Abstract
Aluminum-rich clay minerals are detected across the ancient surface of Mars and record intervals of intense alteration by liquid water. On Earth, these clay minerals can form from hydrothermal alteration or rainfall-driven chemical weathering over thousands to millions of years, but how they formed on Mars remains a mystery. The Perseverance rover discovered light-toned, cobble-sized, aluminum-rich (30-45 wt% Al2O3) “float” rocks (rock fragments), with some exhibiting spectral signatures of kaolinite, an aluminum-rich clay mineral. These rocks now enable an investigation into the ancient kaolinite-bearing terrains of Mars. To interpret their formation, we use data from the SuperCam and Mastcam-Z instruments onboard the rover to compare the chemistry and reflectance spectra of the float rocks with deeply weathered paleosols and hydrothermal kaolin deposits from Earth’s geological record. Aluminum and titanium enrichments coupled with depletion of iron and magnesium are unlike hydrothermal deposits and instead comparable to bleached horizons of paleosols that formed under high rainfall during past greenhouse climates on Earth. These rocks therefore likely represent some of the wettest intervals of Mars’ history.
Similar content being viewed by others
Introduction
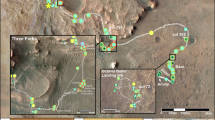
The Mars 2020 Perseverance Rover has discovered several thousand small, light-toned rock fragments, or float rocks, that are spectrally and compositionally distinct from outcrops so far observed on the mission1 (Fig. 1). This unique class of float rock was first observed on the Jezero Crater floor and later on the top of the Jezero sedimentary fan where pebble- to boulder- size clasts were identified2, and so far, a total of ~20 of these light-toned float rocks have been investigated in detail with the SuperCam and Mastcam-Z instruments.
Red arrows in E-J indicate analyses by SuperCam confirming the aluminum-rich ( > ~30 wt% Al2O3) nature of float rocks. A HiRISE Map of Jezero crater showing locations where light-toned float rocks were observed; B Crater floor example (sol 72, zcam 08033); C Crater floor example (sol 388, zcam 08416); Jezero sedimentary fan examples (D sol 711, zcam 08715; E “Dolgoi Island” sol 657, zcam 03505; F “Egegik” sol 662, zcam 03509; G“Unga Island” sol 676, zcam 03517; H “Ouzinkie” sol 691, zcam 03530). Jezero fan top examples (I “Trayfoot Mountain; sol 704, zcam 08704; J “Finch Lake” sol 774, zcam 03625) All images of float rocks are Mastcam-Z enhanced color images (red=630 nm, green=540 nm; blue=480 nm).
A large variety of light-toned float rocks were identified throughout the rover traverse (Fig. 1) ranging from light-to-dark toned, pebble-to-boulder-sized rocks. The anomalous chemistry of the float rocks was first identified by their unique aluminum (Al)-rich compositions as determined by the SuperCam instrument1. A large population was identified at the toe of the western Jezero sedimentary fan (Fig. 1E–H) and again near the ~1-km-wide “Belva crater” on the top of the fan (Fig. 1J). Around mission sol 912, Perseverance encountered several large lag piles (tens of square meters) containing thousands of pebble to cobble-size light-toned float rocks (Fig. 2A), with many examples exhibiting distinct spectral properties in near-infrared spectral wavelengths, likely indicating major differences in composition (Figs. 2B, C, 3).
A Mastcam-Z enhanced color landscape image (R = 630 nm), G = 544 nm), B = 480 nm) showing large pile/lag of pebble to cobble-sized light-toned float rocks (Sol 912, zcam 08919); B Mastcam-Z multispectral image “Lake Blanche” (Sol 913, zcam 03768); showing near-infrared wavelength decorrelation stretch (R = 1022 nm, G = 910 nm, R1 = 800 nm); C Near-infrared wavelength decorrelation stretch of “Learmonth” (sol 920, zcam 03775). Note location for C is not pictured in A.
These targets are the focus of this study. Left to right: Mastcam-Z multispectral images of targets “Chignik” (Sol 680, zcam 03523), “Elk Mountain (Sol 895, zcam 03750), and “Coral Bay” (Sol 920, zcam 03777). Top row: Mastcam-Z natural color images; Second row: Mastcam-Z enhanced color images (red = 630 nm, green = 540 nm; blue = 480 nm); Third row: Mastcam-Z near-infrared wavelength decorrelation stretch (red = 1022 nm; green = 910 nm; blue = 800 nm); bottom row: SuperCam Remote Micro-Imager products showing laser-induced breakdown spectroscopy (LIBS) rasters over Al-rich float rock targets.
A striking discovery was that some of the light-toned rocks have a diagnostic spectral signature of a hydrated kaolin group mineral (e.g., kaolinite, halloysite)1 and are enriched in Al2O3 (~17–35 wt%) (e.g., “Chignik”, Fig. 3) with anomalously low total Fe ( < 1 wt% FeOT, total iron as FeO) compared to other float rocks2. Some of these kaolin-bearing Al-rich rocks also have elevated TiO2 content ( > 1 wt%), indicating Ti within primary and/or or secondary mineral phases. Others show spectral evidence of a dehydrated Al-rich phase and spinel-group minerals, as well as Al2O3 to ~45 wt% and over 1 wt% Ni, (e.g., targets “Dolgoi Island”, “Finch Lake”, Fig. 1E–J), representing ore-grade enrichment of Ni3 (Figs. S1–S2). Some, but not all of the light-toned float rocks are rich in Al; others, such as target AEGIS 910 A (Sol 910), are composed of >90 wt% SiO2 and have a VISIR signature consistent with hydrated silica4.
Deciphering the origin of these float rocks is challenging because they are fully removed from their geological context. However, based purely on the geochemistry of the rocks, it is clear that these materials once experienced intense aqueous alteration1, which could include hydrothermal and/or pedogenic alteration prior to lithification and transport into Jezero crater2. For example, Al enrichments exceeding ~30 wt% coupled with low total Fe are typical of deep weathering profiles5,6,7,8 (Ultisols and Oxisols in US soil taxonomy)9. Specifically, the leaching of Fe2+ from a fluctuating water table and the subsequent loss of total iron (e.g., FeOT > ~1 wt%) contributes to formation of the light-toned, kaolinite-bearing, several -m-thick “pallid zone” in portions of these deep weathering profiles6,10,11. This can occur under diverse chemical conditions including an oxic and slightly acidic, or anoxic/strongly acidic water table12. Another possibility is aqueous alteration of a highly felsic protolith which may not require a lateritic type of weathering13. Ni enrichments > 1 wt% seen in several (but not all) Al-rich float rocks at Jezero crater (e.g., Dolgoi Island, Finch Lake) could also be consistent with deeply weathered soils formed on an ultramafic protolith such as serpentinite or dunite9 (Figs. S2–S3).
Production of pedogenic kaolinite on Mars during the Noachian ( ~ 4.1–3.7 Ga)14,15 has been inferred from orbital observations of phyllosilicate-bearing outcrops that are mineralogically and topographically consistent with weathering profile(s) formed from precipitation-driven pedogenic leaching16. One hypothesis for some of these rocks is that they are pieces of these deep weathering profiles that were later altered by different degrees of heating, possibly from impact1.
Alternatively, these rocks could represent hydrothermal systems rather than a deep weathering profile. On Earth, hydrothermal production of kaolinite tends to occur during extensive subsurface fluid flow through parent rock, typically at temperatures between ~50°−250 °C, with fluids commonly derived from magmatic or geothermal systems17. Hydrothermal alteration of the Martian surface has also been documented by orbital remote sensing18, so it is possible that some of the Jezero Al-rich rocks formed as a result of hydrothermal alteration1.
Although these rocks represent the first clear in-situ detection of kaolin-group minerals by a rover1,19, decades of orbital remote sensing have demonstrated the global abundance of Al-rich clays in outcrops across equatorial to subequatorial latitudes of Mars15,20 dating from the Noachian to early Hesperian21, including locations proximate (tens of kilometers) to Jezero crater22. Al-rich clay minerals have also been identified from orbit within the western Jezero crater rim itself, as well as nearby in incised outcrop exposed within the final meanders of Neretva Vallis23 (Fig. S4). These fortuitous discoveries of Al-rich float rocks by Perseverance now enable initial investigation of kaolinite-bearing terrains of early Mars previously observed only from orbit.
Comparisons with terrestrial analogs (similar materials from Earth) are necessary to explore the possible alteration history of these unique Martian rocks. To begin contextualizing these rocks with analog materials from Earth, we compare the geochemistry and spectral properties of hydrated Al-rich Jezero float rocks to deeply weathered materials from Earth. These include paleosols (ancient, lithified weathering profiles) and ancient hydrothermal kaolin deposits. We hypothesize that some of these float rocks are geochemically and spectrally similar to terrestrial materials that experienced intense and prolonged pedogenic alteration under chemically reducing conditions, followed by burial and lithification. Lastly, to enable future exploration of the source outcrop(s) of this material, we use orbital observations to identify locations in and around Jezero crater from where these rocks could have originated.
Methods
We used images and data from the SuperCam24,25,26 and Mastcam-Z27 instruments onboard Perseverance rover. These included the SuperCam’s laser-induced breakdown spectrometer (LIBS) for major element composition of float rocks (Table S1), visible to near-infrared (VISIR) spectrometer (for constraining qualitative mineralogy and bulk hydration state), and Remote Micro-Imager (RMI) for the highest-resolution remote images. Diagenetic features including rock coatings were excluded from LIBS and VISIR analyses (Supplementary Information). The typical root mean square error of prediction for SuperCam LIBS analysis (in wt%) are: CaO (1.3), FeO (3.1), Na2O (0.5), K2O (0.6), MgO (1.1), SiO2 (6.1), and TiO2 (0.3)28. Mastcam-Z multispectral images were also used to examine the spectral diversity and geologic context of these float rocks. We focused on the “hydrated” class of light-toned, Al-rich float rocks from Royer et al.1, which are characterized by elevated Al2O3 (~20–40 wt%), a ~ 2.21 µm Al-OH band, and the presence of a ~ 1.9 µm hydration band in the IR spectra1. We selected this class since these rocks retain hydration bands and thus may represent targets that have been only minimally altered by later heating related to burial or impact processes1. SuperCam LIBS data for other Al-rich classes of Jezero float rocks (low-hydration and spinel-bearing) are provided in the Supplementary Information and can also be found in Royer et al., 20241. For these comparisons, we included the hydrated Al-rich targets “Chignik” (Sol 680/681); “Rainbow Curve” (Sol 777), “Elk Mountain” (Sol 895) and “Coral Bay” (Sol 924). These targets were remotely observed by both the SuperCam and Mastcam-Z instruments (Fig. 2), but due to their small size (up to ~10 cm) and engineering constraints, no proximity/contact science was conducted with the PIXL or SHERLOC instruments.
We compared SuperCam LIBS observations of composition with previously published geochemical data from terrestrial kaolinite-bearing paleosols and hydrothermal kaolin deposits (Supplementary Information). These comparisons focused on early Eocene (55 Ma) kaolinitic Ultisol paleosols from Blacks Beach, La Jolla, San Diego, CA that formed on mafic to felsic conglomerate, with estimated mean annual precipitation of 1500 ± 299 mm29 and likely only exposed to low temperatures ( < 60–80 °C) during burial diagenesis. We also considered previously published chemical data from drill cores of the Paleoproterozoic ( ~ 2.2 Ga) Hekpoort paleosol in present-day Botswana and South Africa6,30,31, selecting samples from the bleached, low-Fe, several-m-thick “pallid zone” of this well-studied Paleoproterozoic paleosol formed on basaltic protolith30. These pedogenic samples were likely subject to comparably more heating ( ~ 250 °C) during burial diagenesis31. For geochemical comparisons with ancient hydrothermal (hypogene) kaolin deposits from Earth, we used three previously published datasets from hypogene kaolin deposits in Iran32, Malaysia33 and Argentina34 spanning a range of inferred alteration temperatures and protoliths (n = 21 total). The Iran site near Mianeh formed from a parent material of andesite and andesitic tuff during the Oligocene-Miocene at temperatures ranging from 150–240 °C32, whereas the Malaysia sites near Gunung Jerai formed in felsic intrusive rocks (granites and pegmatites) during the Triassic at 94–113 °C33. The Argentina site near Rio Negro, Patagonia formed in mafic to felsic volcanic rocks and ignimbritic tuffs with some clastic sediments at temperatures up to 350 °C during the Triassic- mid Jurassic and are capped by pedogenic or groundwater silcrete34 All data are included as Supplementary Information.
Comparisons of spectral properties between the early Eocene (55 Ma) San Diego paleosol and LIBS VISIR spectra of the “Chignik” target are presented in the short wave-infrared (IR; 1.3–2.5 µm) wavelengths. Methods for operation and calibration of SuperCam’s IR spectrometer can be found in ref. 24,25,26,35. As of mission sol ~1000, Chignik was the only Al-rich float rock with a strongly kaolin-like IR spectra (see Royer et al.1 for more information). No IR reflectance data were available for the Paleoproterozoic Hekpoort paleosol, but even in the case of their availability, direct spectral comparisons with the Paleoproterozoic samples are complicated by burial diagenesis that dehydrated and transformed many of the pedogenic clay minerals and other hydrated phases in these samples30,36.
To contextualize the in-situ detections and explore possible source outcrops for the Al-rich float rocks, we used orbital VISIR (0.35–2.6 µm) reflectance data from the Compact Reconnaissance Imaging Spectrometer for Mars (CRISM)37, which acquires spectral images with spatial scales ~18–200 m/pixel, and searched for the diagnostic signatures of kaolin-group clay minerals (Fig. S1), similar to how the SuperCam VISIR spectrometer detects these clay minerals ( ~ 2.17/2.21 µm bands). To identify putative sources of kaolin-group minerals, we used CRISM High-resolution targeted observations ( < 40 m/pixel) of regions in and around Jezero crater, with CRISM mineral detections projected onto Global Context Camera (CTX) basemap images38. These images cover the Jezero crater and Nili Planum region and were processed using the MOCAAS (Mars Orbital Catalog of Aqueous Alteration Signatures) methodology23, which also includes observations from the Observatoire pour la Minéralogie, l’Eau, les Glaces, et l’Activité (OMEGA) aboard Mars Express. To identify areas of Al clay minerals, large-scale mapping of Al clay distribution was conducted using a global high-resolution map (200 m/pixel) of aqueous minerals from MOCAAS following the methodology of Carter et al.15. The maps classify minerals into five spectral groups including Fe/Mg phyllosilicate clay minerals, Al clays and hydrated silicates, polyhydrated sulfates, monohydrated sulfates, and carbonates23. To investigate potential sources within the Neretva Vallis channel, we used CRISM multispectral data products projected onto CTX basemap images with coverage over the westernmost Neretva Vallis Channel (coverage of this region is not available for full-resolution targeted images). These multispectral Al clay maps were also processed with the MOCAAS methodology, though are associated with larger amounts of uncertainty compared to the high-resolution targeted detections (e.g., 23). These CRISM multispectral data products are included as Supplementary Information (Figs. S4–S5) and a table of all CRISM observations used is included as Table S2.
Results
Spectral and geochemical similarities with intensely weathered paleosols from Earth
The early Eocene (55 Ma) San Diego paleosol samples appear spectrally similar to Chignik (Jezero crater) with features diagnostic of kaolin-group minerals39. Similarities in band position and depth for kaolin-diagnostic features (e.g., presence of a ~ 2.17/2.2 µm doublet) were identified (Fig. 4, Figure S1). Unlike comparisons with pure laboratory standards of kaolinite or halloysite20, the paleosols represent a complex natural mixture with contributions from several different mineral phases29. These mixed-phase terrestrial samples are spectrally similar to the float rocks, which are presumably also composed of a complex mixture of secondary minerals and represent a continuum of Al-rich clay minerals produced from alteration. The main differences in the Chignik target are the loss of contrast of the 2.17 µm doublet feature, the shift of the 1.9 µm feature toward longer wavelengths (possibly from an additional phase like sulfate or Fe-hydroxide), and the addition of a 1.6 µm feature that is likely a residual of atmospheric CO2. Spectral features between 1.9 and 2.1 µm in Chignik are a result of atmospheric corrections to SuperCam IR spectra and thus represent artefacts of processing35. Spectral differences in Chignik versus lab spectra of kaolin minerals could also be due to dehydration, possibly resulting from heating40. The excellent spectral match between the Mars and terrestrial samples allows for consideration of paleosols as a candidate for more in-depth comparison of elemental composition.
A SuperCam RMI showing VISIR raster on Chignik; B Erosional remnant of San Diego paleosol showing truncated E-horizon where VISIR spectra were collected; C Infrared comparisons between Chignik and San Diego paleosol samples. The kaolinite/halloysite diagnostic doublet feature at ~ 2.17 µm/2.2 µm is annotated, and laboratory kaolinite reference spectra are shown in Fig. S1.
The major elemental compositions and inferred weathering intensities were compared between Jezero float rocks and analog samples by plotting each on a molar “A-CN-K” (Al2O3 -CaO+Na2O-K2O) ternary diagram to assess the degree of weathering (Chemical index of alteration, CIA)5, as well as an “A-CNK-FM” (Al2O3 – CaO+Na2O + K2O – FeOT + MgO) ternary diagram, a technique commonly used to constrain the weathering intensity of deeply altered mafic materials where reducing conditions during leaching led to loss of Fe41. To assess the degree of oxidative weathering of mafic materials, we also plotted samples on a “AF-CNK-M” (Al2O3+ FeOT – CaO+Na2O + K2O – MgO) ternary diagram41. The chemical index of alteration (CIA) of the San Diego samples ranges from ~89–9829, while Chignik ranges from ~65–871. The San Diego samples plot in a comparable location on the molar A-CNK-FM ternary, indicating a mafic index of alteration for chemically reducing scenarios (MIAR) of 58–75, comparable to Chignik (MIAR of 35–80) and a similar trend towards loss of Fe and Mg. The index of laterization (IOL) of the bleached E- horizon of the San Diego sample was 25, suggesting kaolinization (Fe loss) rather than laterization (Fe gain)41, and is similar to the light toned regions of Chignik with IOL values ranging from ~14–271. These low IOL values in Chignik also support the hypothesis of kaolinization rather than laterization.
The terrestrial hydrothermal kaolin samples (n = 21) generally plotted at the apex of the A-CN-K ternary and had CIA > 95, unlike the lower values observed in the Jezero float rocks (Fig. 5A). In ternary diagrams for mafic index of alteration under oxidizing and reducing scenarios (AF-CNK-FM and A-CNK-FM, respectively) nearly all of the hydrothermal kaolin samples plot near the apex (n = 19), indicating extreme depletion of soluble cations and MIAO / MIAR ~ 98.
A A-CN-K ternary diagram with the chemical index of alteration (CIA) projected showing early Eocene (~55 Ma) kaolinitic paleosols from San Diego (55 Ma, n = 7) and Paleoproterozoic (~2.2 Ga) “Hekpoort” paleosol from South Africa (n = 21), hydrothermal kaolin samples from three localities in Iran, Malaysia, Argentina (n = 21 total) and comparisons with Chignik (n = 9), Rainbow Curve (n = 5), Elk Mountain (n = 10), and Coral Bay (n = 3) targets from Jezero Crater, Mars; B A-CNK-FM ternary diagram with the mafic index of alteration (MIA) for chemically reducing scenarios (MIA(R)); C AF-CNK-M ternary diagram integrated with the mafic index of alteration for chemically oxidizing scenarios (MIA(O)). Inset panels show lithified hand samples of the Eocene San Diego paleosol (Fig. 4A, point #1) and the sericite zone of the Paleoproterozoic Hekpoort paleosol30.
Perhaps a more striking comparison is with drill core samples from the Paleoproterozoic ( ~ 2.2 Ga) Hekpoort paleosol30, where points from all four of the hydrated Jezero Al-rich float rocks overlap with those from the ~3- meter-thick kaolinitic, bleached, low Fe ( ~ 1–8 wt%), high Al ( > 30 wt%) “pallid zone” of the weathering profile6. These similarities are seen in both ternary diagrams representing A-CN-K and A-CNK-FM for reducing conditions (Fig. 5A, B) and are most apparent with Chignik/Elk Mountain and the Hekpoort paleosol, with several points in the A-CNK-FM (Fig. 5B) plotting atop one another. Less overlap is seen in the MIA(o) ternary (AF-CNK-M) (Fig. 5C), indicating that the Jezero samples experienced less oxidative weathering compared to analog pedogenic samples. By comparison, the extreme depletion of soluble cations characteristic of terrestrial hydrothermal kaolin deposits from Iran, Malaysia and Argentina across a range of estimated alteration temperatures (Fig. 5A–C) are overall unlike the less deeply weathered, though comparably Al-rich, Jezero float rocks.
TiO2 content of Al-rich Jezero float rocks
Titania (TiO2) can serve as a tracer of alteration processes in materials that were subject to chemical alteration. The SuperCam LIBS points from Chignik, Coral Bay and Elk Mountain (minus coatings/ diagenetic features) (n = 22) have TiO2 ranging from ~0.3–2 wt% (Fig. 6; Table S1). Other Al-rich float rock targets described in Royer et al.1 (Dolgoi Island, Unga Island, Finch Lake, Ouzinke) are shown for comparison.
A Boxplot of TiO2 within Chignik, Elk Mountain, Rainbow Curve and Coral Bay targets (diagenetic features/coatings not included); B Comparison with other Al-rich targets from Royer et al1, also shown in Fig. 1 (Dolgoi Island [sol 657], Unga Island [sol 676], Finch Lake [sol 774], and Ouzinke [sol 691]). The orange line represents the median, boxes show quartiles 1-3, the whiskers represent 1.5 times the interquartile range, and lone dots are outliers. Color gradient indicates general value (~1 wt% TiO2) used to distinguish pedogenic versus hydrothermal alteration regimes in Earth analog kaolin deposits and paleosols8,42,45.
Chignik (n = 10) contains the highest TiO2 content of the sample set, with mean and median TiO2 of 1.2 wt% and 1.4 wt%, respectively (standard deviation of 0.36) (Fig. 6; Table S1). By comparison, Elk Mountain (n = 10) and Coral Bay (n = 3) have lower median TiO2 (0.3 and 0.6 wt% TiO2, respectively). Unga Island (n = 10) and Ouzinke (n = 10) have median TiO2 ~ 0.7, whereas Dolgoi Island (n = 10) and Finch Lake (n = 10) have median TiO2 ~ 1 wt%. Several LIBS points on the Al-rich float rocks had TiO2 values that exceeded the range of SuperCam LIBS TiO2 calibration used for the determination of major oxide components28 (e.g., ~3 wt. TiO2 % in a LIBS point on Chignik). However, all of the estimated SuperCam LIBS TiO2 values, including those mentioned above, fell within the ranges of those of the terrestrial samples.
For comparison, the terrestrial pedogenic samples across mafic to felsic protoliths also have elevated TiO2 relative to hydrothermal samples (Supplementary Information). The basaltic Hekpoort paleosol (n = 21) has mean and median TiO2 of 1.48 wt% and 1.52 wt%, respectively. This TiO2 range is highly comparable to Chignik (Fig. 6). The feldspathic San Diego paleosol (n = 7) has mean and median TiO2 of 0.72 wt% and 0.69 wt%, respectively. By contrast, all of the terrestrial hydrothermal samples (n = 21) from three localities across a range of protoliths had lower mean and median TiO2 (0.28 wt% and 0.14 wt%, respectively) (Fig. 6).
Elevated Ti in other classes of Al-rich float rocks such as the “spinel class” (Dolgoi Island, Ouzinkie, Finch Lake) (Fig. 6) could reflect Ti in primary mineral phases (e.g., spinel)1 rather than formation of secondary Ti phases, so caution is necessary when using Ti content to infer alteration environment for the full set of Jezero Al-rich float rocks.
Orbital detections of kaolin-group minerals in and around Jezero crater
To gain an understanding of the possible locations where Al-rich float rocks such as Chignik could have once originated, we used CRISM high-resolution targeted images to identify locations with a candidate kaolin-group mineral signature. At Jezero crater, many outcrops with a kaolin-group mineral signature have a light-toned, patchy, fractured appearance22,23. Previous identifications of kaolinite/halloysite in the SW crater rim are associated with light-toned, layered megabreccia blocks that span approximately 100 m222,23. The CRISM high resolution images show kaolin clay detections on the Jezero crater rim that are approximately 1-2 km from the current rover traverse (as of sol ~1000). A small ( ~ 75 m2) area of kaolin detections is identified as the closest location to the rover traverse (Fig. 7B). When correlating the CRISM detection with HiRISE images, the candidate kaolin areas appear as patchy light-toned outcrop at the base of NW-SE trending ridges (Fig. 7C). Additional larger areas of detections shown in Fig. 7 in the SW Jezero crater rim are associated with comparably rougher terrain inferred from the CTX image basemaps. In contrast to smaller exposures on the western crater rim (Fig. 7B), examination of several candidate kaolin-bearing outcrops exposed in the final meander of the Neretva Vallis channel due west of Jezero crater shows laterally continuous, light-toned outcrop exposed over ~500 meters (Fig. S5).
A CRISM targeted high-resolution detections of kaolin clay minerals across the Jezero crater region; B Zoom of the Jezero sedimentary fan and SW crater rim. The green circle at center highlights a likely kaolin mineral detection in FRS00031442 at a location on the crater rim of Jezero and shows the location of the Perseverance rover traverse (as of sol ~ 1000); C Kaolin mineral detections in FRS00031442 correlate with patchy light toned features on the surface. Subframe of HiRISE image ESP_037119_1985_COLOR; D Example spectra extracted from CRISM FRS00031442 from location on Jezero crater rim shown in B/C) and comparison with laboratory kaolinite23.
Discussion
Although the Jezero rocks are removed from their outcrop, a major result of these comparisons are the geochemical similarities to deeply weathered Paleoproterozoic paleosols once formed from precipitation-driven weathering of mafic rocks. Geochemical dissimilarities with ancient terrestrial hydrothermal kaolin deposits across a range of parent materials and alteration temperatures indicate that the hydrated class of float rocks may have experienced overall less alteration, or alteration at lower temperatures, compared to these materials on Earth. In the following section, we outline distinct lines of evidence supporting or refuting the pedogenic or hydrothermal hypotheses for the alteration history of the float rocks.
Distinguishing pedogenic versus hydrothermal kaolinite
On Earth, either pedogenic (supergene) or hydrothermal alteration (hypogene) can form altered material containing both kaolinite and low Fe content7,42 and distinguishing between the two often requires the geological context provided by outcrop. Pedogenic kaolinite is often formed across laterally extensive stable land surfaces (e.g., hundreds to thousands of meters along strike) or can be eroded and transported to be deposited in horizontal beds at similar scales. By contrast, hypogene kaolinite is often present in more localized networks of veins that represent subsurface fluid flow (typically <100 m wide)7,42,43,44.
Geochemical comparisons with terrestrial kaolin deposits indicate that pedogenic kaolinite can be distinguished from hydrothermal kaolinite by considering bulk TiO2 content, primarily in cases where the protolith(s) are well-constrained45. In general, pedogenic kaolin deposits from Earth typically have TiO2 greater than ~1 wt% due to decreased Ti mobility (relative to other elements) under low temperature ( ~ 25 °C) pedogenic alteration conditions17,42,45,46. Hydrothermal kaolin deposits formed across a range of higher alteration temperatures ( ~ 100–350 °C) do not concentrate Ti to the same degree as pedogenic examples, and typically have TiO2 of approximately 0.5 wt% or less17,42.
However, caution is necessary when using Ti to infer process in cases where the protolith is unknown, because the starting abundance of Ti is a critical constraint33. Further, Ti mobility is related to pH, such that Ti is more readily leached at pH <47. For example, TiO₂ is often higher in pedogenic kaolin because titanium is immobile during weathering, leading to residual enrichment, especially when starting from Ti-poor felsic protoliths where Ti increases as other elements are leached. In contrast, hydrothermal kaolin forms in systems where Ti can be mobile; even if the protolith is Ti-rich (e.g., basalt), alteration may remove Ti so the final kaolin has contents below protolith levels33.
Chignik (n = 10) shows the highest Ti content of the float rocks examined here, with mean and median TiO2 of 1.2 wt% and 1.4 wt%, respectively (standard deviation of 0.36) (Fig. 6; Table S1) along with overlap in composition with a well-characterized, deeply weathered terrestrial paleosol (Fig. 5). When considering TiO2 of all LIBS shots across all Al-rich Jezero float rocks analyzed in Royer et al.1 (n = 118), TiO2 averages 0.9 wt% with a median value of 0.7 (standard deviation of 0.45) (Table S1). Such overall elevated values for Ti could be consistent with some amount of pedogenic kaolinization17,42, but as indicated earlier, elevated Ti estimates could also in part be due to some of the points exceeding the SuperCam LIBS TiO2 calibration28, or, alternatively, a mafic protolith (e.g., basalt) initially rich in Ti. Of the sample set, Chignik stands as distinct from the others because it appears to be the least altered by heat (retention of 1.9 µm and 2.16/2.2 µm features, Fig. 4), so geochemical comparisons with terrestrial paleosols and hydrothermal deposits may be more trustworthy than with other Jezero samples that could have been subject to heating or other unknowns.
The Si-rich float rocks also observed by Perseverance likely formed via hydrothermal fluids4, and thus also might be related to the Al-rich float rocks examined here. For example, the AEGIS 910 A target might represent chemical precipitates from the olivine-dissolving fluids associated with the alteration in Jezero crater and on the Jezero crater rim4. It is therefore possible that the Si-rich float rocks are associated with some of the Al-rich float rocks described in this study, though the geologic context of both materials is currently unknown.
Comparisons with terrestrial laterite
Our analyses suggest that the aluminum-rich Jezero float rocks might represent prolonged pedogenic weathering of rock or sediment (Fig. 5B). This raises the possibility that the float rocks may have experienced weathering akin to terrestrial laterite, which is rock or sediment subject to deep, strongly oxidative pedogenesis47,48, where similar enrichments in Al and Ti are common47,49. In cases of an ultramafic protolith, elevated Ni is also common. Detections of elevated Ni likely > 1 wt% in some targets (Coral Bay, Unga Island, and Finch Lake)3 (Fig. S2–S3) but not others (e.g., Chignik) could be consistent with intense pedogenic alteration of an ultramafic protolith under oxidizing conditions, akin to terrestrial Ni laterite49. However, the depletions of Mg and Fe observed in the float rocks are overall dissimilar to the Fe/Mg-rich saprolite layer of ultramafic Ni laterites from Earth’s geological record, where Ni is either concentrated in veins or diffused within Fe/Mg clay minerals and oxides49. Likewise, the samples from the mafic, Ni-poor Hekpoort paleosol used in our comparisons have low Ni ( ~ 0.05 wt%), high Al ( ~ 30 wt%) and Ti (1–2 wt%), with low Fe/Mg/Mn ( < ~2 wt%)30, unlike Ni-rich laterite from Earth47,49.
It is possible that the “hydrated class” of Jezero float rocks, such as Chignik, are related to the Ni-bearing “spinel class”1 of Jezero float rocks, but the hydrated class does not show the same potential Ni abundances as the spinel class ( > 1 wt%; e.g., Finch Lake; Fig. S3). One possibility is that the float rocks represent a continuum of material derived from a single, or more likely multiple eroded Noachian paleosol profiles of mafic, ultramafic and/or felsic composition. In this way, elemental trends in Al, Fe, Ti and Mg relate to protolith, climate, position/horizon in weathering profile, diagenetic alteration, or other unknowns. Protoliths including felsic material are possible21, but elevated Ni to ore-grade enrichment ( > 1 wt%) is unique to deeply weathered ultramafic parent material, perhaps similar to a Ni-Cu sulfide protolith3,50,51.
Differences between Jezero float rocks and analog samples
There are several major differences between the Jezero float rocks and Earth analog samples, such as trends in total iron and potash (FeOT and K2O) abundance. The analog hydrothermal kaolin samples are depleted in K2O and FeOT relative to pedogenic samples, which could be related to protolith composition, burial diagenesis such as potash metasomatism36, or other unknowns. As indicated in Fig. 5, the elemental compositions of the analog hydrothermal samples are overall dissimilar to the hydrated class of Al-rich float rocks at Jezero. Another difference is that the Jezero Al-rich float rocks are enriched in CaO and Na2O relative to all of the analog samples, which may be related to the above factors, or alternatively, indicate overall less chemical alteration relative to analog samples (e.g., lower chemical index of alteration (Fig. 5A).
The late diagenetic history of the Jezero float rocks also currently remains unclear. For example, the close geochemical similarities with the Paleoproterozoic (2.2 Ga) Hekpoort paleosol are a challenging result to reconcile because those terrestrial samples were likely subject to comparatively more burial diagenesis than currently known Mars examples (e.g., late diagenetic chlorite, sericite and pyrophyllite indicate temperatures > ~250 °C in the “pallid zone”30,31). Unlike the more pristine Chignik target with its preserved kaolinite-like signature (Fig. 4), hydrated Al clay minerals in the Hekpoort paleosol were transformed to higher temperature phases during deep burial diagenesis31. It is hypothesized that these later mineralogical transformations only minimally influenced the original elemental chemical composition of the weathered soil after it was buried and lithified6,30. This phenomenon is common in terrestrial examples of well-lithified, clay-rich paleosols36, which are thought to minimize later diagenetic fluid flow via reduced hydraulic conductivity52.
Subtle spectral differences are also apparent between Chignik and the terrestrial samples. For example, the loss of contrast of the 2.17 µm feature (Fig. 4) could be due to a less crystalline kaolinite or a mixture of kaolinite with Al-smectite40. Furthermore, the 2.2 µm Al-OH band can change due to exposure to heat53, so the subtle differences in band position and depth could also be associated with heat-induced alteration. Shock metamorphism has also been shown to reduce crystallinity and ordering, and is manifested by the doublet band losing its finer structure and decreasing in width54. Impact alteration, as hypothesized for some of these rocks1, could cause variable degrees of either heating or shock.
When, where, and how did these rocks originate?
Kaolin-group minerals have been observed from orbit across the Nili Fossae region outside of Jezero crater, located at the northwest quadrant of the Isidis basin, where they are observed in bedrock units in a stratigraphy always overlying Fe/Mg smectites55. These materials have been mapped as exhumed Noachian ( ~ 4.1–3.7 Ga) basement rocks and are sometimes associated with impact breccias and craters22. They may represent remnants of the regionally extensive Noachian compositional “clay stratigraphy” that is characterized by a distinct Al clay horizon with kaolinite-like spectra (approximately 20–50 m thick) that transitions downward into layered Fe/Mg clay horizons with nontronite-like spectra17,56,57 (up to ~150 m thick). The light-toned, Al-rich upper portions of these regional and globally distributed sequences have previously been interpreted as the products of prolonged precipitation-driven deep weathering of volcanic ash/tuff in subaerial settings58, a process that on Earth leads to kaolinization, the endmember of prolonged pedogenic weathering.
The Al-clay-bearing stratigraphy is most common closest to the Nili Fossae graben and progresses southwest towards Jezero crater, where the kaolinite-bearing outcrops transition to patchy detections distributed across the Noachian basement22,23,59. The kaolinite-bearing materials are commonly tens to hundreds of meters in size in the Nili Planum region, appear stratigraphically above low-calcium-pyroxene--bearing plateaus, and are arguably among the youngest features when observed, though the stratigraphic context is difficult to ascertain22.
One potential source of the Al-rich float rocks examined here include the light-toned, layered megabreccia blocks (area of ~0.1 km2) nearby in the crater rim approximately 2 km from the Jezero sedimentary fan that have a spectral signature of a kaolin-group mineral22,23. Other locations identified in the final meanders of Neretva Vallis are associated with in-place light-toned outcrop that could also be an additional source (Fig. S5). Other possible nearby sources include areas upstream from the Neretva Vallis inlet, where an isolated butte ~16 km west of the Jezero sedimentary fan appears to contain kaolin-group minerals (Fig. S5). If present, these materials may have entered the Neretva Vallis channel as lag deposits during erosion of the butte.
Shallow burial, possibly by Syrtis Major volcanism, or heating from impact processes, may have led to lithification of these presumably soft kaolinized materials to allow their eventual transport into Jezero Crater (Fig. 8). Fluvial, glacial, or impact ejecta transport are plausible mechanisms to explain how they ended up as float rocks in Jezero Crater (Fig. 8).
Aqueous alteration (top-down pedogenic weathering) of a volcanic/volcaniclastic protolith led to the formation of a weathering profile characterized by Al clays overlying Fe/Mg clays; either lithification from impact (top pathway) or lithification from burial enhanced the hardness of the weathered material. Lastly, incision or impact distributed this Al-rich material into Jezero crater.
Future plans for exploration of the crater rim by Perseverance includes targeting and sampling a laterally extensive kaolinite-bearing outcrop60, so it is possible that additional observations of outcrop will further reveal the alteration history of these enigmatic float rocks.
Implications for early Mars climate and hydrological cycle
Regardless of whether these rocks formed from pedogenesis or hydrothermal alteration, from a stoichiometric perspective the presence of kaolin-group minerals and Al2O3 > 30 wt% implies large quantities of liquid water once participated in intense chemical alteration (e.g., water: rock ratios [W:R] > 1000:1) to concentrate Al and Ti7. Such quantities of water are hard to envision for a cold and dry Mars today but have been envisaged for the formation of the “compositional clay stratigraphy”15 under intermittently warm intervals during a largely cold Noachian (4.1-3.7 Ga) climate61.
If the kaolinite is instead due to Noachian impact-driven hydrothermal systems (either the larger Isidis or smaller Jezero-forming impact)62 rather than top-down pedogenic leaching, similarly abundant liquid water would have to be available to drive hydrothermal kaolinization. However, in the hydrothermal scenario, the high W:R settings responsible for kaolin formation may have been much more localized, such as in post-impact hydrothermal vents or fractures within the fresh ejecta63.
Similarities with paleosols formed during greenhouse climates on Earth suggest abundant liquid water (mean annual precipitation > 1000 mm), but the nature of fluid chemistry remains unclear (e.g., pH, redox state). Results from the Al-rich float rocks at Jezero crater confirm there were conditions able to sustain intense chemical weathering processes over geological timescales (103–106 years)41 on Mars. These rocks and their source outcrops, especially the Chignik target, represent a new line of evidence for the possibility of precipitation-driven deep weathering of the Martian crust billions of years ago.
The possibility of weathering-climate feedbacks on early Mars may also be demonstrated by this material. The presence of both a measurable 1.9 µm hydration band and a 2.21 µm Al-OH band in Chignik (Fig. 4) is in-situ evidence that some of these ancient rocks retain mineral water and hydroxyl. This implies some were likely never heated to temperatures needed for dehydroxylation ( > ~450 °C). Although Perseverance is unable to directly measure the absolute water content of rocks on Mars, terrestrial paleosols and hydrothermal kaolin deposits rich in kaolinite/halloysite are typically composed of >10 wt% mineral-bound H2O64. It is therefore possible that regional-scale kaolinization of the Martian surface associated with the formation of these rocks may have consumed large amounts of ancient atmospheric water65. This is water that was once liquid, but is now locked within the structure of kaolin group minerals and other hydrated alteration phases formed during chemical weathering of the Martian crust66. Unlike Earth, Mars lacks subduction-driven plate tectonics to recycle the water and hydroxyl (OH) in hydrated minerals like kaolinite back to the atmosphere via volcanic eruptions with mantle sources67, so the genesis of these minerals billions of years ago may have contributed to the irreversible drying of the planet68. Their presence on the surface of Mars today, as hydrated/hydroxylated Al-rich materials, implies both abundant liquid water during formation and also invokes a pathway for the irreversible sequestration of exchangeable water from the ancient Martian atmosphere. Kaolinite-forming conditions at or near the ancient surface may therefore have been associated with the wettest, and possibly most habitable portions of Mars’ history.
Data availability
The supplementary dataset (Supplementary Data) containing elemental composition and reflectance spectra of all targets examined in this work is available at https://doi.org/10.5281/zenodo.17109674. All Mars 2020 Perseverance rover datasets (including those used in this work) are available on the Planetary Data System (PDS) at the PDS Geosciences Node (https://pds-geosciences.wustl.edu/missions/mars2020/index.html) and the PDS Cartography and Imaging Sciences Node (https://pds-imaging.jpl.nasa.gov/volumes/mars2020.html). Links to specific instrument dataset bundles are listed below:
• Mars 2020 Mission bundle: https://doi.org/10.17189/1522642
• SuperCam Instrument bundle: https://doi.org/10.17189/1522646
• Mastcam-Z Science Imaging bundle: https://doi.org/10.17189/q3ts-c749.
References
Royer, C., Bedford, C. C. & Horgan, B. H. N. Intense alteration on early Mars revealed by high-aluminum rocks at Jezero Crater. Nat. Commun. Earth Environ. 5, 671 (2024).
Bedford, C. C. et al. Discovery of light-toned float rocks in Jezero crater: A tale of aqueous alteration and high-temperature metamorphism. Lunar Planet. Sci. Conf. 2221, 6–7 (2024).
Forni, O. et al. Nickel-Copper deposits on Mars? Discovery of ore-grade abundances and implications on formation and alteration. Lunar Planet. Sci. Conf. 1236, 1–2 (2024).
Beck, P. et al. From hydrated silica to quartz: Potential hydrothermal precipitates found in Jezero crater, Mars. Earth Planet. Sci. Lett. 656, 119256 (2025).
Brimhall, G. H. & Dietrich, W. E. Constitutive mass balance relations between chemical composition, volume, density, porosity, and strain in metasomatic hydrochemical systems: Results on weathering and pedogenesis. Geochim. Cosmochim. Acta. 51, 567–587 (1987).
Beukes, N. J., Dorland, H., Nedachi, M. & Ohmoto, H. Tropical laterites, life on land and the history of atmospheric oxygen in the Paleoproterozoic. Geology 30, 491–494 (2002).
Dill, H. G. Kaolin: Soil, rock and ore: from the mineral to the magmatic, sedimentary and metamorphic environments. Earth Sci. Rev. 161, 16–129 (2016).
Retallack, G. J. Lateritization and bauxitization events. Econ. Geol. 105, 655–667 (2010).
Staff, S. S. Keys to soil taxonomy. United States Dep. Agric. 12, 1–372 (2014).
Abbott, P., Minch, J. & Peterson, G. Pre-Eocene paleosol south of Tijuana, Baja California, Mexico. J. Sediment. Petrol. 46, 355–361 (1976).
Abbott, P. L. Cenozoic Paleosols of the San Diego Area, California. Catena 8, 223–237 (1981).
Drever, J. I. In The Chemistry of Weathering (Springer Nature B.V., 1985).
Wilson, M. J. Weathering of the primary rock-forming minerals: processes, products and rates. Clay Miner 39, 233–266 (2004).
Loizeau, D. et al. Quantifying widespread aqueous surface weathering on Mars: the plateaus south of Coprates Chasma. Icarus 302, 451–469 (2018).
Carter, J., Loizeau, D., Mangold, N., Poulet, F. & Bibring, J. Widespread surface weathering on early Mars: a case for a warmer and wetter climate. Icarus 248, 373–382 (2015).
Klidaras, A. et al. Global topography of clay deposits signify an epoch of warm and humid climate on early Mars. Geology 1516, 3–4 (2025).
Ece, Ö. I., Ercan, H. Ü. Global occurrence, geology and characteristics of hydrothermal-origin kaolin deposits. Minerals. 14 https://doi.org/10.3390/min14040353 (2024).
Ehlmann, B. L., Mustard, J. F. & Murchie, S. L. Geologic setting of serpentine deposits on Mars. Geophys. Res. Lett. 37, 1–5 (2010).
Wang, A. et al. Evidence of phyllosilicates in Wooly Patch, an altered rock encountered at West Spur, Columbia Hills, by the Spirit rover in Gusev crater, Mars. J. Geophys. Res. Planets. 111 https://doi.org/10.1029/2005JE002516 (2006).
Wray, J. J., Ehlmann, B. L., Squyres, S. W., Mustard, J. F. & Kirk, R. L. Compositional stratigraphy of clay-bearing layered deposits at Mawrth Vallis. Mars. Geophys. Res. Lett. 35, 2–7 (2008).
B. Ye, J. R. Michalski, Chemical weathering over hundreds of millions of years of greenhouse conditions on Mars. Commun. Earth Environ. 3 https://doi.org/10.1038/s43247-022-00602-7 (2022).
Scheller, E. L. & Ehlmann, B. L. Composition, stratigraphy, and geological history of the noachian basement surrounding the isidis impact basin. J. Geophys. Res. Planets. 125 https://doi.org/10.1029/2019JE006190. (2020).
Carter, J. et al. A Mars orbital catalog of aqueous alteration signatures (MOCAAS). Icarus 389, 115164 (2023).
Fouchet, F. et al. The SuperCam infrared spectrometer for the perseverance rover of the Mars2020 mission. Icarus. 373 https://doi.org/10.1016/j.icarus.2021.114773 (2022).
Wiens, R. C. et al. The SuperCam Instrument Suite on the NASA Mars 2020 Rover: Body Unit and Combined System Tests (The Author(s) https://doi.org/10.1007/s11214-020-00777-5) 217 (2021).
Maurice, S. et al. The SuperCam Instrument Suite on the Mars 2020 Rover: Science Objectives and Mast-Unit Description (The Author(s) 217 https://doi.org/10.1007/s11214-021-00807-w (2021).
Bell, J. F. et al. The Mars 2020 Perseverance Rover Mast Camera Zoom (Mastcam-Z) Multispectral, Stereoscopic Imaging Investigation. Space Sci. Rev. 217 https://doi.org/10.1007/s11214-020-00755-x (2021).
Anderson, R. B. et al. Post-landing major element quantification using SuperCam laser induced breakdown spectroscopy. Spectrochim. Acta - Part B At. Spectrosc. 188 https://doi.org/10.1016/j.sab.2021.106347 (2022).
Broz, A. P. et al. Eocene (50–55 Ma) greenhouse climate recorded in nonmarine rocks of San Diego, CA, USA. Sci. Rep. 14, 1–19 (2024).
Yang, W. & Holland, H. D. The Hekpoort paleosol profile in strata 1 at Gaborone, Botswana: Soil formation during the great oxidation event. Am. J. Sci. 303, 187–220 (2003).
Driese, S. G. Pedogenic translocation of Fe in modern and ancient Vertisols and implications for interpretations of the Hekpoort paleosol (2.25 Ga). J. Geol. 112, 543–560 (2004).
Abedini, A. & Calagari, A. A. Geochemical characteristics of the Abgarm kaolin deposit, NW Iran. Neues Jahrb. Geol. Palaontol. Abhandlungen. 278, 335–350 (2015).
Baioumy, H. et al. Hypogene kaolin deposits from felsic intrusive rocks (Peninsular Malaysia) with special reference to rare earth elements and stable isotopes geochemistry. Geosci. J. 25, 863–876 (2021).
Grecco, L. E. & Marfil, S. A. Mineralogy and geochemistry of hydrothermal kaolins from the Adelita mine, Patagonia (Argentina); relation to other mineralization in the area. Clay Minerals 47, 131–146 (2012).
Royer, C. et al. Reflectance of Jezero Crater Floor: 1. Data Processing and Calibration of the Infrared Spectrometer (IRS) on SuperCam. J. Geophys. Res. Planets. 128 https://doi.org/10.1029/2022JE007481 (2023).
Novoselov, A. A., Roberto, C. & Filho, D. S. Potassium metasomatism of Precambrian paleosols. Precambr. Res. 262, 67–83 (2015).
Murchie, S. et al. Compact reconnaissance imaging spectrometer for Mars (CRISM) on Mars reconnaissance orbiter (MRO). J. Geophys. Res. 112, 1–57 (2007).
Dickson, J. L., Ehlmann, B. L., Kerber, L., Fassett, C. I. The Global Context Camera (CTX) Mosaic of Mars: a product of information-preserving image data processing. Earth Sp. Sci. 11 https://doi.org/10.1029/2024EA003555. (2024).
Bishop, J. et al. Phyllosilicate diversity and past aqueous activity revealed at Mawrth Vallis, Mars. Science 321, 830–834 (2008).
Cloutis, E. A. et al. Spectral reflectance properties of minerals exposed to simulated Mars surface conditions. Icarus 195, 140–168 (2008).
Babechuk, M. G., Widdowson, M. & Kamber, B. S. Quantifying chemical weathering intensity and trace element release from two contrasting basalt profiles, Deccan Traps, India. Chem. Geol. 363, 56–75 (2014).
Ece, ÖI., Ekinci, B., Schroeder, P. A., Crowe, D. & Esenli, F. Origin of the Düvertepe kaolin-alunite deposits in Simav Graben, Turkey: Timing and styles of hydrothermal mineralization. J. Volcanol. Geotherm. Res. 255, 57–78 (2013).
Dill, H. G., Kaufhold, S., Ehling, A. & Bowitz, J. Oxidized and reduced kaolin fan deposits: their sedimentological-mineralogical facies and physical-chemical regime (North-Bavarian Kaolin Mining District, Germany). Ore Geol. Rev. 72, 459–484 (2016).
Driese, S. G., Medaris, L. G., Kirsimäe, K., Somelar, P. & Stinchcomb, G. E. Oxisolic processes and geochemical constraints on duration of weathering for Neoproterozoic Baltic paleosol. Precambr. Res. 310, 165–178 (2018).
Dill, H. G., Bosse, H. R., Henning, K. H., Fricke, A. & Ahrendt, H. Mineralogical and chemical variations in hypogene and supergene kaolin deposits in a mobile fold belt the Central Andes of northwestern Peru. Miner. Depos. 32, 149–163 (1997).
Ercan, H. Ü, Ece, I. şik, Schroeder, P. A. & Karacik, Z. Differentiating styles of alteration within kaolin-alunite hydrothermal deposits of çanakkale, NW Turkey. Clays Clay Miner 64, 245–274 (2016).
Marsh, E. E., Anderson, E. D., Gray, F. in Mineral Deposit Models for Resource Assessment (2013; http://pubs.er.usgs.gov/publication/sir20105070H), p. 49.
Marsh, E. E., Anderson, E. Ni-Co laterite deposits ─ a deposit model. U.S. Geol. Surv. Open-File Rep. 2011–1259 (2015).
Butt, C. R. M., Cluzel, D. Nickel Laterite Ore Deposits: Weathered Serpentinites, 123–128 (2013).
Baumgartner, R. J. et al. Magmatic controls on the genesis of Ni-Cu±(PGE) sulphide mineralisation on Mars. Ore Geol. Rev. 65, 400–412 (2015).
Baumgartner, R. J., Baratoux, D., Gaillard, F. & Fiorentini, M. L. Numerical modelling of erosion and assimilation of sulfur-rich substrate by martian lava flows: Implications for the genesis of massive sulfide mineralization on Mars. Icarus 296, 257–274 (2017).
Potter-McIntyre, S. L., Chan, M. A. & McPherson, B. J. Concretion formation in volcaniclastic host rocks: evaluating the role of organics, mineralogy, and geochemistry on early diagenesis. J. Sediment. Res. 84, 875–892 (2014).
Cloutis, E. A. et al. Heated kaolinite/halloysite at Barrier Range, Jezero Crater, Mars? In Lunar and Planetary Science Conference, Vol. 2806, 2155 (2023).
Michalski, J. R. et al. Shock metamorphism of clay minerals on Mars by meteor impact. Geophys. Res. Lett. 44, 6562–6569 (2017).
Ehlmann, B. L. et al. Identification of hydrated silicate minerals on Mars using MRO-CRISM: geologic context near Nili Fossae and implications for aqueous alteration. J. Geophys. Res. Planets. 114, 1–33 (2009).
Bishop, J. L. et al. Multiple mineral horizons in layered outcrops at Mawrth Vallis, Mars, signify changing geochemical environments on early Mars. Icarus 341, 113634 (2020).
Ye, B. & Michalski, J. R. Precipitation-driven pedogenic weathering of volcaniclastics on early Mars. Geophys. Res. Lett. 48, 1–10 (2021).
Liu, J. et al. Anoxic chemical weathering under a reducing greenhouse on early Mars. Nat. Astron. https://doi.org/10.1038/s41550-021-01303-5 (2021).
Bramble, M. S., Mustard, J. F. & Salvatore, M. R. The geological history of Northeast Syrtis Major. Mars. Icarus. 293, 66–93 (2017).
Mayhew, L. E. et al. The scientific value of collecting samples from the Jezero Crater rim. Lunar Planet. Sci. Conf. 2602, 8–9 (2024).
Bishop, J. L. et al. Surface clay formation during short-term warmer and wetter conditions on a largely cold ancient Mars. Nat. Astron. 2, 206–213 (2018).
Mandon, L. et al. Refining the age, emplacement and alteration scenarios of the olivine-rich unit in the Nili Fossae region. Mars. Icarus. 336, 113436 (2020).
Schwenzer, S. P. & Kring, D. A. Alteration minerals in impact-generated hydrothermal systems—exploring host rock variability. Icarus 226, 487–496 (2013).
Hwang, H. et al. A role for subducted super-hydrated kaolinite in Earth’s deep water cycle. Nat. Geosci. 10, 947–953 (2017).
Scheller, E. L., Ehlmann, B. L., Hu, R., Adams, D. J. & Yung, Y. L. Long-term drying of Mars by sequestration of ocean-scale volumes of water in the crust. Science 372, 56–62 (2021).
Wernicke, L. J. & Jakosky, B. M. Martian hydrated minerals: a significant water sink. J. Geophys. Res. Planets. 126, 1–24 (2021).
Rolf, T., Weller, M., Gülcher, A. J. P. Venus mantle dynamics and evolution through time. Space Sci. Rev. 218 https://doi.org/10.1007/s11214-022-00937-9 (2022).
Mustard, J. F. Sequestration of volatiles in the Martian crust through hydrated minerals: A significant planetary reservoir of water (Elsevier Inc., 2018) https://doi.org/10.1016/B978-0-12-804191-8.00008-8).
Acknowledgements
The authors are eternally grateful for the Mars 2020 Science and Engineering Teams, and the decades of authors who have studied Earth’s kaolinite. Funding sources: NASA contract NNH13ZDA018O for SuperCam (C.R., C.C.B., R.C.W., A.O., S.C.); SuperCam, (1532432) and Mastcam-Z (15-707, 1511125) (JRJ); Mastcam-Z (15-707, 1511125) (BK); Texaco Postdoctoral prize fellowship awarded by the division of Geological and Planetary Sciences of Caltech (L.M.); Spanish Agency for Research AEI/MCIN/FEDER, Grant No. PID2022-142750OB-I00 (J.M.M.), and NASA RSS Participating Scientist grant 80NSSC20K0239 (E.M.H.). A portion of this research was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration (80NM0018D0004).
Author information
Authors and Affiliations
Contributions
A.P.B., B.H.N.H., C.B., C.R., H.M., S.C., R.C.W., E.C.C., J.M.M., L.M., A.K., M.B., B.K., O.F., J.C., E.D., C.Q.-N., J.R.J., J.I.N., L.H., U.W., E.A.C., P.B., J.F.B., J.I.S., A.C., A.P.B., B.H.N.H., C.B., C.R., H.M., S.C., R.C.W., E.C.C., J.M.M., L.M., A.K., M.B., O.F., J.C., E.D., J.R.J., J.I.N., L.H., E.A.C., B.H.N.H., J.F.B., R.C.W., A.C., A.P.B., B.H.N.H., C.B., C.R., H.M., S.C., R.C.W., E.C.C., J.M.M., L.M., A.K., M.B., B.K., O.F., J.C., E.D., C.Q.-N., J.R.J., J.I.N., L.H., U.W., E.A.C., P.B., J.F.B., J.I.S., A.C., A.P.B., C.B., C.R., M.B., J.C., A.P.B., C.B., C.R., B.H.H., R.C.W., A.P.B., B.H.N.H., C.B., C.R., H.M, S.C., R.C.W., E.C.C., J.M.M., L.M., A. K., M.B., B.K., O.F., J.C., E.D., C.Q.-N., J.R.J., J.I.N., L.H., U.W., E.A.C., P.B., J.F.B., J.I.S., A.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. Co-author Candice C. Bedford is an Editorial Board Member for Communications Earth & Environment, but was not involved in the editorial review of, nor the decision to publish this article.
Peer review
Peer review information
Communications Earth and Environment thanks John Edgar and Lindsay McHenry for their contribution to the peer review of this work. Primary Handling Editor: Joe Aslin. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Broz, A.P., Horgan, B.H.N., Bedford, C. et al. Alteration history of aluminum-rich rocks at Jezero crater, Mars. Commun Earth Environ 6, 935 (2025). https://doi.org/10.1038/s43247-025-02856-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-025-02856-3