Abstract
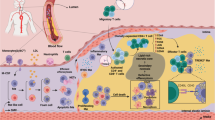
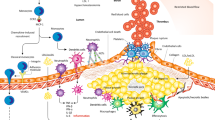
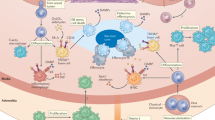
Vascular smooth muscle cells, endothelial cells and macrophages undergo phenotypic conversions throughout atherosclerosis progression, both as a consequence of chronic inflammation and as subsequent drivers of it. The inflammatory hypothesis of atherosclerosis has been catapulted to the forefront of cardiovascular research as clinical trials have shown that anti-inflammatory therapy reduces adverse cardiovascular events. However, no current therapies have been specifically designed to target the phenotype of plaque cells. Fate mapping has revealed that plaque cells convert to detrimental and beneficial cell phenotypes during atherosclerosis, with cumulative evidence highlighting that vascular cell plasticity is intimately linked with plaque inflammation, ultimately impacting lesion stability. Here we review vascular cell plasticity during atherosclerosis in the context of the chronic inflammatory plaque microenvironment. We highlight the need to better understand how plaque cells behave during therapeutic intervention. We then propose modulating plaque cell phenotype as an unexplored therapeutic paradigm in the clinical setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2100–2132 (2024).
GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2133–2161 (2024).
GBD 2021 Forecasting Collaborators.Burden of disease scenarios for 204 countries and territories, 2022–2050: a forecasting analysis for the Global Burden of Disease Study 2021. Lancet 403, 2204–2256 (2024).
Libby, P. The changing landscape of atherosclerosis. Nature 592, 524–533 (2021).
Ridker, P. M. How common is residual inflammatory risk? Circ. Res. 120, 617–619 (2017).
Ridker, P. M. et al. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet 401, 1293–1301 (2023). This study suggests that residual inflammatory risk predicts adverse cardiovascular events among individuals receiving statin therapy.
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017). This clinical trial provided proof of principle that anti-inflammatory therapy can improve cardiovascular outcomes independently of lipid lowering.
Tardif, J.-C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019). This study showed that colchicine reduces adverse cardiovascular events in patients with myocardial infarction.
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020). This study demonstrated that colchicine reduces adverse cardiovascular events in patients with stable coronary artery disease.
Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 117, 2525–2536 (2021). This review summarizes the role of inflammation in atherosclerosis.
Fiolet, A. T. L. et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur. Heart J. 42, 2765–2775 (2021).
Li, J. et al. Colchicine in patients with acute ischaemic stroke or transient ischaemic attack (CHANCE-3): multicentre, double blind, randomised, placebo controlled trial. Br. Med. J. 385, e079061 (2024).
Kelly, P. et al. Long-term colchicine for the prevention of vascular recurrent events in non-cardioembolic stroke (CONVINCE): a randomised controlled trial. Lancet 404, 125–133 (2024).
Misawa, T. et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 14, 454–460 (2013). This paper showed that colchicine suppresses NLRP3 inflammasome activation via microtubule destabilization.
Martinon, F., Pétrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Libby, P. Interleukin-1β as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J. Am. Coll. Cardiol. 70, 2278–2289 (2017).
Opstal, T. S. J. et al. Colchicine attenuates inflammation beyond the inflammasome in chronic coronary artery disease. Circulation 142, 1996–1998 (2020).
Vaidya, K. et al. Colchicine inhibits neutrophil extracellular trap formation in patients with acute coronary syndrome after percutaneous coronary intervention. J. Am. Heart Assoc. 10, e018993 (2021).
Schwarz, N. et al. Colchicine exerts anti-atherosclerotic and ‑plaque-stabilizing effects targeting foam cell formation. FASEB J. 37, e22846 (2023).
Li, W. et al. Colchicine promotes atherosclerotic plaque stability independently of inflammation. Preprint at bioRxiv https://doi.org/10.1101/2023.10.03.560632 (2023).
Ridker, P. M. et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 380, 752–762 (2018).
Micha, R. et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am. J. Cardiol. 108, 1362–1370 (2011).
van der Heijden, T. et al. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice—brief report. Arterioscler. Thromb. Vasc. Biol. 37, 1457–1461 (2017).
Ridker, P. M. et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 397, 2060–2069 (2021).
Menu, P. et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2, e137 (2011).
Lin, A. et al. Clonal expansion in cardiovascular pathology. JACC Basic Transl. Sci. 9, 120–144 (2024).
Misra, A. et al. Integrin β3 regulates clonality and fate of smooth muscle-derived atherosclerotic plaque cells. Nat. Commun. 9, 2073 (2018). This study showed how VSMCs clonally expand during atherosclerotic plaque development.
Betsholtz, C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 15, 215–228 (2004).
Newman, A. A. C. et al. Multiple cell types contribute to the atherosclerotic lesion fibrous cap by PDGFRβ and bioenergetic mechanisms. Nat. Metab. 3, 166–181 (2021). This paper showed that the protective fibrous cap is formed from multiple cell sources.
Owens, G. K., Kumar, M. S. & Wamhoff, B. R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767–801 (2004).
Kwartler, C. S. et al. Nuclear smooth muscle α-actin participates in vascular smooth muscle cell differentiation. Nat. Cardiovasc. Res. 2, 937–955 (2023).
Guo, D. C. et al. Mutations in smooth muscle α-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am. J. Hum. Genet. 84, 617–627 (2009).
Kaw, K. et al. Smooth muscle α-actin missense variant promotes atherosclerosis through modulation of intracellular cholesterol in smooth muscle cells. Eur. Heart J. 44, 2713–2726 (2023).
Pan, H. et al. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation 142, 2060–2075 (2020). This research identified a stem cell-like transitional phenotype during smooth muscle cell transdifferentiation.
Alencar, G. F. et al. Stem cell pluripotency genes Klf4 and Oct4 regulate complex SMC phenotypic changes critical in late-stage atherosclerotic lesion pathogenesis. Circulation 142, 2045–2059 (2020). This study showed that multiple smooth muscle cell-derived phenotypes arise via a transitional stem cell-like phenotype.
Chen, P. Y. et al. Smooth muscle cell reprogramming in aortic aneurysms. Cell Stem Cell 26, 542–557 (2020).
Cheng, P. et al. Smad3 regulates smooth muscle cell fate and mediates adverse remodeling and calcification of the atherosclerotic plaque. Nat. Cardiovasc. Res. 1, 322–333 (2022).
Rattazzi, M. et al. Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: potential role of chondrocyte-like cells. Arterioscler. Thromb. Vasc. Biol. 25, 1420–1425 (2005).
Kawai, K., Kawakami, R., Finn, A. V. & Virmani, R. Differences in stable and unstable atherosclerotic plaque. Arterioscler. Thromb. Vasc. Biol. 44, 1474–1484 (2024). This review summarizes key pathological differences between stable and unstable plaques.
Rong, J. X., Shapiro, M., Trogan, E. & Fisher, E. A. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc. Natl Acad. Sci. USA 100, 13531–13536 (2003). This study showed that VSMCs can express macrophage-related genes in response to cholesterol.
Vengrenyuk, Y. et al. Cholesterol loading reprograms the microRNA-143/145–myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler. Thromb. Vasc. Biol. 35, 535–546 (2015).
Conklin, A. C. et al. Meta-analysis of smooth muscle lineage transcriptomes in atherosclerosis and their relationships to in vitro models. Immunometabolism https://doi.org/10.20900/immunometab20210022 (2021).
Li, Y. et al. Smooth muscle-derived macrophage-like cells contribute to multiple cell lineages in the atherosclerotic plaque. Cell Discov. 7, 111 (2021).
Nagesh, P. T. et al. HDL regulates TGFβ-receptor lipid raft partitioning, restoring contractile features of cholesterol-loaded vascular smooth muscle cells. Preprint at bioRxiv https://doi.org/10.1101/2023.10.19.562786 (2023).
Allahverdian, S., Chehroudi, A. C., McManus, B. M., Abraham, T. & Francis, G. A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 129, 1551–1559 (2014).
Wang, Y. et al. Smooth muscle cells contribute the majority of foam cells in ApoE (apolipoprotein E)-deficient mouse atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 39, 876–887 (2019).
Chen, J., Kitchen, C. M., Streb, J. W. & Miano, J. M. Myocardin: a component of a molecular switch for smooth muscle differentiation. J. Mol. Cell. Cardiol. 34, 1345–1356 (2002).
Liu, Y. et al. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J. Biol. Chem. 280, 9719–9727 (2005).
Shankman, L. S. et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 21, 628–637 (2015). This study established the importance of VSMC plasticity in atherosclerosis.
Pekayvaz, K. et al. Mural cell-derived chemokines provide a protective niche to safeguard vascular macrophages and limit chronic inflammation. Immunity 56, 2325–2341 (2023).
Farina, F. M. et al. The epigenetic enzyme DOT1L orchestrates vascular smooth muscle cell–monocyte crosstalk and protects against atherosclerosis via the NF-κB pathway. Eur. Heart J. 43, 4562–4576 (2022).
Gu, L. et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor deficient mice. Mol. Cell 2, 275–281 (1998). This study established the importance of CCL2 in monocyte recruitment.
Owsiany, K. M., Deaton, R. A., Soohoo, K. G., Tram Nguyen, A. & Owens, G. K. Dichotomous roles of smooth muscle cell-derived MCP1 (monocyte chemoattractant protein 1) in development of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 42, 942–956 (2022).
Gräbner, R. et al. Lymphotoxin β receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J. Exp. Med. 206, 233–248 (2009).
Hu, D. et al. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin β receptors. Immunity 42, 1100–1115 (2015).
Alexander, M. R., Murgai, M., Moehle, C. W. & Owens, G. K. Interleukin-1β modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-κB-dependent mechanisms. Physiol. Genomics 44, 417–429 (2012).
Eun, S. Y., Ko, Y. S., Park, S. W., Chang, K. C. & Kim, H. J. IL-1β enhances vascular smooth muscle cell proliferation and migration via P2Y2 receptor-mediated RAGE expression and HMGB1 release. Vasc. Pharmacol. 72, 108–117 (2015).
Kabir, I. et al. The age of bone marrow dictates the clonality of smooth muscle-derived cells in atherosclerotic plaques. Nat. Aging 3, 64–81 (2023).
Gomez, D. et al. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat. Med. 24, 1418–1429 (2018). This study suggested that IL-1β has a protective role in plaque VSMC derivatives.
Karnewar, S. et al. IL-1β inhibition partially negates the beneficial effects of diet-induced atherosclerosis regression in mice. Arterioscler. Thromb. Vasc. Biol. 44, 1379–1392 (2024).
Fidler, T. P. et al. Suppression of IL-1β promotes beneficial accumulation of fibroblast-like cells in atherosclerotic plaques in clonal hematopoiesis. Nat. Cardiovasc. Res. 3, 60–75 (2024).
Ma, S., Yang, D., Li, D., Tang, B. & Yang, Y. Oleic acid induces smooth muscle foam cell formation and enhances atherosclerotic lesion development via CD36. Lipids Health Dis. 10, 53 (2011).
Wirka, R. C. et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat. Med. 25, 1280–1289 (2019).
Carramolino, L. et al. Cholesterol lowering depletes atherosclerotic lesions of smooth muscle cell-derived fibromyocytes and chondromyocytes. Nat. Cardiovasc. Res. 3, 203–220 (2024).
Sharma, D. et al. Comprehensive integration of multiple single-cell transcriptomic data sets defines distinct cell populations and their phenotypic changes in murine atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 44, 391–408 (2024).
Winkels, H. et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ. Res. 122, 1675–1688 (2018).
Hume, D. A., Millard, S. M. & Pettit, A. R. Macrophage heterogeneity in the single-cell era: facts and artifacts. Blood 142, 1339–1347 (2023).
Piollet, M. et al. TREM2 protects from atherosclerosis by limiting necrotic core formation. Nat. Cardiovasc. Res. 3, 269–282 (2024).
Adkar, S. S. & Leeper, N. J. Efferocytosis in atherosclerosis. Nat. Rev. Cardiol. 21, 762–779 (2024).
Tabas, I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 (2010).
Flores, A. M. et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat. Nanotechnol. 15, 154–161 (2020).
Kojima, Y. et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536, 86–90 (2016).
Jarr, K.-U. et al. Effect of CD47 blockade on vascular inflammation. N. Engl. J. Med. 384, 382–383 (2021).
Ceneri, N. et al. Rac2 modulates atherosclerotic calcification by regulating macrophage interleukin-1β production. Arterioscler. Thromb. Vasc. Biol. 37, 328–340 (2017).
Shao, J. et al. Regulation of macrophage polarization by mineralized collagen coating to accelerate the osteogenic differentiation of mesenchymal stem cells. ACS Biomater. Sci. Eng. 8, 610–619 (2022).
Loi, F. et al. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res. Ther. 7, 15 (2016).
Sun, Y. et al. Smooth muscle cell-specific Runx2 deficiency inhibits vascular calcification. Circ. Res. 111, 543–552 (2012).
Byon, C. H. et al. Runx2-upregulated receptor activator of nuclear factor κB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler. Thromb. Vasc. Biol. 31, 1387–1396 (2011).
Nakashima, Y., Raines, E. W., Plump, A. S., Breslow, J. L. & Ross, R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 18, 842–851 (1998).
Hernandez, G. E. et al. Aortic intimal resident macrophages are essential for maintenance of the non-thrombogenic intravascular state. Nat. Cardiovasc. Res. 1, 67–84 (2022).
Hashimoto, K., Kataoka, N., Nakamura, E., Tsujioka, K. & Kajiya, F. Oxidized LDL specifically promotes the initiation of monocyte invasion during transendothelial migration with upregulated PECAM-1 and downregulated VE-cadherin on endothelial junctions. Atherosclerosis 194, e9–e17 (2007).
Valente, A. J., Irimpen, A. M., Siebenlist, U. & Chandrasekar, B. OxLDL induces endothelial dysfunction and death via TRAF3IP2: inhibition by HDL3 and AMPK activators. Free Radic. Biol. Med. 70, 117–128 (2014).
Mollace, V. et al. Oxidized LDL attenuates protective autophagy and induces apoptotic cell death of endothelial cells: role of oxidative stress and LOX-1 receptor expression. Int. J. Cardiol. 184, 152–158 (2015).
Cushing, S. D. et al. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc. Natl Acad. Sci. USA 87, 5134–5138 (1990).
Li, D. & Mehta, J. L. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation 101, 2889–2895 (2000).
Smalley, D. M. et al. Native LDL increases endothelial cell adhesiveness by inducing intercellular adhesion molecule-1. Arterioscler. Thromb. Vasc. Biol. 16, 585–590 (1996).
Takei, A., Huang, Y. & Lopes-Virella, M. F. Expression of adhesion molecules by human endothelial cells exposed to oxidized low density lipoprotein: influences of degree of oxidation and location of oxidized LDL. Atherosclerosis 154, 79–86 (2001).
Apostolov, E. O., Shah, S. V., Ok, E. & Basnakian, A. G. Carbamylated low-density lipoprotein induces monocyte adhesion to endothelial cells through intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. Arterioscler. Thromb. Vasc. Biol. 27, 826–832 (2007).
Toma, L., Sanda, G. M., Deleanu, M., Stancu, C. S. & Sima, A. V. Glycated LDL increase VCAM-1 expression and secretion in endothelial cells and promote monocyte adhesion through mechanisms involving endoplasmic reticulum stress. Mol. Cell. Biochem. 417, 169–179 (2016).
Georgakis, M. K., Bernhagen, J., Heitman, L. H., Weber, C. & Dichgans, M. Targeting the CCL2–CCR2 axis for atheroprotection. Eur. Heart J. 43, 1799–1808 (2022).
Pickett, J. R., Wu, Y., Zacchi, L. F. & Ta, H. T. Targeting endothelial vascular cell adhesion molecule-1 in atherosclerosis: drug discovery and development of vascular cell adhesion molecule-1-directed novel therapeutics. Cardiovasc. Res. 119, 2278–2293 (2023).
Chen, P.-Y. et al. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J. Clin. Invest. 125, 4514–4528 (2015). This paper showed that the endothelial to mesenchymal transition drives inflammation and atherosclerotic plaque burden.
Evrard, S. M. et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat. Commun. 7, 11853 (2016).
Chen, P.-Y. et al. Endothelial TGF-β signalling drives vascular inflammation and atherosclerosis. Nat. Metab. 1, 912–926 (2019).
Chen, Y.-C. et al. A novel mouse model of atherosclerotic plaque instability for drug testing and mechanistic/therapeutic discoveries using gene and microRNA expression profiling. Circ. Res. 113, 252–265 (2013).
Andueza, A. et al. Endothelial reprogramming by disturbed flow revealed by single-cell RNA and chromatin accessibility study. Cell Rep. https://doi.org/10.1016/j.celrep.2020.108491 (2020).
Ivan, L. & Antohe, F. Hyperlipidemia induces endothelial-derived foam cells in culture. J. Recept. Signal Transduct. 30, 106–114 (2010).
Kim, K. et al. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ. Res. 123, 1127–1142 (2018).
Yao, Y. et al. A role for the endothelium in vascular calcification. Circ. Res. 113, 495–504 (2013).
Yao, J. et al. Serine protease activation essential for endothelial–mesenchymal transition in vascular calcification. Circ. Res. 117, 758–769 (2015).
Medici, D. et al. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 16, 1400–1406 (2010).
Yung, L. M., Sánchez-Duffhues, G., Ten Dijke, P. & Yu, P. B. Bone morphogenetic protein 6 and oxidized low-density lipoprotein synergistically recruit osteogenic differentiation in endothelial cells. Cardiovasc. Res. 108, 278–287 (2015).
Sánchez-Duffhues, G. et al. Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J. Pathol. 247, 333–346 (2019).
Boström, K. I., Yao, J., Guihard, P. J., Blazquez-Medela, A. M. & Yao, Y. Endothelial–mesenchymal transition in atherosclerotic lesion calcification. Atherosclerosis 253, 124–127 (2016).
Liang, G. et al. Tenascin-X mediates flow-induced suppression of EndMT and atherosclerosis. Circ. Res. 130, 1647–1659 (2022).
Santovito, D. et al. Noncanonical inhibition of caspase-3 by a nuclear microRNA confers endothelial protection by autophagy in atherosclerosis. Sci. Transl. Med. 12, eaaz2294 (2020).
Takagaki, Y. et al. Endothelial autophagy deficiency induces IL6-dependent endothelial mesenchymal transition and organ fibrosis. Autophagy 16, 1905–1914 (2020).
Singh, K. K. et al. The essential autophagy gene ATG7 modulates organ fibrosis via regulation of endothelial-to-mesenchymal transition. J. Biol. Chem. 290, 2547–2559 (2015).
Helmke, A. et al. Endothelial-to-mesenchymal transition shapes the atherosclerotic plaque and modulates macrophage function. FASEB J. 33, 2278–2289 (2019).
Woo, K. V. et al. Endothelial FGF signaling is protective in hypoxia-induced pulmonary hypertension. J. Clin. Invest. https://doi.org/10.1172/JCI141467 (2021).
Chen, P. Y. et al. FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2, 1684–1696 (2012).
Fu, Z. et al. Oxidized low-density lipoprotein-induced microparticles promote endothelial monocyte adhesion via intercellular adhesion molecule 1. Am. J. Physiol. Cell Physiol. 313, C567–C574 (2017).
Chen, P. Y., Schwartz, M. A. & Simons, M. Endothelial-to-mesenchymal transition, vascular inflammation, and atherosclerosis. Front. Cardiovasc. Med. 7, 53 (2020).
Haynes, B. A. et al. Endothelial-to-mesenchymal transition in human adipose tissue vasculature alters the particulate secretome and induces endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 39, 2168–2191 (2019).
Swirski, F. K. et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc. Natl Acad. Sci. USA 103, 10340–10345 (2006).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Robbins, C. S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
Galkina, E. et al. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J. Exp. Med. 203, 1273–1282 (2006).
Lim, H. Y. et al. Hyaluronan receptor LYVE-1-expressing macrophages maintain arterial tone through hyaluronan-mediated regulation of smooth muscle cell collagen. Immunity 49, 326–341 (2018).
Williams, J. W. et al. Limited proliferation capacity of aortic intima resident macrophages requires monocyte recruitment for atherosclerotic plaque progression. Nat. Immunol. 21, 1194–1204 (2020).
Gerhardt, T. & Ley, K. Monocyte trafficking across the vessel wall. Cardiovasc. Res. 107, 321–330 (2015).
Moore, K. J., Sheedy, F. J. & Fisher, E. A. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13, 709–721 (2013). This review summarizes the role of macrophages throughout atherogenesis.
Bäck, M., Yurdagul, A. Jr., Tabas, I., Öörni, K. & Kovanen, P. T. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 16, 389–406 (2019).
Zernecke, A. et al. Meta-analysis of leukocyte diversity in atherosclerotic mouse aortas. Circ. Res. 127, 402–426 (2020). This scRNA-seq meta-analysis highlighted the transcriptional heterogeneity of macrophages.
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010). This study demonstrated the importance of the NLRP3 inflammasome during atherogenesis.
Spann, N. J. et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151, 138–152 (2012).
Patterson, M. T. et al. Trem2 promotes foamy macrophage lipid uptake and survival in atherosclerosis. Nat. Cardiovasc. Res. 2, 1015–1031 (2023).
Dib, L. et al. Lipid-associated macrophages transition to an inflammatory state in human atherosclerosis, increasing the risk of cerebrovascular complications. Nat. Cardiovasc. Res. 2, 656–672 (2023).
Barrett, T. J. Macrophages in atherosclerosis regression. Arterioscler. Thromb. Vasc. Biol. 40, 20–33 (2020).
Oesterle, A., Laufs, U. & Liao, J. K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 120, 229–243 (2017).
Liu, C. et al. Statins improve endothelial function via suppression of epigenetic-driven EndMT. Nat. Cardiovasc. Res. 2, 467–485 (2023).
Miano, J. M. & Berk, B. C. Retinoids: versatile biological response modifiers of vascular smooth muscle phenotype. Circ. Res. 87, 355–362 (2000).
Zhou, B. et al. All-trans-retinoic acid ameliorated high fat diet-induced atherosclerosis in rabbits by inhibiting platelet activation and inflammation. J. Biomed. Biotechnol. 2012, 259693 (2012).
Wu, Y. et al. ATRA improves endothelial dysfunction in atherosclerotic rabbits by decreasing CAV‑1 expression and enhancing eNOS activity. Mol. Med. Rep. 17, 6796–6802 (2018).
Huize, P. et al. Retinoic acid signaling modulates smooth muscle cell phenotypic switching in atherosclerosis through epigenetic regulation of gene expression. Preprint at bioRxiv https://doi.org/10.1101/2022.11.09.515888 (2022).
Langlois, M., Duprez, D., Delanghe, J., De Buyzere, M. & Clement, D. L. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation 103, 1863–1868 (2001).
Nakata, Y. & Maeda, N. Vulnerable atherosclerotic plaque morphology in apolipoprotein E-deficient mice unable to make ascorbic acid. Circulation 105, 1485–1490 (2002).
Gokce, N. et al. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 99, 3234–3240 (1999).
Arakawa, E. et al. l-Ascorbic acid stimulates expression of smooth muscle-specific markers in smooth muscle cells both in vitro and in vivo. J. Cardiovasc. Pharmacol. 42, 745–751 (2003).
Pakala, R., Stabile, E., Jang, G. J., Clavijo, L. & Waksman, R. Rapamycin attenuates atherosclerotic plaque progression in apolipoprotein E knockout mice: inhibitory effect on monocyte chemotaxis. J. Cardiovasc. Pharmacol. 46, 481–486 (2005).
Castro, C. et al. Rapamycin attenuates atherosclerosis induced by dietary cholesterol in apolipoprotein-deficient mice through a p27 Kip1-independent pathway. Atherosclerosis 172, 31–38 (2004).
Liu, X., Tang, Y., Cui, Y., Zhang, H. & Zhang, D. Autophagy is associated with cell fate in the process of macrophage-derived foam cells formation and progress. J. Biomed. Sci. 23, 57 (2016).
Varghese, Z., Fernando, R., Moorhead, J. F., Powis, S. H. & Ruan, X. Z. Effects of sirolimus on mesangial cell cholesterol homeostasis: a novel mechanism for its action against lipid-mediated injury in renal allografts. Am. J. Physiol. Renal Physiol. 289, F43–48, (2005).
Liu, R. et al. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 128, 2047–2057 (2013).
Liu, M. et al. H3K4 di-methylation governs smooth muscle lineage identity and promotes vascular homeostasis by restraining plasticity. Dev. Cell 56, 2765–2782 (2021).
Chen, W. Q. et al. Oral rapamycin attenuates inflammation and enhances stability of atherosclerotic plaques in rabbits independent of serum lipid levels. Br. J. Pharmacol. 156, 941–951 (2009).
Luo, Z. et al. Moderate autophagy inhibits vascular smooth muscle cell senescence to stabilize progressed atherosclerotic plaque via the mTORC1/ULK1/ATG13 signal pathway. Oxid. Med. Cell. Longev. 2017, 3018190 (2017).
Mehlen, P. et al. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 395, 801–804 (1998).
Llambi, F., Causeret, F., Bloch‐Gallego, E. & Mehlen, P. Netrin‐1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 20, 2715–2722 (2001).
Lengrand, J. et al. Pharmacological targeting of netrin-1 inhibits EMT in cancer. Nature 620, 402–408 (2023).
Cassier, P. A. et al. Netrin-1 blockade inhibits tumour growth and EMT features in endometrial cancer. Nature 620, 409–416 (2023).
van Gils, J. M. et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat. Immunol. 13, 136–143 (2012).
Schlegel, M. et al. Silencing myeloid netrin-1 induces inflammation resolution and plaque regression. Circ. Res. 129, 530–546 (2021).
Wawruszak, A. et al. Histone deacetylase inhibitors and phenotypical transformation of cancer cells. Cancers https://doi.org/10.3390/cancers11020148 (2019).
Lecce, L. et al. Histone deacetylase 9 promotes endothelial–mesenchymal transition and an unfavorable atherosclerotic plaque phenotype. J. Clin. Invest. 131, e131178 (2021).
Cao, Q. et al. Histone deacetylase 9 represses cholesterol efflux and alternatively activated macrophages in atherosclerosis development. Arterioscler. Thromb. Vasc. Biol. 34, 1871–1879 (2014).
Yoshida, T., Gan, Q., Shang, Y. & Owens, G. K. Platelet-derived growth factor-BB represses smooth muscle cell marker genes via changes in binding of MKL factors and histone deacetylases to their promoters. Am. J. Physiol. Cell Physiol. 292, C886–C895 (2007).
Zhang, M. et al. HDAC6 regulates the MRTF-A/SRF axis and vascular smooth muscle cell plasticity. JACC Basic Transl. Sci. 3, 782–795 (2018).
Hubbert, C. et al. HDAC6 is a microtubule-associated deacetylase. Nature 417, 455–458 (2002).
Teixeira, A. F., Ten Dijke, P. & Zhu, H. J. On-target anti-TGF-β therapies are not succeeding in clinical cancer treatments: what are remaining challenges? Front. Cell Dev. Biol. 8, 605 (2020).
Patel, A. P., Wang, M., Kartoun, U., Ng, K. & Khera, A. V. Quantifying and understanding the higher risk of atherosclerotic cardiovascular disease among South Asian individuals. Circulation 144, 410–422 (2021).
Warmbrunn, M. V. et al. Networks of gut bacteria relate to cardiovascular disease in a multi-ethnic population: the HELIUS study. Cardiovasc. Res. 120, 372–384 (2024).
Shen, X. et al. Gut microbiota and atherosclerosis-focusing on the plaque stability. Front. Cardiovasc. Med. 8, 668532 (2021).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Seldin, M. M. et al. Trimethylamine N‐oxide promotes vascular inflammation through signaling of mitogen‐activated protein kinase and nuclear factor‐κB. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.115.002767 (2016).
Sharma, N. et al. Myeloid Krüppel-like factor 4 deficiency augments atherogenesis in ApoE−/− mice—brief report. Arterioscler. Thromb. Vasc. Biol. 32, 2836–2838 (2012).
Chin, D. D. et al. Long-term, in vivo therapeutic effects of a single dose of miR-145 micelles for atherosclerosis. Bioact. Mater. 27, 327–336 (2023).
Chin, D. D. et al. miR-145 micelles mitigate atherosclerosis by modulating vascular smooth muscle cell phenotype. Biomaterials 273, 120810 (2021).
Cordes, K. R. et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460, 705–710 (2009).
Deloukas, P. et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 45, 25–33 (2013).
Döring, Y. et al. Vascular CXCR4 limits atherosclerosis by maintaining arterial integrity: evidence from mouse and human studies. Circulation 136, 388–403 (2017).
Cimen, I. et al. Targeting a cell-specific microRNA repressor of CXCR4 ameliorates atherosclerosis in mice. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.adf3357 (2023).
Virchow, R. C. Cellular Pathology. As based upon Physiological and Pathological History [Translated from the 2nd edn of the original by Chance, F.] (John Churchill, 1860).
Anitschkow, N. Uber die veranderungen der kaninchenaorta bei experimenteller cholesterinsteatase. Beitr. Pathol. Anat. 56, 379–404 (1913).
Poole, J. C. & Florey, H. W. Changes in the endothelium of the aorta and the behaviour of macrophages in experimental atheroma of rabbits. J. Pathol. Bacteriol. 75, 245–251 (1958).
Ross, R. & Glomset, J. A. Atherosclerosis and the arterial smooth muscle cell. Science 180, 1332–1339 (1973).
Jonasson, L., Holm, J., Skalli, O., Bondjers, G. & Hansson, G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 6, 131–138 (1986).
Boring, L., Gosling, J., Cleary, M. & Charo, I. F. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897 (1998).
Ridker, P. M. et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New Engl. J. Med. 359, 2195–2207 (2008).
Altschul, R. Selected Studies on Arteriosclerosis (Charles C. Thomas, 1950).
Feil, S. et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circulation Res. 115, 662–667 (2014).
Acknowledgements
A.M. is supported by a Heart Foundation Vanguard grant (NHF1017) and a New South Wales Cardiovascular Research Capacity Program grant (DOH1024). E.A.F. is supported by a National Heart, Lung and Blood Institute grant (National Institutes of Health R01HL084312). A.L. is supported by an Australian Government Research Training Program Scholarship.
Author information
Authors and Affiliations
Contributions
A.L. drafted the manuscript with input from J.M.M., E.A.F. and A.M. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Jason Kovacic, Nathan Palpant and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, A., Miano, J.M., Fisher, E.A. et al. Chronic inflammation and vascular cell plasticity in atherosclerosis. Nat Cardiovasc Res 3, 1408–1423 (2024). https://doi.org/10.1038/s44161-024-00569-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44161-024-00569-y
This article is cited by
-
Nano-therapeutics targeting the macrophage-based microenvironment in the treatment of atherosclerosis
Journal of Translational Medicine (2025)
-
Ultrasound-driven atherosclerosis nanomedicine: from mechanical, cavitation, and sonodynamic therapies to bedside translation
Journal of Nanobiotechnology (2025)
-
Sentrin‐specific protease 3 (SENP3)-mediated Krüppel-like factor 4 (KLF4) deSUMOylation regulates vascular smooth muscle cell phenotypic switching in atherosclerosis
Molecular Biomedicine (2025)
-
Preoperative red cell width distribution to albumin ratio predicts early radial artery occlusion following coronary intervention via conventional transradial access
Scientific Reports (2025)
-
Single-cell RNA-seq analysis of mouse carotid artery under disturbed flow and human carotid plaques identifies key cell populations in atherosclerosis development
Scientific Reports (2025)