Wastian, J. & Lütz, C. Hydrurus-related golden algae (Chrysophyceae) cause yellow snow in polar summer snowfields. Phycol. Res. 61, 277–285 (2013).
Soto, D. F., Franzetti, A., Gómez, I. & Huovinen, P. Functional filtering and random processes affect the assembly of microbial communities of snow algae blooms at Maritime Antarctic. Sci. Total Environ. 805, 150305 (2022).
Engstrom, C. B., Raymond, B. B., Albeitshawish, J., Bogdanovic, A. & Quarmby, L. M. Rosetta gen. nov. (Chlorophyta): resolving the identity of red snow algal rosettes. J. Phycol. 60, 275–298 (2024).
Remias, D., Procházková, L., Nedbalová, L., Benning, L. G. & Lutz, S. Novel insights in cryptic diversity of snow and glacier ice algae communities combining 18S rRNA gene and ITS2 amplicon sequencing. FEMS Microbiol. Ecol. 99, fiad134 (2023).
Onuma, Y., Yoshimura, K. & Takeuchi, N. Global simulation of snow algal blooming by coupling a land surface and newly developed snow algae models. J. Geophys. Res.127, e2021JG006339 (2022).
Hoham, R. W. & Remias, D. Snow and glacial algae: a review. J. Phycol. 56, 264–282 (2020).
Soto, D. F., Gómez, I. & Huovinen, P. Antarctic snow algae: unraveling the processes underlying microbial community assembly during blooms formation. Microbiome 11, 200 (2023).
Ding, L. et al. Timing and mechanisms of Tibetan Plateau uplift. Nat. Rev. Earth Environ. 3, 652–667 (2022).
Huang, X., Deng, J., Wang, W., Feng, Q. & Liang, T. Impact of climate and elevation on snow cover using integrated remote sensing snow products in Tibetan Plateau. Remote Sens. Environ. 190, 274–288 (2017).
Jin, H. et al. Extraction of snow melting duration and its spatiotemporal variations in the Tibetan Plateau based on MODIS product. Adv. Space Res. 70, 15–34 (2022).
Qi, J. et al. Effect of Indian monsoon on the glacial airborne bacteria over the Tibetan Plateau. Sci. Total Environ. 831, 154980 (2022).
Ramel, C. et al. Integrating ecosystem services within spatial biodiversity conservation prioritization in the Alps. Ecosyst. Serv. 45, 101186 (2020).
Dorji, T., Moe, S. R., Klein, J. A. & Totland, Ø Plant species richness, evenness, and composition along environmental gradients in an Alpine meadow grazing ecosystem in central Tibet, China. Artic Antarct. Alp. Res. 46, 308–326 (2014).
Matsuzaki, R., Nozaki, H., Takeuchi, N., Hara, Y. & Kawachi, M. Taxonomic re-examination of “Chloromonas nivalis (Volvocales, Chlorophyceae) zygotes” from Japan and description of C. muramotoi sp. nov. PLoS ONE 14, e0210986 (2019).
Muramoto, K., Nakada, T., Shitara, T., Hara, Y. & Nozaki, H. Re-examination of the snow algal species Chloromonas miwae (Fukushima) Muramoto et al., comb. nov. (Volvocales, Chlorophyceae) from Japan, based on molecular phylogeny and cultured material. Eur. J. Phycol. 45, 27–37 (2010).
Beniston, M. Mountain climates and climatic change: an overview of processes focusing on the European Alps. Pure Appl. Geophys. 162, 1587–1606 (2005).
Tesson, S. V. M., Skjøth, C. A., Šantl-Temkiv, T. & Löndahl, J. Airborne microalgae: insights, opportunities, and challenges. Appl Environ. Microbiol. 82, 1978–1991 (2016).
Ren, W., Yao, T. & Xie, S. Key drivers controlling the stable isotopes in precipitation on the leeward side of the central Himalayas. Atmos. Res. 189, 134–140 (2017).
Yoichi, W. et al. The evolutionary history of rice azaleas (Rhododendron tschonoskii alliance) involved niche evolution to a montane environment. Am. J. Bot. 110, e16166 (2023).
Schwob, G. et al. Exploring the microdiversity within marine bacterial taxa: Toward an integrated biogeography in the Southern Ocean. Front. Microbiol. 12, 703792 (2021).
Archer, S. D. J. et al. Airborne microbial transport limitation to isolated Antarctic soil habitats. Nat. Microbiol. 4, 925–932 (2019).
Kuttippurath, J., Patel, V. K. & Sharma, B. R. Observed changes in the climate and snow dynamics of the Third Pole. npj Clim. Atmos. Sci. 7, 162 (2024).
Procházková, L., Leya, T., Křížková, H. & Nedbalová, L. Sanguina nivaloides and Sanguina aurantia gen. et spp. nov. (Chlorophyta): the taxonomy, phylogeny, biogeography and ecology of two newly recognised algae causing red and orange snow. FEMS Microbiol. Ecol. 95, fiz064 (2019).
Gálvez, F. E., Saldarriaga-Córdoba, M., Huovinen, P., Silva, A. X. & Gómez, I. Revealing the characteristics of the Antarctic snow alga Chlorominima collina gen. et sp. nov. through taxonomy, physiology, and transcriptomics. Front. Plant Sci. 12, 662298 (2021).
Ninglian, W., Tandong, Y., Thompson, L. G. & Davis, M. E. Indian monsoon and North Atlantic Oscillation signals reflected by Cl– and Na+ in a shallow ice core from Dasuopu glacier, Xixabangma,. Himal. Ann. Glaciol. 35, 273–277 (2002).
Lutz, S., Anesio, A. M., Edwards, A. & Benning, L. G. Linking microbial diversity and functionality of arctic glacial surface habitats: Arctic glacial surface habitats. Environ. Microbiol. 19, 551–565 (2017).
McCutcheon, J. et al. Mineral phosphorus drives glacier algal blooms on the Greenland Ice Sheet. Nat. Commun. 12, 570 (2021).
Grzesiak, J. et al. Microbial community changes along the Ecology Glacier ablation zone (King George Island, Antarctica). Polar Biol. 38, 2069–2083 (2015).
Jorgenson, K. L., Hotaling, S., Tronstad, L. M., Finn, D. S. & Collins, S. M. Trophic flexibility and hydrology structure alpine stream food webs: Implications for a fading cryosphere. Ecosphere 15, e70039 (2024).
Klaveness, D. & Lindstrøm, E. Hydrurus foetidus (Chromista, Chrysophyceae): A large freshwater chromophyte alga in laboratory culture. Phycol. Res. 59, 105–112 (2011).
Lutz, S., McCutcheon, J., McQuaid, J. B. & Benning, L. G. The diversity of ice algal communities on the Greenland Ice Sheet as revealed by oligotyping. Microb. Genomics 4, e000159 (2018).
Ji, M. et al. Similar heterotrophic communities but distinct interactions supported by red and green-snow algae in the Antarctic Peninsula. N. Phytol. 233, 1358–1368 (2022).
Krug, L., Erlacher, A., Markut, K., Berg, G. & Cernava, T. The microbiome of alpine snow algae shows a specific inter-kingdom connectivity and algae-bacteria interactions with supportive capacities. ISME J. 14, 2197–2210 (2020).
Li, W. et al. Combined effects of elevated carbon dioxide and temperature on phytoplankton-zooplankton link: a multi-influence of climate change on freshwater planktonic communities. Sci. Total Environ. 658, 1175–1185 (2019).
Lyons, W. B., Welch, K. A. & Doggett, J. K. Organic carbon in Antarctic snow. Geophys. Res. Lett. 34, 2006GL028150 (2007).
Liebner, S., Rublack, K., Stuehrmann, T. & Wagner, D. Diversity of aerobic methanotrophic bacteria in a permafrost active layer soil of the Lena Delta, Siberia. Microb. Ecol. 57, 25–35 (2009).
Chen, Y. et al. Temporal variation of bacterial community and nutrients in Tibetan glacier snowpack. Cryosphere 16, 1265–1280 (2022).
Hu, Y., Che, T., Dai, L. & Xiao, L. Snow depth fusion based on machine learning methods for the Northern Hemisphere. Remote Sens. 13, 1250 (2021).
Fang, S. et al. Dataset of daily near-surface air temperature in China from 1979 to 2018. Earth Syst. Sci. Data 14, 1413–1432 (2022).
Che, T., Hu, Y., Dai, L. & Xiao, L. Long-term series of daily snow depth dataset over the Northern Hemisphere based on machine learning (1980–2019). National Tibetan Plateau/Third Pole Environment Data Center. https://cstr.cn/18406.11.Snow.tpdc.271701 (2021).
Fang, S. & Mao, K. A dataset of daily near-surface air temperature in China from 1979 to 2018. National Tibetan Plateau/Third Pole Environment Data Center. https://doi.org/10.5281/zenodo.5502275 (2022).
Khan, A. et al. Disparity in soil bacterial community succession along a short time-scale deglaciation chronosequence on the Tibetan Plateau. Soil Ecol. Lett. 2, 83–92 (2020).
Amaral-Zettler, L. A., McCliment, E. A., Ducklow, H. W. & Huse, S. M. A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small-subunit ribosomal RNA genes. PLoS ONE 4, e6372 (2009).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ. Microbiol 75, 7537–7541 (2009).
Callahan, B. J., McMurdie, P. J. & Holmes, S. P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 11, 2639–2643 (2017).
Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930 (2003).
Legendre, P. & Anderson, M. J. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 69, 1–24 (1999).
Liu, Y. et al. Plant colonization mediates the microbial community dynamics in glacier forelands of the Tibetan Plateau. iMeta 2, e91 (2023).
Freedman, Z. & Zak, D. R. Soil bacterial communities are shaped by temporal and environmental filtering: evidence from a long-term chronosequence. Environ. Microbiol. 17, 3208–3218 (2015).
Acknowledgements
This work was supported by the National Science Foundation of China (32161123004, 42330410, and 42171138) and the European Research Council, H2020 European Research Council (grant no. 856416).
Author information
Authors and Affiliations
Contributions
Y.L. and M.J. designed the study. L.G.B. and A.M.A. collected the Arctic snow samples. D.Y. performed the analysis and wrote the first draft of the paper with M.J. A.M.A., B.F., Y.C., N.T., P.L. and Y.L. assisted with analysis and helped improve the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Francisca Gálvez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Ilka Peeken and Mengjie Wang. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, D., Ji, M., Anesio, A.M. et al. Cosmopolitan and endemic species jointly enhance the potential of producing algal blooms in Tibetan glaciers. Commun Earth Environ 6, 584 (2025). https://doi.org/10.1038/s43247-025-02528-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-025-02528-2