Abstract
Concerns about climate change-influenced tree growth declines and world tree mortality raise questions about potential reductions in tree longevity. However, the global influences of climate and growth patterns on tree longevity remain poorly understood. Here we analyzed 219,000 tree-ring widths from 4880 globe sites, encompassing 246 species, to investigate tree longevity patterns. Gymnosperms exhibited significantly greater average longevity (366 ± 240 years) than angiosperms (216 ± 81 years), with the oldest individual exceeded 3000 years. Globally, gymnosperm longevity was negatively correlated with precipitation. Arid-adapted trees exhibited significantly higher longevity, likely due to their conservative growth strategy, characterized by slow growth rates and enhanced drought resilience. Trees in harsh environments defined by high altitude, nutrient-poor soils, and minimal human impact were more likely to attain greater longevity. These findings highlight the impact of climate change on tree longevity and the necessity for targeted conservation strategies to protect these vital ecosystem components.
Similar content being viewed by others
Introduction
Old trees, often referred to as ancient guardians of forests, provide numerous ecosystem services. They provide specialized habitats and shelters for certain plants, animals and fungi, share nutrients with neighboring young trees through the mycorrhizal networks, and serve as reservoirs for genetic diversity and carbon sequestration1,2,3,4. The death of old trees can have cascading effects throughout the ecosystem, with serious implications for its integrity and biodiversity2,5. The recent global warming and the related extreme climate events, particularly drought, have increased catastrophic tree mortality worldwide6,7, thus reducing tree longevity. While recent studies have highlighted the impact of climate change on tree mortality, the precise global distribution of tree longevity and the factors that govern it are not yet fully elucidated.
Trees, unlike animals, exhibit a theoretically limitless lifespan due to the modular architecture8 and the ability of meristematic tissues to regenerate annually or remain dormant for long periods9,10. In reality, trees die from hydraulic failure or carbon starvation under climate stress11, and other disturbances like biotic and mechanical factors12. Consequently, the trees have developed an early life acclimation to environmental pressures and phenotypic plasticity as effective strategies for achieving tree longevity13. Recent research suggests that cold alpine and boreal environments promote tree longevity by reducing growth rates14, highlighting the importance of temperature15,16,17. Also, arid climates are associated with extended tree longevity18. While both coldness and aridity contribute to slowing down growth, the primary factor promoting longevity on a global scale remains unclear. Furthermore, tree longevity is influenced by various disturbances throughout their lives13. Limited resilience to ecoclimatic stress increases tree mortality rate and consequently shortens tree longevity19,20,21,22. However, our understanding of how long-lived trees effectively manage disturbances and achieve their remarkable longevity remains limited23.
The traditional investigations of tree longevity often employed historical records and forest inventories inferred from factors like stem size and tree height, which present limitations for accurately estimating the cambial age of old trees16,24,25. Instead, tree-ring data used for dendroclimatic reconstructions offers a robust estimation of longevity due to its accurate dating and collection of locally oldest trees. We herein use a tree-ring network to assess the effects of climate and growth resilience on tree longevity, which we define as the 99th quantile of the age distribution for trees within each site, on a global scale.
Results
Influences of climate and species on global tree longevity
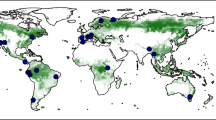
We compiled and analyzed a comprehensive dataset encompassing 4880 tree-ring sites across various biomes and ecosystems (see Methods). Cold (61%) and temperate biomes (21%) are most represented, while arid, polar and tropical biomes account for 11, 5, and 1%, respectively (Supplementary Fig. 1). Mature trees, defined as those with ages younger than 75th percentiles are most prevalent (72.7%), followed by old trees, with ages between 75th percentiles and 1.5 times the interquartile range (23.8%), and ancient trees, defined as those with ages older than 1.5 times the interquartile range of the longevity distribution in each clade, representing approximately 3.5% of the total. (Supplementary Fig. 1). Ancient and old trees exhibit a concentrated spatial distribution around 40° N and S (Fig. 1b), with tree longevity declining towards the tropics and the polar regions.
a Geographical distribution of the 4880 tree-ring width series. b–e Longevity distribution based on latitude, clades, Köppen climate zone, and aridity index. Blue and orange circles in figures a–c represent gymnosperm (N = 3956) and angiosperm (N = 924), respectively. Violin plots illustrate the spread and density of data points around the median. Boxplots display the 25th percentile, median and 75th percentile, with whiskers extending to 1.5 times the interquartile range and showing outliers. Statistically significant differences among age groups are indicated by different letters (Tukey’s HSD tests, p < 0.05). Letters at panel bottoms specify the number of tree-ring series.
Our analysis reveals a strong association between extreme longevity and arid environments. Over 60% of ancient trees are found in arid regions with mean annual precipitation (MAP) < 600 mm and annual temperature (MAT) around 10 °C (Fig. 2). Old gymnosperms are predominantly located in sites with lower precipitation, while precipitation has no significant impact on angiosperms longevity (Fig. 2a, c, Supplementary Table 1). Gymnosperms longevity exhibits a significant negative correlation with MAP, with ancient and old trees associated with lower MAP values (mean ± SD, 753 ± 600 mm and 799 ± 624 mm, respectively) compared to mature trees (859 ± 517 mm). Although there was no significant difference in MAT across gymnosperm age classes, except between old and mature trees, angiosperm age increased with MAT, with old trees exhibiting higher MAT (11 ± 5 °C) than mature trees (10 ± 6 °C) (Fig. 2b, d). In diverse biomes, gymnosperms showed significantly lower precipitation in old and ancient tree habitats compared to mature trees, with the exception of temperate zones (Supplementary Fig. 4). These older gymnosperms also experienced significantly reduced growth temperatures in arid, tropical, and temperate regions. Conversely, angiosperms exhibited no age-related differences in precipitation or temperature (Supplementary Fig. 4). These findings indicate a pronounced negative effect of MAP on tree longevity at a global scale, particularly for gymnosperms, while MAT has a negligible impact on both clades, as evidenced by regression slopes approaching to zero (Supplementary Fig. 5).
a,c Mean annual precipitation (MAP) and mean annual temperature (MAT) in gymnosperms. b,d MAP and MAT in angiosperms. Older populations are positioned above younger ones to highlight their distribution. Inserted boxplots summarize the data distribution for each age group, with boxes showing the 25th percentile, median and 75th percentile, and whiskers indicating 1.5 times the interquartile range. Statistically significant differences among age groups are indicated by different letters (Tukey’s HSD tests, p < 0.05).
Gymnosperms exhibit greater longevity (366 ± 240 years) compared to angiosperms (216 ± 81 years) (Fig. 1c). Tropical regions harbor the youngest trees on average (Fig. 1d), while arid and semi-arid regions support the oldest trees for both gymnosperms and angiosperms (Fig. 1d, e). Cupressaceae (587 ± 405 years) and Taxodiaceae (543 ± 347 years) are the two longest-lived gymnosperm families, although Pinaceae include exceptionally old individuals (Supplementary Fig. 6). Among angiosperms, Nothofagaceae (264 ± 75 years) and Magnoliaceae (233 ± 108 years) exhibit the greatest longevity (Supplementary Fig. 6). Notably, Sequoiadendron giganteum and Juniperus przewalskii exceed 3000 years in the US and China, respectively. However, even older individuals of Pinus longaeva in West US16 and Fitzroya cupressoides individuals in South Chile26 have been documented with ages of 4800 and 3620 years, respectively.
Climate stress during the juvenile period promotes tree longevity
The location of old-growth trees in harsh environments aligns with a consistent negative relationship between slow growth rates and tree longevity observed across both gymnosperms and angiosperms (Fig. 3a–c) and all biomes (Fig. 3d–f). This negative association follows a non-linear, negative exponential pattern, evident even during the juvenile period, specifically for cambial age less than 25 and 50 years. The link suggests that slower juvenile growth rates may contribute to increased longevity in trees. Notably, trees in arid biomes demonstrate a stronger correlation between juvenile growth stress and increased longevity compared to those in cold environments (Fig. 3e, f).
The negative growth rate-longevity relationship is evident at both the species (Supplementary Fig. 7) and individual site levels (Supplementary Figs. 8–10) throughout tree lifespans and during juvenile phases, although not always statistically significant. Several long-lived gymnosperms, such as J. prezewalskii and P. longaeva, are primarily from arid environments and show low growth rates. Gymnosperms generally display slower growth and greater longevity in arid compared with humid habitats (Supplementary Figs. 8–10), with Taxodium distichum as an exception, being native to humid sites. Angiosperms like Quercus douglasii can grow in both humid and arid environments, and achieve greater longevity in arid sites. Trees typically maximize their lifespan potential via a strategy of slower juvenile growth, and longevity also varies across differing environmental contexts.
Strong tree resilience of old trees
Old trees not only undergo early-life climate stress acclimation but also often demonstrate enhanced resilience to climate stress through their growth. Old trees exhibit greater inter-annual growth fluctuations and increased resilience to growth disturbances, as supported by a significant positive correlation (r = 0.21, p < 0.05) between mean tree-ring chronology sensitivity and longevity in gymnosperms (Fig. 4).
The color, shape and orientation of ellipses are mapped to coefficient values. Abbreviations: MS, mean sensitivity; AC1, first-order autocorrelation; Rbar, mean inter-series correlation; EPS, express population signal; SNR, signal-noise ratio; Rt, resistance; Rc, recovery; Rs, resilience. Only statistically significant correlation coefficients (p < 0.05) are displayed.
Old gymnosperms exhibit a more pronounced growth decline during disturbances (r = −0.18, p < 0.05) but also demonstrate a stronger recovery capacity (r = 0.25, p < 0.05), resulting in greater overall growth resilience (r = 0.12, p < 0.05) (Fig. 4). Trees in arid regions display enhanced overall resilience to stress, characterized by reduced resistance but increased recovery capacity (Supplementary Fig. 11). In contrast to gymnosperms, relationships between resistance, recovery, resilience, and longevity are insignificant in angiosperms (Fig. 4). Additionally, a positive correlation was identified in gymnosperms between site elevation and longevity (r = 0.34, p < 0.05), and between wind speed and longevity (r = 0.12, p < 0.05), while negative associations emerge with canopy height, soil organic carbon, the human influence index (HII) and lightning density (LD) (r = −0.13, r = −0.14, r = −0.25, and r = −0.09, respectively, p < 0.05) (Supplementary Fig. 12). The longevity of angiosperms demonstrated a significant positive relationship with elevation (r = 0.09, p < 0.05) and wind speed (r = 0.13, p < 0.05) and a statistically significant negative relationship with the HII (r = −0.15, p < 0.05).
Discussion
Tree mortality is often attributed to various environmental disturbances rather than programmed senescence. Therefore, the maintenance of sustained, albeit slow, growth under environmental stress, coupled with the avoidance of mortality events induced by episodic extreme environmental disturbances, appears to be a crucial strategy for trees to achieve extended longevity. Our findings indicate that trees in arid environments exhibit significantly higher average longevity compared to those in resource-rich regions such as tropical and temperate zones, as well as cold regions. This disparity may be attributed to the lower growth rates and enhanced resilience to disturbances observed in arid-adapted trees. Water limitation in arid environments compels trees to adopt a conservative growth strategy characterized by high hydraulic safety but low hydraulic transport efficiency, resulting in slow growth, manifested as smaller tree size, lower tree height, and higher wood density. Furthermore, trees in arid environments demonstrate lower resistance but greater recovery capacity when subjected to drought disturbances. This conservative strategy facilitates rapid adjustments to prevent hydraulic failure during drought events and promotes swift recovery of growth post-disturbance.
Sustained slow growth is recognized as a critical determinant for trees to achieve exceptional longevity. Our findings of slower growth rates in older trees support a trade-off between radial growth and tree longevity14,17,27. Rapid tree growth, by accelerating the attainment of larger stature, can increase mortality risk primarily due to heightened susceptibility to mechanical vulnerabilities from environmental and pest/pathogen stresses28. For conifers, specifically, this rapid stem growth may also compromise wood density accumulation, further increasing the probability of mechanical failure29. Furthermore, rapid growth often intensifies competition for environmental resources. For instance, competition for light, whereby canopy interference regulates light availability, has been identified as a crucial limiting factor for trees reaching their potential lifespan in numerous forest environments15,30,31.
A key findings is a trade-off between growth rate and longevity in trees inhabiting arid and cold environments, where slower growth rates correlate with extended lifespans. Both limited water availability and low temperatures constrain xylem cell development. Reduced water potential in arid conditions impedes cell turgor, thereby inhibiting cell division and elongation, resulting in diminished radial growth rates and narrow tree-rings32,33. Similarly, photosynthetic capacity and growth are curtailed when ambient temperatures fall below 0 °C and 5 °C, respectively34. Consequently, inherent growth rate limitations in trees from arid and cold regions contribute to their extended longevity. Environmental conditions prevalent in cold or arid climates may alleviate pressures from pests and diseases, or attenuate senescence processes within tree functions35. Additionally, these conditions can lead to more open forest or woodland structures, reducing the likelihood of damage from wind disturbances or neighboring tree falls36, thereby further promoting longevity. Finally, lower human influence in these regions minimizes threats from deforestation and land-use change.
Our results show higher longevity in arid compared to cold forests on a global scale. This aligns with previous research demonstrating lower mortality risk in arid environments32, but contrast with studies emphasizing temperature’s role in longevity at landscape or region scales14,15. In arid environments, trees exhibit narrower xylem tracheids and increased wood density with decreasing precipitation and water availability, reflecting a shift towards prioritizing hydraulic safety over efficient water transport37,38. Maintaining favorable water status often necessitates a reduction in photosynthetic carbon gain, which subsequently limits height growth39,40. This dual constraint on growth in both size and height, coupled with the prioritization of hydraulic safety, likely contributes to the extended longevity observed in arid ecosystems18. Several additional factors, such as twisted stems and fissured barks, often associated with partial cambial dieback, may extend longevity in old arid-region conifers41. Conversely, despite experiencing prolonged and frigid winters, trees in boreal forests have evolved phenological adaptations to minimize exposure to sub-zero temperatures, and rapidly capitalize on favorable temperatures during June and July42,43. Additionally, the relatively abundant water resources in boreal forests promote increased tree height, resulting in taller canopies compared to arid biomes (Supplementary Fig. 13). Consequently, while radial growth is slow in boreal trees, the attainment of maximum height at a relatively young age may ultimately limit longevity due to heightened vulnerability to hydraulic failure, increased respiration rates, and elevated evapotranspiration demands39. In contrast, trees in polar environments, including subarctic and subalpine regions, exhibit both slow stem and height growth, contributing to longevity despite their often dwarfed, shrub-like stature.
A further finding supports a link between stress resilience and longevity. Trees from arid forests, exhibiting greater stress resilience, particularly drought tolerance, tend to be older than those from wetter forests (Supplementary Fig. 11). Intra-annual density fluctuations in arid and semi-arid regions indicate abrupt growth declines during drought10,44. Trees can rapidly adjust their cellular osmotic potential to cope with drought, protecting against hydraulic failure at the cost of reduced growth and potentially lower drought resistance. However, arid-adapted trees demonstrate strong recovery, indicating adaptability to climate extremes. This aligns with population-level observations, where arid-adapted trees exhibit increased resilience compared to wetter-adapted counterparts in common garden experiments and ecotones11,45. Local genetic adaptation allows arid-adapted trees to recover rapidly from extreme drought events compared to wet-adapted species45. Moreover, repeated drought exposure may enhance growth resilience through ecological memory of past drought events24,46, contributing to extended longevity. In contrast, while boreal trees prioritize xylem growth during droughts, their recovery is slower compared to arid-adapted trees. The combination of tall stature, exposure to greater solar radiation, and evaporative demand renders boreal trees more vulnerable to drought stress47,48. Frequent and more intense droughts, often coupled with insect outbreaks and increased fire activity, are likely to further exacerbate tree mortality in boreal forests49. The risk of mechanical failure and mortality in trees is elevated in boreal forests due to exposure to extreme windthrow events50,51. Interestingly, drought resistance increases in some wet environments dominated by angiosperms52, suggesting a balance between drought resistance and recovery is crucial for extreme longevity, even in wetter climates with seasonal dry periods. The capacity for extended longevity is also supported by a suite of functional traits exhibited by certain tree species. Waxy-cuticle needles, persisting for decades, can potentially compensate for lower photosynthetic capacity in older conifers38,53,54. Short stature, denser wood, and lower tree density decrease water transport demands, competition, and vulnerability to wind, insects, and diseases.
Protecting old-growth trees from future climate threats is increasingly critical55. Whether trees in arid regions are more vulnerable to climate change than those from cold environments is still not clear52. Our study indicates that old trees in arid environments possess superior long-term drought resilience, potentially enabling them to maintain survival and remarkable longevity. Conversely, cold environment trees, with lower overall drought tolerance, may be more susceptible. Nevertheless, even drought-adapted species or individuals may face challenges when water deficits exceed their tolerance thresholds.
Understanding the adaptations of long-lived arid trees provides valuable insights for forest management strategies aimed at enhancing resilience to future climate stresses. The rich genetic diversity accumulated by ancient trees during unique historical circumstances can bridge the gap between normal and extreme conditions. Potential management strategies include selecting drought-resistant genotypes with high genetic diversity from long-lived arid tree provenances, conducting species or population translocation within suitable locations, and thinning stand densities to reduce competition and the risk of insect outbreaks and wildfires. Many of these old trees exist outside protected areas, placing them at high risk of extinction. Comprehensive conservation efforts are essential, ranging from preserving large, intact old-growth forests to safeguarding individual trees within human-dominated landscapes16,25.
Conclusion
Our study reveals a global pattern of tree longevity and highlights the predominant influences of aridity, instead of coldness, on promoting tree longevity. In contrast to cold environments, water scarcity exerts a more pronounced negative impact on xylem development and height growth, thereby contributing to slower growth rates and enhanced tree longevity. Early-life drought exposure, coupled with rapid growth recovery and strong resilience, further promotes exceptional longevity in arid-adapted trees. Protecting old-growth trees and implementing management strategies informed by these findings are crucial for mitigating future climate impacts and preserving these invaluable genetic resources.
Methods
Tree-ring data acquisition
To investigate the influence of climatic conditions on tree lifespan, our study utilized tree-ring width data from the International Tree-Ring Data Bank (ITRDB) (ITRDB, https://www.ncei.noaa.gov/products/paleoclimatology/tree-ring, last accessed September 4th, 2023), and supplemented with 14 collected tree-ring width series from Southeast China. Tree growth data from landscapes heavily impacted by human activities, such as trees growing in temples, rural areas, and tourist sites, were not collected. The ITRDB is a global tree-ring data repository primarily used for climate reconstruction studies. Dendrochronologists typically prioritize sampling large, old trees at the population level to obtain the longest possible tree-ring records. Consequently, tree-ring width data within the ITRDB predominantly represent the oldest individuals at each site. Tree-ring cores were basically collected using standardized protocols, prioritizing pith inclusion when feasible. A total of 5876 tree-ring sites from 309 species were downloaded globally. Metadata associated with each site, including file ID, spatial coordinates, altitude, and species, were extracted.
To ensure data quality, a rigorous verification process was implemented. This included checking and correcting data formats for consistency, removing duplicate records. All tree-ring width series were analyzed using the dplR package (version 1.7.7)56 in R (version 4.4.1)57 for subsequent analysis. The number of records for each site was determined, and the tree core age was calculated as the difference between the start and end years. However, the precise establishment year of some trees may be inaccurate due to missing piths or hollow trunks, potentially leading to a slight underestimation of tree age. Therefore, we selectively included sites with a minimum of 15 records and excluded sites with a maximum age below 100 years. The final dataset comprised exclusively old-growth populations characterized by a substantial number of records per site, ensuring robust estimates of maximum tree age. This filtering resulted in a final dataset 4880 tree-ring width sites from 240 species across 73 genera, encompassing over 219,000 individual records spanning latitudes from 60 °S to 75 °N (Supplementary Table 1, Supplementary Fig. 1), with gymnosperms and angiosperms constituted 89.1 and 18.9%, respectively. Conversely, existing literature documents that angiosperms constitute approximately 89.4% of extant plant species58. This discrepancy arises because certain angiosperms, particularly those in tropical and subtropical regions, exhibit indistinct annual ring boundaries due to prolonged growing seasons with continuous cambial activity and complex xylem anatomical structures. Consequently, angiosperms are significantly underrepresented in ITRDB databases, and subsequently, in our analysis. This under-representation within our dataset may have introduced a potential underestimation of the tree longevity. However, the average longevity of trees in tropical environments derived from our study (193 ± 86 years) closely aligns with the mean tree longevity reported in a previous investigation on tropical tree longevity (186 ± 138 years)17. Therefore, while acknowledging the substantial disparity between the sampled angiosperm population (predominantly from tropical regions) and their actual prevalence in natural ecosystems, we posit that the lifespan estimations derived from our angiosperm samples, despite potential sampling bias, likely reflect the approximate longevity of these species within the studied tropical environments.
To evaluate the minimum sample size required for a representative estimation of the true maximum age of a species or sites, we investigated the relationship between tree-ring sampling sites and records with the maximum age of tree species (Supplementary Fig. 2a, b). The results revealed a significant linear relationship between the maximum age of angiosperms and increased sample size (p < 0.001, R2 of 0.38 and 0.37 for sampling sites and records, respectively). The sample size did not significantly affect the maximum age of gymnosperms. For angiosperms, each increase of 100 sampling sites and records led to an increase in maximum tree age of 199 and 5 years, respectively. To rigorously determine the minimum tree-ring width records necessary for accurate species lifespan assessment. We examined the maximum age distribution of a representative gymnosperm and angiosperm species by randomly resampling 500 times from their respective age distributions, with sample sizes ranging from 25 to 500 (Supplementary Fig. 2c, d). Comparing the maximum ages of these random tree subsets with the true observed maximum ages revealed that the average maximum age of the subsets exceeded the 95th percentile of the true age of the tree species when the sample size was 25 records. When the sample size was 100, the average maximum age of the subsets exceeded the species-level lifespan, i.e., the 99th percentile of the age distribution of the tree species. This indicates that when the sample size is greater than 100 tree age records, we can consider the species-level lifespan to be close to its true lifespan. Thus, for species-level lifespan analysis, species with at least 3 sites and a total of more than 150 records were included, resulting in a dataset of 125 species from 39 genera and 14 families (Supplementary Table 2). At the species level, the average lifespans of gymnosperms and angiosperms were 611 ± 345 year and 303 ± 205 years, respectively.
Environmental data collection
To comprehensively assess global tree longevity patterns across biomes, tree longevity was categorized based on temperature and moisture conditions using the Köppen climate classifications59: (1) tropical, (2) temperate, (3) cold, (4) polar and (5) arid. Additionally, aridity index (AI)60 was employed for further classification: (1) hyper-arid (AI < 0.03), (2) arid (0.03 ≤ AI ≤ 0.2), (3) semi-arid (0.2 < AI ≤ 0.5), (4) dry sub-humid (0.5 < AI ≤ 0.65) and (5) humid (AI > 0.65). Site-level average annual temperature (MAT, °C), precipitation (MAP, mm) and wind speed (WS, m s−1) for 1970–2000 were obtained from WorldClim61 at a 30 arc-second resolution (~ 1 km2). The average annual wind speed in the study area ranged from 1 to 10 m s−1, corresponding to wind forces ranging from calm to fresh breeze on the Beaufort scale. Site-level topsoil (0–30 cm) properties from the Harmonized World Soil Database62 at the same resolution were also acquired. Soil texture (ST) was classified into 13 groups based on USDA system, ranging from heavy clay to sand. Soil organic carbon (SOC) content (SOC, %) and soil pH served as indicators of soil health. Human influence was assessed using the HII63 from 1995–2004 at a 1 km resolution. This index incorporates nine indicators, including population pressure (population density), human land use and infrastructure (built-up areas, nighttime lights, land use/land cover), and human accessibility (coastlines, roads, railroads, navigable rivers). Site-level forest canopy height data for 2019 at a 30 m resolution64 was obtained, with values ranged from 0 to 60 m and recorded as 0 when the values less than 3 m. Site-level LD (LD, stokes km−2 day−1) was extracted from WWWLLN65 repository for the period 2010–2024 at a spatial resolution of 0.5 degree. To calculate the site-level annual average number of forest fires, we utilized the MCD14ML Collection 6 MODIS Thermal Anomalies/Fire data product66, spanning the years 2002 to 2023. To ensure the reliability of the fire detection data, a quality control measure was implemented by filtering the dataset to include only fire pixels with a confidence level exceeding 10%. Linear regression and Spearman’s rank correlation coefficients were then calculated to quantify the relationships between tree longevity and the various environmental variables.
Estimation of tree longevity
To estimate the maximum lifespan for trees at each study site, the 99th quantile of the age distribution within each site was used as a representative measure of longevity. This approach provides a robust estimate of tree longevity that is less susceptible to outliers. Tree longevity was subsequently analyzed across various groups (gymnosperms vs. angiosperms, Köppen climate zones, and aridity classifications). Tukey’s HSD tests (two-sided) was employed to compare mean longevity differences among these groups.
Age classification and potential environmental drivers
To explore the relationship between age and climate in more detail, a nuanced age classification system was developed for each taxonomic group. Trees were categorized into three age groups22: mature trees, defined as those with ages younger than 75th percentile are most prevalent, old trees, with ages between 75th percentiles and 1.5 times the interquartile range, and ancient trees, defined as those with ages older than 1.5 times the interquartile range of the longevity distribution in each clade. This classification resulted in mature (<450 years), old (≥450 and <814 years), and ancient (≥814 years) gymnosperms. For angiosperms, mature and old trees were separated at 273 years, with trees older than 453 years classified as old trees due to the limited sample size of only three individuals in the ancient category. The distribution of MAP and MAT across age groups within gymnosperm and angiosperm was analyzed and Tukey’s HSD tests (two-sided) used to compare mean value among groups. The climate-longevity relationships were fitted by the 95th quantile regressions.
Examining the association between growth and longevity
To account for potential variations in growth rates due to age and historical climate, mean and juvenile specifically for cambial age less than 25 and 50 years, growth rates were calculated using standardized tree-ring width indices. A negative exponential detrending approach was applied to address age-related growth trends. The relationship between growth rate and tree longevity was assessed across clades and species using exponential regressions. To further explore this relationship within species, the six longest-lived gymnosperms and four longest-lived angiosperms were selected for in-depth analysis. Relative age within a species was calculated by dividing a tree’s actual age by the species’ longevity. Similarly, the relative growth rate was determined by dividing a tree’s mean ring width by the species’ maximum ring width.
Growth traits based on tree-ring data
Tree-ring width measurements were processed using standard dendrochronological methods67. Individual tree-ring width series were detrended with a negative exponential function and averaged into site-level chronologies of ring-width indices using a hierarchical approach (from tree to site) and bi-weight robust means. To assess the influence of climate on tree age through radial growth variations, site-level tree-ring statistics were calculated using the dplR packages56 in R57. Mean sensitivity (MS) quantified year-to-year variability, with higher values indicating stronger climatic fluctuations. First-order autocorrelation (AC1) assessed the temporal dependence between consecutive rings67. Inter-records correlation (Rbar) measured similarity among tree-ring records68, while expressed population signal (EPS) evaluated the representation of a common climate signal in the chronology (higher EPS indicating a stronger common signal)69. Signal-noise ratio (SNR) represented the strength of the climate signal relative to background noise. To evaluate tree responses and overall growth resilience to ecological disturbance during their whole lifespan, tree resistance (Rt), recovery (Rc), and resilience (Rs) were calculated70 using established formulas considering tree-ring width during and around disturbance events within two years71.
Where Dr is the tree-ring width during the ecological disturbance year, which is regarded as the relative growth decline year (also called pointer year) when more than 75% of tree series reached at least 30% of growth declines than the year before. Pre Dr and Post Dr represent the average tree-ring width from 2 years before and after the year of external disturbance. This approach considers the potential legacy effect of the natural disturbance on tree growth and avoids contamination from the influence of any subsequent disturbance event. These metrics assessed a tree’s ability to withstand (Rt), recover from (Rc), and maintain growth (Rs) after disturbances. In this study, we aimed to evaluate the general ability of trees to resist and/or recover from external disturbances. Therefore, we did not distinguish the pointer events based on the specific type of growth decline. Multiple comparisons were performed using Tukey’s HSD test (two-sided). Spearman’s rank correlation coefficients were used to examine relationships between tree longevity and these tree-ring statistics.
Linear regression was performed to investigate the relationship between tree age and size. Spearman’s rank correlation coefficients were calculated to determine the role of both tree size and age in influencing early growth rate (juvenile growth rates at 25 and 50 years) and ecological resilience traits (resilience, resistance, and recovery). Our analysis indicated a strong linear correlation between age and tree diameter in tropical regions for both gymnosperms (R² = 0.57) and angiosperms (R² = 0.24) (Supplementary Fig. 3a, b). In contrast, these linear relationships were weaker in gymnosperms from cold and arid regions and non-significant in angiosperms from arid and temperate environments (Supplementary Fig. 3a, b). These findings suggest that tree size does not consistently increase linearly with age across all environments and tree clades. The correlations between longevity and these growth rate and resilience traits were consistently and substantially stronger than those observed between diameter and the same metrics (Supplementary Fig. 3c). This discrepancy likely arises from the decoupled relationship between tree age and diameter in certain ecosystems, resulting in a less stable correlation between size and the examined variables compared to age.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw tree-ring width data are accessible on the ITRDB (https://www.ncei.noaa.gov/products/paleoclimatology/tree-ring). Processed datasets supporting the findings of this study have been deposited in Figshare and are accessible at https://doi.org/10.6084/m9.figshare.29436140.
Code availability
The codes used to calculate the tree-ring parameters in this study have been deposited in Figshare and are accessible at https://doi.org/10.6084/m9.figshare.29436140.
References
Luyssaert, S. et al. Old-growth forests as global carbon sinks. Nature 455, 213–215 (2008).
Lindenmayer, D. B., Laurance, W. F. & Franklin, J. F. Global decline in large old trees. Science 338, 1305–1306 (2012).
Pickles, B. J. et al. Transfer of 13C between paired Douglas-fir seedlings reveals plant kinship effects and uptake of exudates by ectomycorrhizas. N. Phytol. 214, 400–411 (2017).
Bezemer, N., Krauss, S. L., Roberts, D. G. & Hopper, S. D. Conservation of old individual trees and small populations is integral to maintain species’ genetic diversity of a historically fragmented woody perennial. Mol. Ecol. 28, 3339–3357 (2019).
Lindenmayer, D. B. Conserving large old trees as small natural features. Biol. Conserv. 211, 51–59 (2017).
Wardle, D. A., Walker, L. R. & Bardgett, R. D. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305, 509–513 (2004).
Martínez-Vilalta, J., Lloret, F. & Breshears, D. D. Drought-induced forest decline: causes, scope and implications. Biol. Lett. 8, 689–691 (2012).
Peñuelas, J. & Munné-Bosch, S. Potentially immortal? N. Phytol. 187, 564–567 (2010).
Gao, J., Rossi, S. & Yang, B. Origin of intra-annual density fluctuations in a semi-arid area of Northwestern China. Front. Plant Sci. 12, 777753 (2021).
Gao, J., Yang, B., Peng, X. & Rossi, S. Tracheid development under a drought event producing intra-annual density fluctuations in the semi-arid China. Agr. For. Meteorol. 308–309, 108572 (2021).
McDowell, N. et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? N. Phytol. 178, 719–739 (2008).
Das, A. J., Stephenson, N. L. & Davis, K. P. Why do trees die? Characterizing the drivers of background tree mortality. Ecology 97, 2616–2627 (2016).
Piovesan, G. & Biondi, F. On tree longevity. N. Phytol. 231, 1318–1337 (2021).
Brienen, R. J. W. et al. Forest carbon sink neutralized by pervasive growth-lifespan trade-offs. Nat. Commun. 11, 4241 (2020).
Di Filippo, A. et al. The longevity of broadleaf deciduous trees in Northern Hemisphere temperate forests: insights from tree-ring series. Front. Ecol. Evol. 3, 46 (2015).
Liu, J. et al. Age and spatial distribution of the world’s oldest trees. Conserv. Biol. 36, e13907 (2022).
Locosselli, G. M. et al. Global tree-ring analysis reveals rapid decrease in tropical tree longevity with temperature. P. Natl Acad. Sci. USA 117, 33358–33364 (2020).
Xu, C. & Liu, H. Hydraulic adaptability promotes tree life spans under climate dryness. Glob. Ecol. Biogeogr. 31, 51–61 (2022).
Johnstone, J. F. et al. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 14, 369–378 (2016).
Anderegg, W. R. L., Trugman, A. T., Badgley, G., Konings, A. G. & Shaw, J. Divergent forest sensitivity to repeated extreme droughts. Nat. Clim. Change 10, 1091–1095 (2020).
DeSoto, L. et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 11, 545 (2020).
Au, T. F. et al. Younger trees in the upper canopy are more sensitive but also more resilient to drought. Nat. Clim. Change 12, 1168–1174 (2022).
Munné-Bosch, S. Limits to tree growth and longevity. Trends Plant Sci. 23, 985–993 (2018).
Mu, Y. et al. Size-focused conservation may fail to protect the world’s oldest trees. Curr. Biol. 33, 4641–4649 (2023).
Huang, L. et al. Human activities and species biological traits drive the long-term persistence of old trees in human-dominated landscapes. Nat. Plants 9, 898–907 (2023).
Lara, A. & Villalba, R. A 3620-year temperature record from Fitzroya cupressoides tree rings in Southern South America. Science 260, 1104–1106 (1993).
Büntgen, U. et al. Limited capacity of tree growth to mitigate the global greenhouse effect under predicted warming. Nat. Commun. 10, 2171 (2019).
Gora, E. M. & Esquivel-Muelbert, A. Implications of size-dependent tree mortality for tropical forest carbon dynamics. Nat. Plants 7, 384–391 (2021).
Rose, K. E., Atkinson, R. L., Turnbull, L. A. & Rees, M. The costs and benefits of fast living. Ecol. Lett. 12, 1379–1384 (2009).
Metcalf, C. J. E., Horvitz, C. C., Tuljapurkar, S. & Clark, D. A. A time to grow and a time to die: a new way to analyze the dynamics of size, light, age, and death of tropical trees. Ecology 90, 2766–2778 (2009).
Pavlin, J. et al. Disturbance history is a key driver of tree life span in temperate primary forests. J. Veg. Sci. 32, e13069 (2021).
Stephenson, N. L. et al. Causes and implications of the correlation between forest productivity and tree mortality rates. Ecol. Monogr. 81, 527–555 (2011).
Allen, C. D., Breshears, D. D. & McDowell, N. G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6, 129 (2015).
Körner, C. Plant adaptation to cold climates. F1000Res 5, 2769 (2016).
Stephenson, N. L. & van Mantgem, P. J. Forest turnover rates follow global and regional patterns of productivity. Ecol. Lett. 8, 524–531 (2005).
Kholdaenko, Y. A. et al. Stand density effects on tree growth and climatic response in Picea obovata Ledeb. plantations. For. Ecol. Manag. 519, 120349 (2022).
Willson, C. J., Manos, P. S. & Jackson, R. B. Hydraulic traits are influenced by phylogenetic history in the drought-resistant, invasive genus Juniperus (Cupressaceae). Am. J. Bot. 95, 299–314 (2008).
Adler, P. B. et al. Functional traits explain variation in plant life history strategies. Proc. Natl Acad. Sci. USA 111, 740–745 (2014).
Koch, G. W., Sillett, S. C., Jennings, G. M. & Davis, S. D. The limits to tree height. Nature 428, 851–854 (2004).
Ryan, M. G. & Yoder, B. J. Hydraulic limits to tree height and tree growth. BioScience 47, 235–242 (1997).
Matthes, U., Kelly, P. E., Ryan, C. E. & Larson, D. W. The formation and possible ecological function of stem strips in Thuja occidentalis. Int. J. Plant Sci. 163, 949–958 (2002).
Jarvis, P. & Linder, S. Constraints to growth of boreal forests. Nature 405, 904–905 (2000).
Rossi, S., Deslauriers, A., Lupi, C. & Morin, H. Control over growth in cold climates. In Trees in a changing environment: Ecophysiology, Adaptation, and Future Survival 191–219 (Springer, 2014).
Zhang, J. et al. Extended xylogenesis and stem biomass production in Juniperus przewalskii Kom. during extreme late-season climatic events. Ann. Sci. 77, 99 (2020).
Depardieu, C. et al. Adaptive genetic variation to drought in a widely distributed conifer suggests a potential for increasing forest resilience in a drying climate. N. Phytol. 227, 427–439 (2020).
Cole, L. E. S., Bhagwat, S. A. & Willis, K. J. Recovery and resilience of tropical forests after disturbance. Nat. Commun. 5, 3906 (2014).
McGregor, I. R. et al. Tree height and leaf drought tolerance traits shape growth responses across droughts in a temperate broadleaf forest. N. Phytol. 231, 601–616 (2021).
Bennett, A. C., McDowell, N. G., Allen, C. D. & Anderson-Teixeira, K. J. Larger trees suffer most during drought in forests worldwide. Nat. Plants 1, 15139 (2015).
Peng, C. et al. A drought-induced pervasive increase in tree mortality across Canada’s boreal forests. Nat. Clim. Change 1, 467–471 (2011).
Girard, F., De Grandpré, L. & Ruel, J.-C. Partial windthrow as a driving process of forest dynamics in old-growth boreal forests. Can. J. Res. 44, 1165–1176 (2014).
Frelich, L. E., Montgomery, R. A. & Reich, P. B. Seven ways a warming climate can kill the Southern boreal forest. Forests 12, 560 (2021).
Gazol, A. et al. Forest resilience to drought varies across biomes. Glob. Change Biol. 24, 2143–2158 (2018).
Magney, T. S. et al. Mechanistic evidence for tracking the seasonality of photosynthesis with solar-induced fluorescence. Proc. Natl Acad. Sci. USA 116, 11640–11645 (2019).
Stephenson, N. L. et al. Rate of tree carbon accumulation increases continuously with tree size. Nature 507, 90–93 (2014).
Cannon, C. H., Piovesan, G. & Munné-Bosch, S. Old and ancient trees are life history lottery winners and vital evolutionary resources for long-term adaptive capacity. Nat. Plants 8, 136–145 (2022).
Bunn, A. G. A dendrochronology program library in R (dplR). Dendrochronologia 26, 115–124 (2008).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. (2021).
Crepet, W. L. & Niklas, K. J. Darwin’s second “abominable mystery”: Why are there so many angiosperm species? Am. J. Bot. 96, 366–381 (2009).
Beck, H. E. et al. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 5, 180214 (2018).
Zomer, R. J., Xu, J. & Trabucco, A. Version 3 of the global aridity index and potential evapotranspiration database. Sci. Data 9, 409 (2022).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005).
Fischer, G. et al. Global agro-ecological zones assessment for agriculture (GAEZ 2008). IIASA, Laxenburg, Austria and FAO. Rome, Italy. (2008).
Wildlife Conservation Society-WCS & Center for International Earth Science Information Network-CIESIN-Columbia University. Last of the Wild Project, Version 2, 2005 (LWP-2): Global Human Influence Index (HII) Dataset (Geographic). NASA Socioeconomic Data and Applications Center (SEDAC, 2005).
Potapov, P. et al. Mapping global forest canopy height through integration of GEDI and Landsat data. Remote Sens. Environ. 253, 112165 (2021).
Kaplan, J. O. & Lau, K. H.-K. The WGLC global gridded lightning climatology and time series. Earth Syst. Sci. Data 13, 3219–3237 (2021).
NASA VIIRS Land Science Team. VIIRS (NOAA-21/JPSS-2) I Band 375 m Active Fire Product NRT (Vector data). NASA LANCE MODIS at the MODAPS (NASA, 2021).
Fritts, H. C. Tree rings and climate. (Blackburn Press, 2001).
Hughes, M. K., Swetnam, T. W. & Diaz, H. F. Dendroclimatology: progress and prospects. (Springer, 2010).
Buras, A. A comment on the expressed population signal. Dendrochronologia 44, 130–132 (2017).
Lloret, F., Keeling, E. G. & Sala, A. Components of tree resilience: effects of successive low-growth episodes in old ponderosa pine forests. Oikos 120, 1909–1920 (2011).
Becker, M., Nieminen, T. M. & Gérémia, F. Short-term variations and long-term changes in oak productivity in northeastern France. The role of climate and atmospheric CO2. Ann. Sci. 51, 477–492 (1994).
Acknowledgements
This work was funded by the National Natural Science Foundation of China (42425101 and 42301058), the China Postdoctoral Science Foundation (2023M730602), Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (GZC20230450) and Fujian Institute for Cross-Straits Integrated Development (LARH24JBO7). We thank all contributors to the ITRDB to make this analysis feasible. We thank Yu Zhou, Hongling Yang, and Hang Xing for their assistance with data collection and analysis.
Author information
Authors and Affiliations
Contributions
J.G. and K.F. conceived the research, J.G., K.F., and L.J. designed the study, J.G. and R.S. performed the data analyses. J.G. produced the figures and led the writing. J.G., K.F., C.J.M., L.J., R.S., C.D., L.H.W., C.J.J., E.J., D.N.K., A.T.F., and G.Z. contributed to the interpretation of the results and the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Pieter Zuidema and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Mengjie Wang. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, J., Fang, K., Chen, J.M. et al. Climate-driven patterns of global tree longevity. Commun Earth Environ 6, 610 (2025). https://doi.org/10.1038/s43247-025-02609-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-025-02609-2
This article is cited by
-
Contrasting pathways to tree longevity in gymnosperms and angiosperms
Nature Communications (2025)