Abstract
Background:
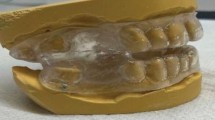
The clinical symptoms of obstructive sleep apnea (OSA) are poorly correlated with disease severity based on the apnea-hypopnea index (AHI). The cumulative duration of respiratory effort assessed by mandibular jaw movement monitoring with automated analysis (REMOV) may better capture the clinical burden of OSA. This cross-sectional study assessed the association between REMOV and patient-reported outcomes (PROs), including sleepiness, fatigue, and depression.
Methods:
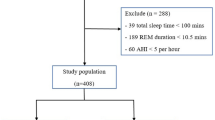
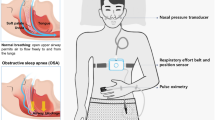
One thousand adults referred for suspected OSA underwent polysomnography, REMOV analysis, and PRO assessment using validated questionnaires. Relationships between REMOV, AHI, and PROs were examined using principal component analysis and regression models.
Results:
Median REMOV values align with OSA severity (6.5%, 23.4%, 28.8%, and 42.8% of total sleep time at AHI values of <5, 5–15, 15– < 30, and ≥30 events/h, respectively). REMOV is significantly associated with sleepiness, fatigue, and depression. These associations are most evident in patients with an AHI ≤ 15 events/h. AHI is not significantly associated with any PROs.
Conclusions:
These data suggest that REMOV may serve as a complementary metric in OSA, especially in patients with mild disease. Incorporating REMOV into OSA severity grading may improve the alignment between PROs and therapeutic decisions.
Plain language summary
Obstructive sleep apnea (OSA) can cause excessive daytime sleepiness, fatigue, or depressed mood. However, these symptoms often do not align with the conventional apnea-hypopnea index (AHI), which measures the number of breathing interruptions per hour of sleep. This study tested a new metric called REMOV, which quantifies the percentage of sleep time spent with increased respiratory effort. We studied 1,000 adults referred for suspected OSA. Each participant underwent an overnight polysomnography with simultaneous REMOV measurement based on mandibular jaw movement analysis and completed questionnaires about their symptoms. We found that higher REMOV values were significantly associated with more severe symptoms, especially in patients with mild OSA. Our findings suggest that REMOV could complement the AHI in routine practice, supporting earlier treatment decisions, and thereby improving OSA management outcomes.
Similar content being viewed by others
Data availability
Due to patient confidentiality, the dataset is not publicly available. However, de-identified data may be obtained from the corresponding author upon reasonable request, subject to a data-sharing agreement and approval by the relevant ethics committee.
References
Kapur, V. K. et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 13, 479–504 (2017).
Lévy, P. et al. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Prim. 1, 15015 (2015).
Bailly, S. et al. Obstructive sleep apnea: a cluster analysis at time of diagnosis. PLoS ONE 11, e0157318 (2016).
Bailly, S. et al. Clusters of sleep apnoea phenotypes: a large pan-European study from the European Sleep Apnoea Database (ESADA). Respirology 26, 378–387 (2021).
Zinchuk, A. & Yaggi, H. K. Phenotypic subtypes of OSA: a challenge and opportunity for precision medicine. Chest 157, 403–420 (2020).
Edwards, C., Almeida, O. P. & Ford, A. H. Obstructive sleep apnea and depression: a systematic review and meta-analysis. Maturitas 142, 45–54 (2020).
Emsellem, H. A., Colwell, H. H., Cronin, J., Farkas, R. H. & Mathias, S. D. Fatigue is distinct from sleepiness and negatively impacts individuals living with obstructive sleep apnea (OSA): results from qualitative research of individuals with OSA. Health Qual. Life Outcomes 23, 26 (2025).
Lal, C., Weaver, T. E., Bae, C. J. & Strohl, K. P. Excessive daytime sleepiness in obstructive sleep apnea. mechanisms and clinical management. Ann. Am. Thorac. Soc. 18, 757–768 (2021).
Mjelle, K. E. S., Lehmann, S., Saxvig, I. W., Gulati, S. & Bjorvatn, B. Association of excessive sleepiness, pathological fatigue, depression, and anxiety with different severity levels of obstructive sleep apnea. Front. Psychol. 13, 839408 (2022).
Zhang, D., Zhang, Z., Li, H. & Ding, K. Excessive daytime sleepiness in depression and obstructive sleep apnea: more than just an overlapping symptom. Front. Psychiatry 12, 710435 (2021).
Javaheri, S. & Javaheri, S. Update on persistent excessive daytime sleepiness in OSA. Chest 158, 776–786 (2020).
Bailes, S. et al. Fatigue: the forgotten symptom of sleep apnea. J. Psychosom. Res. 70, 346–354 (2011).
Chervin, R. D. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest 118, 372–379 (2000).
Chen, Y. H., Keller, J. K., Kang, J. H., Hsieh, H. J. & Lin, H. C. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J. Clin. Sleep Med. 9, 417–423 (2013).
Jackson, M. L. et al. Clinical depression in untreated obstructive sleep apnea: examining predictors and a meta-analysis of prevalence rates. Sleep Med. 62, 22–28 (2019).
Vanek, J. et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 72, 50–58 (2020).
Malhotra, A. et al. Metrics of sleep apnea severity: beyond the apnea-hypopnea index. Sleep 44, zsab030 (2021).
Malhotra, A., Martinot, J. B. & Pépin, J. L. Insights on mandibular jaw movements during polysomnography in obstructive sleep apnea. J. Clin. Sleep Med. 20, 151–163 (2024).
Martinot, J. B. et al. The key role of the mandible in modulating airflow amplitude during sleep. Respir. Physiol. Neurobiol. 279, 103447 (2020).
Pepin, J. L. et al. Mandibular movements are a reliable noninvasive alternative to esophageal pressure for measuring respiratory effort in patients with sleep apnea syndrome. Nat. Sci. Sleep 14, 635–644 (2022).
Pépin, J. L. et al. Mandibular jaw movement automated analysis for oral appliance monitoring in obstructive sleep apnea: a prospective cohort study. Ann. Am. Thorac. Soc. 21, 814–822 (2024).
Pépin, J. L. et al. Assessment of mandibular movement monitoring with machine learning analysis for the diagnosis of obstructive sleep apnea. JAMA Netw. Open 3, e1919657 (2020).
Martinot, J. B., Le-Dong, N. N., Malhotra, A. & Pépin, J. L. Respiratory effort during sleep and prevalent hypertension in obstructive sleep apnoea. Eur. Respir. J. 61, 2201486 (2023).
Martinot, J. B. et al. Respiratory effort during sleep and the rate of prevalent type 2 diabetes in obstructive sleep apnoea. Diab. Obes. Metab. 25, 2815–2823 (2023).
Pépin, J. L. et al. Exploring the dose-response relationship between mandibular protrusion and respiratory effort burden in oral appliance therapy for obstructive sleep apnea. Ann. Am. Thorac. Soc. 22, 915–924 (2025).
Berry, R. B. et al. AASM scoring manual updates for 2017 (version 2.4). J. Clin. Sleep Med. 13, 665–666 (2017).
Pichot, P. & Brun, J. P. Brief self-evaluation questionnaire for depressive, asthenic and anxious dimensions. Ann. Med. Psychol. 142, 862–865 (1984).
Pichot, P. in Assessment of Depression (eds Sartorius, N. & Ban, T. A.) (Springer, 1986).
Arel-Bundock, V., Greifer, N. & Heiss, A. How to interpret statistical models using marginaleffects for R and Python. J. Stat. Softw. 111, 1–32 (2024).
Husson, F., Le, S. & Pagès, J. Exploratory Multivariate Analysis by Example Using R (Chapman & Hall/CRC, 2017).
Mikis, D. et al. Flexible Regression and Smoothing: Using GAMLSS in R (Chapman & Hall/CRC, 2017).
Azarbarzin, A. et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the osteoporotic fractures in men study and the sleep heart health study. Eur. Heart J. 40, 1149–1157 (2019).
Kainulainen, S. et al. Severe desaturations increase psychomotor vigilance task-based median reaction time and number of lapses in obstructive sleep apnoea patients. Eur. Respir. J. 55, 1901849 (2020).
Labarca, G., Gower, J., Lamperti, L., Dreyse, J. & Jorquera, J. Chronic intermittent hypoxia in obstructive sleep apnea: a narrative review from pathophysiological pathways to a precision clinical approach. Sleep Breath. 24, 751–760 (2020).
Dewan, N. A., Nieto, F. J. & Somers, V. K. Intermittent hypoxemia and OSA: implications for comorbidities. Chest 147, 266–274 (2015).
Bennett, L. S., Barbour, C., Langford, B., Stradling, J. R. & Davies, R. J. Health status in obstructive sleep apnea: relationship with sleep fragmentation and daytime sleepiness, and effects of continuous positive airway pressure treatment. Am. J. Respir. Crit. Care Med. 159, 1884–1890 (1999).
Alomri, R. M., Kennedy, G. A., Wali, S. O., Ahejaili, F. & Robinson, S. R. Differential associations of hypoxia, sleep fragmentation, and depressive symptoms with cognitive dysfunction in obstructive sleep apnea. Sleep 44, zsaa213 (2021).
Ferreira, C. B., Schoorlemmer, G. H., Rocha, A. A. & Cravo, S. L. Increased sympathetic responses induced by chronic obstructive sleep apnea are caused by sleep fragmentation. J. Appl. Physiol. 129, 163–172 (2020).
Patil, S. P. et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med.15, 335–343 (2019).
Ramar, K. et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J. Clin. Sleep Med. 11, 773–827 (2015).
Rizzo, D. et al. The role of fatigue and sleepiness in drivers with obstructive sleep apnea. Transp. Res Part F Traffic Psychol. Behav. 62, 796–804 (2019).
Peppard, P. E., Szklo-Coxe, M., Hla, K. M. & Young, T. Longitudinal association of sleep-related breathing disorder and depression. Arch. Intern. Med. 166, 1709–1715 (2006).
Arnold, W. C. & Guilleminault, C. Upper airway resistance syndrome 2018: non-hypoxic sleep-disordered breathing. Expert Rev. Respir. Med. 13, 317–326 (2019).
Guilleminault, C., Stoohs, R., Clerk, A., Cetel, M. & Maistros, P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest 104, 781–787 (1993).
Pelin, Z., Karadeniz, D., Oztürk, L., Gözükirmizi, E. & Kaynak, H. The role of mean inspiratory effort on daytime sleepiness. Eur. Respir. J. 21, 688–694 (2003).
Tobias, L. & Won, C. PRO: upper airway resistance syndrome represents a distinct entity from obstructive sleep apnea syndrome. J. Dent. Sleep Med. 3, 21–24 (2016).
Yanagimori, M. et al. Respiratory arousal threshold among patients with isolated sleep apnea and with comorbid insomnia (COMISA). Sci. Rep. 13, 7638 (2023).
Arora, N., Meskill, G. & Guilleminault, C. The role of flow limitation as an important diagnostic tool and clinical finding in mild sleep-disordered breathing. Sleep Sci. 8, 134–142 (2015).
Mann, D. L. et al. Flow limitation is associated with excessive daytime sleepiness in individuals without moderate or severe obstructive sleep apnea. Ann. Am. Thorac. Soc. 21, 1186–1193 (2024).
Jordan, A. S., O’Donoghue, F. J., Cori, J. M. & Trinder, J. Physiology of arousal in obstructive sleep apnea and potential impacts for sedative treatment. Am. J. Respir. Crit. Care Med. 196, 814–821 (2017).
Patil, S. P. et al. Long-term health outcomes for patients with obstructive sleep apnea: placing the agency for healthcare research and quality report in context—a multisociety commentary. J. Clin. Sleep Med. 20, 135–149 (2024).
Zinchuk, A., Gentry, M., Concato, J. & Yaggi, K. Phenotypes in obstructive sleep apnea: a definition, examples and evolution of approaches. Sleep Med. Rev. 35, 113–123 (2016).
Carretta, T. R. & Ree, M. J. Correction for range restriction: lessons from 20 research scenarios. Mil. Psychol. 34, 551–569 (2022).
Bonsignore, M. R., Saaresranta, T. & Riha, R. L. Sex differences in obstructive sleep apnoea. Eur. Respir. Rev. 28, 190030 (2019).
Guilleminault, C., Davis, K. & Huynh, N. T. Prospective randomized study of patients with insomnia and mild sleep disordered breathing. Sleep 31, 1527–1533 (2008).
Lee, M. H. et al. Gender differences in the effect of comorbid insomnia symptom on depression, anxiety, fatigue, and daytime sleepiness in patients with obstructive sleep apnea. Sleep Breath. 18, 111–117 (2014).
Schwarz, E. I. & Schiza, S. Sex differences in sleep and sleep-disordered breathing. Curr. Opin. Pulm. Med. 30, 593–599 (2024).
Martinot, J. B. et al. Mandibular movement analysis to assess efficacy of oral appliance therapy in OSA. Chest 154, 1340–1347 (2018).
Acknowledgements
The authors wish to thank Ms. Mathilde Leval and Ms. Bao Truc Nguyen Ham for their assistance in data collection. Assistance for manuscript formatting was provided by Nicola Ryan (New Zealand), an independent medical writer.
Author information
Authors and Affiliations
Contributions
J.-B.M., J.-L.P., and N.-N.L.-D. designed the study. J.-B.M., S.B., and D.C. conducted the research procedure and had full access to all study data. N.-N.L.-D. and J.-B.M. performed the data analysis and interpretation. J.-B.M., N.-N.L.-D., S.B., and J.-L.P. prepared the first draft of the manuscript. J.-L.P., J.-B.M., D.C., and S.B. reviewed and edited the final manuscript. All authors made the decision to submit the manuscript for publication and assume responsibility for the accuracy and completeness of the analyses and for the fidelity of this report to the study protocol.
Corresponding author
Ethics declarations
Competing interests
J.-B.M. is a scientific advisor to Sunrise and is an investigator in pharmaceutical trials for Jazz Pharmaceuticals, SMB Lab, Takeda, and Alkermes. N.-N.L.-D. is an employee of Sunrise. D.C. declares no financial competing interests. S.B. reports income related to medical education from ResMed and Vitalaire, and has received financial support for seminars and congress travel from Bioprojet, Vitalaire, and Jazz Pharmaceuticals. J.-L.P. is supported by the French National Research Agency (ANR) in the framework of the “FRANCE 2030” program, the “e-health and integrated care” chair of Grenoble Alpes University Foundation, and the “Sleep Health-AI chair” within the “MIAI Cluster” for artificial intelligence (ANR-23-IACL-0006). He also reports income related to medical education from ResMed, Sefam, Zoll-Respicardia, Eli Lilly, Idorsia, Pharmanovia, Biosency, and Bioprojet. There was no funding or other financial support for this research from Sunrise. Non-financial competing interests: The authors declare that they have no non-financial competing interests, including but not limited to personal relationships, academic affiliations, or institutional involvements that could have influenced the work reported in this manuscript.
Peer review
Peer review information
Communications Medicine thanks Harald Hrubos-Strøm, Sailaja Sunkara and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Martinot, JB., Le-Dong, NN., Clause, D. et al. Respiratory effort burden measured by mandibular jaw movements as a digital marker with clinical insights in obstructive sleep apnea. Commun Med (2026). https://doi.org/10.1038/s43856-026-01378-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-026-01378-z