Abstract
Ecological restoration is an increasing global priority and is critical to reverse ecosystem decline caused by anthropogenic impacts. We outline the approach to marine restoration in Gökova Bay, Türkiye and present observations from lesser to more highly assisted restoration interventions and trials, including enforcing multi-habitat No Fishing Zones (including Posidonia oceanica, macroalgae and rocky reef) within a wider Marine Protected Area network. We consolidate evidence from ecological monitoring, small-scale fisheries catch data, marine patrol threat monitoring and a macroalgae trial intervention. It remains difficult to decipher interactions in this system where anthropogenic threats persist and change is not linear but we observe that areas of restoration (No Fishing Zones) demonstrated a lower proportion of Non Indigenous Species compared to fished areas. This work provides a new case study of the progression of multi-habitat interventions and the potential for marine ecosystem recovery in the eastern Mediterranean Sea, informing restoration at scale.
Similar content being viewed by others
Introduction
Sustainable Development Goal 14 (Life Below Water)1 highlights the importance of safeguarding and reviving marine ecosystems, while Target 2 of the 2022 Kunming-Montreal Global Biodiversity Framework commits to ‘Restore 30% of all Degraded Ecosystems’2. Though open to interpretation on the nature and mechanisms of restoration3, a growing number of international commitments target the restoration of degraded and fragmented ecosystems including the EU Nature Restoration Law (2024) and UN Decade of Ecosystem Restoration (2021–2030)4. Biodiversity remains under anthropogenic pressures and beyond the conservation community, stakeholders are recognising ecological restoration is necessary, not only for ecological and climate goals but also for social priorities5. Understanding the potential for marine restoration in different regions and across habitats is important to accelerate scaling and implementation. More holistic, reliable documentation of restoration goals, approaches and the progress of ecological indicators is therefore needed6.
The Mediterranean Sea is the largest semi-enclosed Sea on earth, and the region provides complex environmental and cultural connections between Europe, Asia and Africa7. Its marine and coastal ecosystem is characterised by varied bathymetry, diverse threatened and commercially important biodiversity8, high endemism8 and critical habitats9,10. However, long-term and intensive anthropogenic influence has shaped the region11,12 and although reference baselines for ecosystems here are limited, food-webs13 and habitats9,10 in the Mediterranean Sea now demonstrate significant and sustained degradation14,15.
Threats to the Mediterranean region include development along the Mediterranean coastline which has expanded with impacts from disturbance and habitat loss16. The eastern Mediterranean also remains one of the most overfished regions in the world15 with implications at both regional and local scale13,14. Impacts of fishing are complex and can be highly specific for species and habitats but destructive fishing practices17 have added to the widescale destruction and fragmentation of habitats as ecosystem integrity, resilience and complexity continue to decline18,19,20. As a result there are high pressures on fish which provide essential but diminishing livelihoods for small-scale fishers (SSF) of this region21, including on the Mediterranean coast of Türkiye22,23 where the present study was conducted.
Despite near-total isolation, important links through the Gibraltar Strait and Turkish Straits open the Mediterranean Sea to the Atlantic Ocean and Black Sea. In addition, the Suez Canal construction in 1869 artificially opened the eastern Mediterranean Sea to the Red Sea, instigating a one-way migration of an estimated 1000 Non-Indigenous Species (NIS) into the eastern Mediterranean, growing each year24. This Lessepsian migration has altered food-webs and ecological diversity25 as Invasive Alien Species (IAS) lacking natural predation outcompete native biota with an estimated annual cost of USD 1 billion24,26,27,28. Climate change has increased the speed of this migration26,29,30.
The Mediterranean is recognised as a climate change hotspot and the region is warming faster than the global mean31,32. Seasonality is distinct, but environmental gradients are strong and the eastern Mediterranean already experiences higher temperature and salinity, and lower productivity than the west7,33. Marine heatwaves (anomalous, sustained high temperatures)34 have been linked to negative socio-ecological impacts including mass mortality of marine species35 and toxic algal blooms36. Immobile species and habitats are particularly vulnerable, for example slow-growing, endemic seagrass Posidonia oceanica is predicted to regress under future climate scenarios, impacting the co-benefits and ecosystem functions it provides7,33,37.
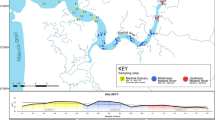
The study area considered is Gökova Bay, recognised as a highly biodiverse part of the Turkish coast38,39. It forms part of the coastline of Muğla Province of south west Türkiye, at the south eastern limit of the oligotrophic Aegean Sea (Fig. Fig 1). The coastline of Gökova Bay extends ~ 400 km and has distinct bathymetry including two deep basins40,41. Small-scale fisheries here are vital for coastal communities, characterised by their use of small fishing vessels (<12 m), traditional small-scale fishing gears and limited inshore range, supporting livelihoods and local economies.
The coastal zone of Gökova Bay includes rocky shores, shingle beaches and sea cliffs38. Near-shore habitats within MPAs include meadows of P. oceanica43,44,45, important not only for biodiversity but also climate priorities, sequestering carbon into biomass and trapping organic matter within roots and rhizomes over longer time frames46,47 and therefore acting as both an autochthonous source of organic matter and a store of allochthonous organic matter48. Remote sensing mapping increasingly shows greater spatial and temporal resolution on P. oceanica in the Mediterranean49 but mapping within Gökova Bay has not encompassed entire ecosystems or multiple habitats so is not yet sufficient resolution or accuracy45 to inform marine spatial planning or connectivity assessment. Macroalgae is another important vegetated benthic habitat found in Gökova Bay, with varied species and forms such as canopy-forming, coralline and turf algae, essential for a diversity of vertebrates and invertebrates at different life stages50. Knowledge on historical composition of macroalgae in the Mediterranean is limited50 although coverage is assessed as regionally low within Gökova Bay51, and canopy-forming species are particularly under threat38,50,51. In addition to these vegetated habitats, areas of rocky reef provide a substrate for macroalgae and a complex structure which provides refuge, including for juvenile fish52. In total, 73% of all marine fish species in Türkiye have been observed in Gökova Bay53 and over 700 species of macrofauna and macroflora were identified during surveys in 2006 within the supralittoral zone to 55 m depth38,54. Coastal caves act as breeding and resting sites for Mediterranean monk seal (Monachus monachus)55, whose eastern Mediterranean sub-population has less than 450 individuals56 and Boncuk Bay in the south of Gökova Bay is now recognised as an Important Shark and Ray Area (ISRA)57 for sandbar shark (Carcharhinus plumbeus), Endangered in the Mediterranean58. In the restoration efforts described below we consider Gökova Bay as a coastal seascape, i.e. ‘a physical mosaic of interacting habitats occupying the coastal environment across time and space’59,60.
In 1988, the inner part of Gökova Bay was officially gazetted by the Turkish Government as a Special Environmental Protection Area (SEPA, Gazette No. 19863) to protect both natural and cultural assets of the region (e.g. Kadın and Akçapınar streams, and Liquidambar orientalis sweet gum forest). SEPA legislation provides a contiguous legal protection to protect environmental values, define usage and protection, and determine responsibilities for zoning61,62. The SEPA was extended first in 1990 (Gazette No. 20702) and then in 2010 (Gazette No. 27793) to cover both marine and land area ( ~ 1900 km2).
Despite this designation, fish census transects between 2006 and 2007 demonstrated overfishing and depletion, with Gökova Bay presenting the lowest fish biomass ( ~ 4 g/m2) of 34 sites sampled across five Mediterranean countries51. Biomass figures51 were aligned with urgent, anecdotal reports from SSF of declines in fish catch in Gökova Bay42. Concerns centred on illegal fishing activities and fishing in bays close to shore, including semi-industrial purse seine fishing and trawling42. The study site also demonstrated amongst the lowest macroalgal biomass51 suggesting the degraded state of the Bay, with a high proportion of herbivorous IAS rabbitfish observed (Siganus luridus and Siganus rivulatus) indicating altered or unbalanced trophic webs and grazing pressures51,63. While temperate native herbivorous fish consume macroalgae at a high rate, they have been observed to feed exclusively on established macroalgae64. On the other hand, Lessepsian IAS feed both on established macroalgae and macroalgal recruits, depleting macroalgal cover and disturbing the natural ecosystem balance51. Cystoseira spp. provide an important but declining habitat, under pressure from IAS such as Siganus spp. with limited research and implications for marine restoration63,65. In the wider eastern Mediterranean, IAS grazing has created areas devoid of erect algae with resulting declines in the biodiversity they support and function of these habitats51,63 showing the need to understand the restoration potential and approaches required.
The restoration approach applied in Gökova Bay was developed in a step-wise manner in response to the reported declines in fish, and has evolved through collaborative effort between local, national and international stakeholders. This initially included fisheries cooperatives, scientific institutions, Non-Governmental Organisations and Government bodies resulting in gazettement of six No Fishing Zones (NFZs) in 2010 (Gazette No. 27637). NFZs were designated under Turkish national law, situated within existing Fisheries Restricted Areas (FRAs, 1991, Gazette No. 20800) to protect marine ecosystems (which include rocky reef, P. oceanica and macroalgae habitats) and support restoration efforts, with details of the process previously documented42. In 2020, an additional NFZ was added to the network (Gazette No. 31221), bringing the total to seven (Table 1, Fig. 1).
In recognition of the ecosystem degradation and urgency for action, threat reduction to enable natural recovery was the objective of the incremental expansion of the MPA network, aiming to enhance fish catch66 and protect higher trophic level predators67 of commercial and conservation concern (including Epinephelus spp. of grouper, M. monachus and C. plumbeus). The network therefore had multiple simultaneous yet informal goals related to the recovery of ecological, cultural and socio-economic values, including re-instating ecological integrity and associated resilience68 of historically degraded marine habitats and fisheries. While MPAs are commonly viewed as central tools in conservation, here the MPA network establishment, enforcement and expansion are considered as key steps in an ongoing process of ecological restoration due to their starting condition as highly degraded53,63 areas with clear evidence of disruption to natural ecological processes both within the protected areas and in surrounding waters. The ambition of the Gökova MPA network is aligned with the Society of Ecological Restoration definition of ecological restoration in that it is ‘assisting the recovery of an ecosystem that has been degraded, damaged, or destroyed’66. That said, no ecological reference model or reference site66 was selected in the MPA network design process, pre-degradation baselines (sensu CBD2) were largely unavailable and there was no stated ecosystem restoration target set at the outset.
With the ambition of building functionality and resilience while enhancing the scale of the network and restoration potential, a collaboration of stakeholders (led by local NGO the Mediterranean Conservation Society hereafter AKD by their Turkish acronym) pursued implementation, establishing patrols, employing local community members as marine rangers. Rangers recorded incidences of illegal fishing to track threat, sometimes in collaboration with the Turkish Coast Guard Command bringing the authority to issue sanctions as deterrents. Focus was placed on implementing localized and increasingly assisted interventions at key sites to fill knowledge gaps, inform interventions and complement natural regeneration69 in NFZ. For example, a macroalgae experimental trial presented in this study aimed to understand pressures from herbivorous IAS and potential for restoration63, while other project activities not presented include underwater clean ups removing discarded or lost fishing gears70 which smother benthic habitats and pose a risk to marine life71, establishment and strengthening of markets for IAS aiming to reduce pressures on native biota72 and the creation of abiotic habitat through construction of a dry resting/breeding ledge in a remote coastal cave complimenting restoration activities, with M. monachus now documented using the ledge73.
A management plan was established for Gökova SEPA (2020–2024) covering broad socio-cultural targets and although commitments to species monitoring were outlined, specific ecological recovery targets were not defined74. Indicators monitoring species, habitats, threats and local fisheries were formalised within a monitoring framework established in 2019 alongside defined, project-driven targets. Ecological monitoring is ongoing (e.g. M. monachus cave use, C. plumbeus observations, macroalgae coverage, P. oceanica shoot count density and expansion) complimented by small-scale fisheries cooperative fish catch data collection, to record an ongoing process of restoration towards recovery and wider fisheries impacts in Gökova Bay (Figs. 2, 3). Monitoring aims to move beyond spatial protection targets to inform multiple aspects of restoration effectiveness.
Restoration interventions are ordered on a continuum (after Chazdon et al.69) estimated by intensity of intervention needed for recovery, ranging between lightly (top) and intensively (bottom) assisted recovery.
Though widely used, the dichotomy of active versus passive restoration is increasingly criticised6,69 and a more nuanced restoration continuum has been proposed representing multiple levels of assistance, of equal potential value for restoration, recovery and ecological succession75. We adopt the terms natural regeneration and assisted regeneration to reflect this continuum69, in lieu of the active and passive restoration terminology66.
Natural regeneration66 (or unassisted natural recovery69) as a restoration approach can include activities to remove the source of degradation or human disturbance to prevent further ecosystem decline (e.g. according to Chazdon et al.’s69 classification for river ecosystems and Gann et al.66 who state natural (or spontaneous) regeneration encompasses passive restoration ‘which may include removal of contamination, inappropriate grazing, over-fishing’). In the marine ecosystem however, restoration activities along this continuum of assistance are not yet well-defined. MPA and establishment with the ambition of ecological recovery through threat reduction within degraded marine ecosystems arguably fit on this continuum (see Fig. 4). No significant reconstruction of biota (sensu Gann et al.66; e.g. major reintroductions) have been implemented in Gökova Bay to-date, though the construction of an artificial monk seal ledge provides a notable example of successful physical habitat creation73.
Individual threats to the Mediterranean marine environment have been broadly characterised, yet less is known about interactions between multiple threats through time and space7. The designation and implementation of highly and fully restricted MPAs has been unevenly distributed, with efforts and legislation focussed less in the southern and eastern Mediterranean ecoregions9. Evidence of change taking place in the eastern Mediterranean Sea is therefore limited, which inhibits impactful learning from restoration and conservation action in this region9. To contribute to this knowledge gap we present data from observations in Gökova Bay between 2013 and 2023, and reflect on threat, recovery potential and change within this MPA network as part of a broad restoration effort. Though baseline assessments have emerged from the Aegean76, to our knowledge this is the first attempt to present observations from an eastern Mediterranean seascape-scale restoration project synthesising long-term data across trophic levels, habitats and restoration approaches and considering progress towards ecosystem recovery over eleven years.
Methods
Fish Biomass
An underwater visual survey method was used, to estimate the number, species and length of fish observed during monitoring transects within and outside NFZ51. Data collection took place annually in June between 2013 to 202351. Three NFZs in Gökova Bay were surveyed, İngiliz Limanı NFZ (6.13 km²), Löngöz NFZ (0.36 km²) and Karacasöğüt NFZ (6.08 km²) and three sites outside NFZ were surveyed (within FRAs) including Ballısu, Körmen-Çiçekli and Löngöz (Fig. 3).
Eight sites within the three NFZs were selected and three sites outside NFZ, maximising distance between sites (at least 500 m where possible within the size of the NFZ) on areas of rocky reef habitat. One 50 x 10 m dive transect was completed at each site, at a depth of 15 to 20 m. The observer swam once along the transect at a constant speed, recording the species and estimating the length of each fish observed in 5 to 10 cm increments67,77. Surveys were completed by an experienced diver familiar with fish taxonomy and identification of local taxa. The same individual completed this monitoring every year to maximise consistency of visual length estimates between years.
Fish biomass (wet mass) was estimated from fish length data using a length-weight relationship (Weight = a Lengthb). Species specific a and b values were obtained preferentially from available literature or from the FishBase website if unavailable77. If a and b values were used from FishBase, the most geographically relevant values were selected and the mean value was calculated. Fish trophic level was classified from Sala et al.51.
Fish biomass statistical analysis
NIS are alien species whose presence is a result of human actions that have facilitated their movement through biogeographical barriers78, and we categorised NIS according to Richardson et al.78. Among these NIS, species showing biodiversity, human health and socio-economic impacts were categorized as IAS79. Our aim was to test the relationship between the proportion of indigenous species and NIS in the presence or absence of NFZ designations, and understand change between data collection years. Fish biomass was calculated from the sum of monthly observations, for months with data. We used the proportion of NIS per month as the dependent variable, and the year of data collection and presence of NFZ designations (represented as a dummy variable), as the independent variables. NFZs were assigned the value of 1 to represent sampling sites within NFZs, and 0 to represent sampling sites outside NFZs to differentiate two levels of legal fishing restriction and facilitate analysis on the potential influence of designations.
The Fractional Logit method80 was used for data analyses with robust standard errors, to estimate the proportional change in IAS biomass over time. This method was selected due to its capability of handling the fractional nature of the dependent variable (NIS biomass proportion)80 and the values of 0 and 1, without transforming the data81,82. Robust standard errors account for non-independent and non-identically distributed errors, mitigating their impact on the standard errors and significance tests associated with the fractional logit estimation.
Since the direct interpretation of the fractional logit estimation result is not feasible it was necessary to calculate the marginal effects of the year variable and the dummy variable representing NFZ. The marginal effect of the year variable quantifies change in the proportion of NIS biomass per year. The marginal effect of the dummy variable (NFZ) measures the difference in the proportion of NIS between sites inside and outside NFZ. Assessment of the marginal effects therefore provides potential insight into the specific impacts of the year variable and the presence of NFZ restriction on the proportion of NIS biomass, contributing to our understanding of the dynamics of in NIS and NFZ impact and function.
Marine patrols
Marine patrols were used for NFZ enforcement, surveillance and monitoring of key threat (illegal fishing). Patrols took place by boat, from boat stations in İngiliz Limani, Akyaka and Karacasöğüt (Fig. 3), and data reported was collected between 2017 to 2023 within NFZ boundaries only (Fig. 1). Marine rangers used the SMART mobile application (latest version 1.0.478, desktop version 7.5.6)83 which is integrated with the Cybertracker mobile technology to track patrols generating GPS integrated data on their mobile phone devices (models varied as rangers used their own GPS enabled Android and IOS devices). Patrol route, effort and illegal fishing incidences encountered were recorded.
We analyzed the relationship between patrol effort and the number of illegal fishing activities observed in NFZ (standardised by patrol effort), to understand threat level within NFZ. Python was used for data processing and analyses (including Geopandas, Pandas, Shapely and PyProj libraries). Raw patrol data was processed and transformed into point data and labelled according to NFZ location, excluding points outside NFZ. Points from the same patrol in the same NFZ were converted to LineString format. Patrol durations were calculated from date-time variables using the first and last GPS point of each patrol, and line lengths were calculated to give patrol distance (EPSG:5637 projection). Distance and duration values provide monthly patrol effort for each NFZ. Lost tracks without GPS data, or with incomplete GPS tracks were removed from the data as no information on correct route was available.
Analyses focussed on assessing changes in patrol effort and illegal fishing to understand more about effectiveness of patrols and levels of threat over time. The dependent variable used was the number of detected illegal fishing activities and the independent variable was patrol effort (time or distance patrolled). Count regression analysis was used since the number of illegal fishing incidences is a count variable. Negative Binomial Regression (NBR) analysis was applied following the alpha test. There are instances where the dependent variable takes on integer values but is not categorical. Based on observations, the frequency of an event occurring can be represented as count data. NBR is the preferred count data model when overdispersion makes the Poisson regression model unsuitable.
NBR model (log-lin):
The estimation of NBR models is carried out using the maximum likelihood method.
Fish landings
Fish catch landings data from Akyaka Fishery Cooperative (Fig. 3) was used to determine the catch obtained per day of fishing effort (CPUE)84 in Gökova Bay. The data was collected between 2015 to 2023 and provides information directly from local small-scale fisheries using gillnets and trammel nets (collectively referred to as nets) and longlines. Data on the catch landed by local fishers and sold to the Cooperative provides the CPUE for net (kg/1000 m net/day) and longline fishing (kg/1000 hooks/day). Only species which have economic value within the market are recorded, so this data does not include non-edible species or NIS with no market value. Information on spatial location of fishing grounds, target species, benthic habitat or distance from shore were not recorded and the assumption was made that all catch was from outside NFZ but within Gökova Bay, based on legal restrictions and inshore range.
Analyses looked at overall trends, key species and non-key species to understand the fish catch landed using different fishing gears, and to present annual and seasonal (quarterly) trends. The data were categorised into overall CPUE, CPUE of key species (grouper - Epinephelus aeneus, Epinephelus costae, Myscteroperca rubra - and Penaeid shrimps) and CPUE of non-key species (all other commercial species sold in the cooperative, n = 60).
Linear and logarithmic regression models were used. The linear model provides absolute change, while the logarithmic model shows relative change, interpretable as percentage change. Seasonal effects were analyzed by comparing seasonal (quarterly) CPUE values against winter (quarter four). Winter was used as the baseline season due to adverse weather conditions limiting fishing effort and accessibility to fishing grounds, with fewer fishing trips undertaken and lower overall fishing effort compared to other seasons.
Macroalgae
Macroalgae monitoring was conducted between 2019 to 2023, twice a year during northern hemisphere spring and autumn which are the main seasons for algal reproduction in Turkish coastal waters85. The aim was to examine the effects of NFZs on macroalgae growth and communities, and define average coverage. Three macroalgae monitoring sites were selected to monitor potential differences of macroalgae habitats within and outside NFZ (Fig. 3), including two sites monitored inside NFZs (Ayın and Karacaada) and one site monitored outside the NFZs (Çiçekli). Sites were selected on rocky substratum to be representative of the general macroalgae coverage of Gökova Bay according to previous research38,86, and based on similar hydrodynamic conditions, depth (3–5 meters), presence of macroalgae habitat and coastal structure85. Specific placement of transects was based on snorkel assessment to determine suitable areas.
Monitoring was performed using SCUBA diving in the upper littoral zone where a non-destructive method was used. Transects (50 m length) were marked at each site and 10 quadrats (20 x 20 cm) were placed at equal distances apart on the benthos and photographed (using Canon PowerShot G9 X Mark II, Fantasea Line housing). Total macroalgae relative surface coverage (relative abundance) was quantified by placing a digital grid layered on to the photographic images, using Adobe Photoshop software87. Whenever possible the species of macroalgae was determined using identification guides, or the genus was recorded if species identification was not possible from the images88,89. Coverage was calculated by summing each whole digital grid square covered by each taxon identified, and recorded as percentage coverage. Since macroalgae often have complex forms growing both horizontally and vertically, some species were overlapping and as a result the coverage of macroalgae often exceeds 100% (e.g. macroalgae with a thalli which can grow on coralline algae, or epiphytic macroalgae)88,90.
Macroalgae restoration trial
A trial restoration intervention was established in 2020 to 2021, to understand herbivorous grazing pressure82 (predominantly IAS S. rivulatus and S. luridus) and the potential for recovery of overgrazed macroalgae vegetated ecosystems. Two sites were selected at 10 m depth (Fig. 3) including one site outside NFZs (Ballısu) and one inside Karacasöğüt NFZ (Karacaada). Five fish exclusion cages (60 x 40 x 20 cm) were established at each site in triplicate (15 cages at each site), attached to the hard rocky substrate allowing light and water to enter but excluding fish of a certain size (1 cm2 mesh size). The thallus of Cystoseira sp. were collected from a donor site in Datça, Muğla, and transferred in a box of seawater to the experimental site within one hour of collection. Thallus length measurements were taken before the transplantation. Three thallus were transplanted into each cage and attached using cable ties and underwater epoxy (Fig. 5). Every three months, cages were checked and cleaned and three cages were removed. Thallus measurements were taken starting from the third month of transplantation (Fig. 6).
C. plumbeus
In April 2021 a high-definition solar-powered continuous shark monitoring system was installed in Boncuk Bay (Fig. 3) to understand the presence of C. plumbeus. This system provides data during daylight hours via two un-baited cameras (Paran Tek DCC-960) at a depth of 3 m, and one remotely adjustable camera (Dahua 45x 2MP IP PTZ Speed Dome Camera) positioned on land close to the study site to observe activity from the shore. Continuous monitoring began from April 2021 but disruption during the set-up period resulted in patchy data in 2021, so results presented here are for 2022 only.
Artificial Intelligence (AI) solutions for data analyses (C. plumbeus identification) were trialled but not used due to challenges with the size of the data and the connectivity to internet at the study site, and data was therefore analyzed manually. All footage was viewed by video analysts who recorded the number of shark observations. Individual sharks were not identifiable by visible characteristics, so each observation was counted as a unique sighting of a single shark, unless sharks appeared in one frame together or passed in the same direction immediately after one another and were without doubt unique individuals. If multiple sharks were seen in the same or consecutive footage in this way, this would be counted as one observation. If no sharks were observed within one hour of monitoring this was counted as one No Observation.
Results
Fish biomass
In total, 40 fish species were observed over the study period, with the 14 most abundant species contributing over 90% of the biomass observed (Table 2). NIS accounted for 16.55% of total biomass over the study period, and IAS accounted for 83.37% of the NIS biomass recorded.
The proportions of NIS within and outside NFZ were examined, annually (Supplementary Table 1). The estimated fractional logit model demonstrates statistical significance (Wald test (χ^2) = 14.78, p = 0.000) and the marginal effects of the year variable and the dummy variable representing the NFZs are calculated (Supplementary Table 2 and 3). The marginal effect of the year variable is 0.0199 (SE = 0.039488, p = 0.000), suggesting the proportion of NIS biomass increases annually by 1.99%. This implies that over time, across all sites inside and outside NFZ, the proportion of NIS biomass gradually grows, with a decline in the proportion of native fish biomass during the same period. With 95% probability, the proportion of NIS biomass is projected to increase in future years, within the range of 0.7% and 3.2% per year. The calculated marginal effect of the dummy variable for NFZs is -0.11997 (SE = 0.2214382, p = 0.000), indicating that the proportion of NIS biomass in NFZs is 12% lower compared to out of NFZ. With 95% probability, a decrease in the proportion of NIS biomass in NFZ is expected, within the range of 19.4% to 4.6% during the study period.
Marine Patrols
In total, marine rangers at AKD undertook 25,621 km of SMART patrols within NFZ between 2017 to 2023, covering 3,045 hours of patrolling, encountering and intervening in 483 incidences of illegal fishing during these patrols (Supplementary Table 4). Total patrol effort and incidences within the NFZ are presented (Fig. 7). SMART patrol distance and duration varied within the study years and between NFZ. Higher patrol distance was recorded in Akyaka, Karaca and İngiliz Limanı NFZ due to the location of boat stations here (after 2019, the marine ranger station in İngiliz Limanı relocated to Karaca). Highest patrol effort was recorded in 2019, and lowest in 2017 but there is no trend in the distance and duration of patrols over time. There is a decreasing trend in illegal fishing activities after 2019 with decreasing patrol effort, with a peak in 2019 when patrol effort was highest. The majority of illegal fishing incidences (57 %) were recorded in Akyaka NFZ.
In total, 74% of illegal fishing incidences were classified as professional or amateur, with 26% of unknown fishing classification. Of the 74% known, the majority of incidences of illegal fishing were of amateur fishing rather than professional fishing (87.3%). Incidences of illegal fishing using a fishing line, trolling line or longline made up the majority of incidences recorded (71.5%).
When examining patrol distance, the estimation results of the NBR model are statistically significant (Log-likelihood = -482.64, p = 0.000) and the alpha parameter is significant, confirming the NBR model is appropriate (Supplementary Table 5). The trend coefficient (z = -2.179, p = 0.0293) shows that the number of detected illegal fishing activities decreased by an average of 0.17 incidents per year. The coefficient for patrol distance indicates that for every 1 km increase in patrol distance, the number of detected illegal fishing incidents rises by 0.0069599 (z = 6.289, p = 0.000). An increase of 143.68 km in patrol distance results in the detection of one additional illegal fishing incident.
When examining patrol duration the estimation results of the NBR model are statistically significant (Log-likelihood = -488.8960, p = 0.000) and the alpha parameter is significant, confirming the NBR model is appropriate (Supplementary Table 6). When patrol hours increase by one hour, the detected illegal fishing activity increases by 0.0551 incidents (SE. = 0.0629, p = 0.000) according to marginal effect value of trend variable. When patrol effort increases by 20 hours, the detected illegal fishing activity increases by one additional incident.
To standardise the change between years, the number of illegal fishing incidences was normalised by presenting incidences per patrol distance and duration (Fig. 8). When formal SMART patrols began, incidences were higher (~0.2 incidences per hour and per 10 km patrolled). Despite an initial reduction in 2018, incidences rose again in 2019, 2020 and 2021. There is a decrease in 2022, to the lowest number of incidences per hour and 10 km patrolled in 2023 (<0.1 incidences per hour and per 10 km patrolled, or approximately a quarter of the number of incidences observed from our 2017 baseline).
Fish Landings
The analysis of annual change in CPUE highlights differences between fishing methods and species categories. The overall linear trend for CPUE using nets showed a significant decline over the study period, with an average annual decrease of 10% (p = 0.001). For non-key species, CPUE using nets showed a decline of 13.1% annually (p < 0.001), but a small but significant increase was observed when examining key species caught using nets, with a 1.83% rise per year (p = 0.001). In contrast, CPUE for longlines showed a positive trend overall with an average annual increase of 20.3% (p = 0.001). CPUE for key species showed a strong upward trend, with an annual increase of 51.1% (p = 0.001) and CPUE for non-key species showed an average annual increase of 13.9% (p = 0.001) (Supplementary Table 7).
Using logarithmic and linear regression models for CPUE shows annual change across different fishing methods and species groups. For net fishing overall, there is a significant 10% annual decline (t = -5.14, p = 0.000). For key species (mainly shrimp) for fishing nets, there is a modest but significant annual increase of 1.84% (t = 4.45, p = 0.000). However, non-key species caught by net exhibit a 13% (t = -5.95, p = 0.000) annual decline, signalling a decrease in the catch of other commercially important species. In contrast, longline fishing shows a significant 20.2% (t = 9.56, p = 0.000) annual increase, indicating improved efficiency or greater availability of fish. Longline fishing shows a substantial 51% (t = 4.98, p = 0.000) annual increase in the catch of key species and non-key species also display an annual increase of 13.9% (t = 4.76, p = 0.000).
Examining seasonal trends, the overall CPUE for nets and key species in spring and summer shows a significant increase of 37.4% (t = 0.374, p = 0.022) and 60.3% (t = 2.414, p = 0.0178) respectively, compared to winter season. In autumn, the CPUE for key species caught using nets showed an increase in CPUE of 173.5% (t = 4.703, p = 0.000). The highest increase for key species is observed in autumn, highlighting the most productive fishing season.
Macroalgae
At all three macroalgae survey sites the dominant macroalgae genus observed from photo quadrats was the turf algae Cladophora sp. (filamentous green algae), which showed the majority of coverage. Coralligenous algae such as Lithophyllum sp. and Amphiroa rigida, alongside erect algae Padina pavonica, Laurencia obtusa and Liagora viscida were also observed in much lower quantities ( < 15% at all sites). The comparison between the sites outside and inside NFZ did not show any correlation with macroalgae coverage.
Between 2019 and 2023 the coverage of macroalgae fluctuated between years with no clear trend overall (Fig. 9). In 2019, the total macroalgae coverage was 46.1% in Ayın Koyu, 36.8% in Karacaada and 36.1% in Çiçekli. In 2020 there was a large increase of coverage observed at all the stations. In 2021 and 2022, coverage significantly decreased. In 2023, an increase was again observed at all the stations. Overall from five years of sampling, Ayın Koyu showed the highest total macroalgae coverage followed by Çiçekli and Karacaada.
The mean coverage (%) of macroalgae at three sites (Ayın Koyu and Karacaada inside No Fishing Zone (NFZ), Çiçekli outside NFZ) between 2019 and 2023. The coverage exceeds 100% in some years and sites due to multiple layers of overlapping macroalgae90.
Macroalgae restoration trial
During the experiment, loss of cages was experienced during heavy storms after six months of monitoring, and cages lost were re-established for the following six months. According to the thallus measurements taken, the average growth rate of the thalli in each cage was calculated as 1.22 ± 0.33 cm per three months. After removal of the cages at six months, the thallus were eaten by Siganus spp. immediately. To confirm observations, we set a GoPro camera after the cage removal and left it recording for 12 hours. The camera recordings showed that the thallus were eaten by Siganus species (Fig. 10).
C. plumbeus
C. plumbeus observations were categorised by season and temperature but data in 2022 do not provide a full year of monitoring due to system upgrades and maintenance (Table 3) so analyses therefore focussed on the assessment of C. plumbeus temperature preferences (Table 4).
The sea temperature in the study area fluctuated between 15 °C and 29 °C, presenting a range of thermal conditions and sharks were observed between 17 to 29 °C. Table 4 presents the number of C. plumbeus observations at different sea temperatures, categorised as ≤20 °C or > 20 °C91. A majority of observations (82%) occurred when the water temperature was above 20 °C, indicating a preference of sandbar shark for relatively warmer water temperatures.
The regression analysis suggests higher temperature (>20o C) significantly influences the number of C. plumbeus observations. Higher temperatures are strongly associated with an increase in shark sightings (p = 0.000), meaning that as temperatures rise, more sharks are observed. (Supplementary Table 8).
Discussion
This study observed ecological recovery and restoration in Gökova Bay, an area with spatial protections on degraded marine habitats. Information on the potential for restoration and impact of restoration interventions has historically been overlooked69 or difficult to obtain92, and measuring the effectiveness of restoration at habitat or ecosystem level is challenging93,94, particularly at large spatial scales and for coastal seascapes. Using data spanning eleven years we synthesise ecological change observed in a large-scale marine restoration initiative built upon natural recovery and more recent lightly assisted restoration interventions within a well-enforced MPA network, considering ecological indicators (fish biomass, macroalgae, sharks), fish catch, threat monitoring and the experimental exclusion of herbivorous fish. Below we indicate some avenues for further research, restoration action and ongoing monitoring.
Using NFZ as a tool for restoration within a degraded network, marine patrols are intended as a deterrent to reduce degradation from destructive and illegal fishing activities. Patrols are therefore an essential element of recovery of degraded coastal MPA sites95. High fishing pressure in the eastern Mediterranean14,15, including small-scale fishing, has shaped fish communities in the Aegean sea96 so active enforcement is expected to remain essential to recover native assemblages. We documented marine patrols and illegal fishing incidences encountered inside NFZ, to quantify enforcement effort and threat. Annual patrol effort was varied, including slow initial adoption of SMART in 2017, increased resources for enforcement from 2019 and new focal areas of work beyond Gökova Bay from 2020 in light of MPA expansions. Legal restrictions on patrolling but not on professional fishing in 2020 during the COVID-19 pandemic may have resulted in a lower deterrent effects97, with higher rates of illegal fishing recorded. We observe lower illegal fishing incidences per hour and per 10 km patrolled between 2021 to 2023 suggesting illegal fishing pressures were reducing in NFZ during this period, and that patrol effort may have been sufficient. That said, other external factors (e.g. fisher engagement, community education, high costs of fishing gears during economic instability) may also be influencing rates of illegal fishing. Enforcement agencies and NGO staff cannot be continuously present within the NFZs and although unpredictable patrol schedules are adopted, familiarity with the patrol boat and rangers could affect fishing incidences encountered so alternative observation mechanisms may complement patrols and engagement activities98. Our results provide a reference point66 to understand future MPA implementation and effectiveness95 but future work to look across different levels of enforcement effort in different contexts will help to standardise terminology for different levels of assistance6 and place MPA enforcement alongside other restoration interventions99.
The study identified progress and challenges in moving towards, understanding and evidencing ecological restoration including natural regeneration. Eleven years of fish biomass data collected in Gökova Bay shows an increasing proportion of NIS (majority IAS) within total biomass reflecting pressures from Lessepsian species which are widely reported elsewhere in the southern and eastern Mediterranean regions79,100. On the other hand, highly restricted and enforced NFZ in Gökova Bay (covering multiple benthic habitats including priority rocky reef, P.oceanica and macroalgae habitats) have a higher proportion of native species compared to sites outside NFZ. Coastal areas of the Southern Aegean Sea have been highly affected by impacts from IAS101 and our findings may indicate NFZ here have the potential to bring higher resilience to pressures of trophic imbalance or instability which IAS bring102. Previous research has highlighted the scarcity of information on the effects of MPAs on invasive species, with molluscs and algae more highly represented in literature103 and mixed findings103,104,105 on directions of correlation between MPAs and invasives. Our findings support existing research that coastal MPAs (in our case well-enforced NFZ) can be negatively correlated with NIS (in our case, fish species), within small to medium MPAs104. Fish populations can take years to decades106,107 to respond to well-enforced NFZ and illegal fishing in our study area appears to be reducing only in recent years. The potential of NFZ to help manage pressures from IAS in the eastern Aegean therefore warrants further, longer-term monitoring and could have important implications for prioritizing enforcement of NFZ, marine spatial planning and design of multi-habitat MPAs to provide refuge for species vulnerable to IAS pressures51. Targeted IAS catch within NFZ (e.g. for generalist feeders such as Pterois spp., or herbivores such as Siganus spp.) could enhance the differences seen between NFZ and outside NFZ sites and ensure NFZ do not act as havens for IAS. That said, careful management would be necessary to ensure no detrimental effects on native species and habitats.
On the other hand, the restoration and regeneration of vegetated habitats which support diverse species and are essential within a well-functioning marine ecosystem50 appears to remain constrained. Fish biomass surveys suggested the invasive species with the highest proportion of total biomass is herbivorous IAS S. rivulatus, consistent with previous research in Gökova Bay63 and the experimental trial documenting restoration potential within degraded macroalgal habitat. Within this trial we hypothesized that restoration of the macroalgal forests in NFZs would be more successful than at sites outside NFZ due to expected lower pressures from herbivorous IAS grazers. Fish exclusion cages did help Cystoseira sp. to grow and reproduce, but following removal of the cages Siganus spp. grazed the full thallus with no difference observed inside or outside NFZ. Our results align with work elsewhere (including sites south east from Gökova Bay) suggesting grazing pressure by Siganus spp. remains a primary reason for the shift to a barren landscape in macroalgal assemblages of Gökova Bay63,64. One promising observation after cage removal and grazing was a germinated sprout of Cystoseira sp. detected hidden and protected in the epoxy, demonstrating suitable conditions for recruitment do exist here, and aligning with previous studies suggesting Cystoseira sp. canopy has the potential to recover if herbivore pressure was reduced in Gökova Bay63. Since IAS pressures appear to be lower within NFZ sites and threats from illegal fishing appear to be declining in recent years, a repeat of this trial in future under changing conditions could be insightful. Continuous video recording during future trials could also help us to understand potential impacts on macroalgae from other herbivores108,109.
Macroalgae monitoring showed no correlation between NFZs and macroalgal coverage, suggesting other abiotic or biotic factors beyond fishing pressures are controlling macroalgal regeneration and communities. Results showed that Cladophora sp. (filamentous green algae) was the dominant macroalgae at all stations, probably because of its fast growth rate resulting in higher ability to withstand grazing110. This provides different observations from previous research in 2006 between 0 to 45 m depth in Gökova Bay where branching red alga Liagora viscida constituted 98 % of the total macroalgal biomass observed in Çiçekli (the out of NFZ site, within our study), brown algae Cystoseira corniculata was reported in Ayın Koyu station and Cladophora coelothrix (17%), Jania longifurca (57%) and Amphiroa rigida (17%) were recorded in Karacaada86. Although difference in study depth will account for some of these differences with our study111, the very low density and less diverse erect macroalgae we observed in comparison to this previous work reiterates the significance of overgrazing now observed in Gökova Bay. Previous research in the Red Sea112 suggested S. luridus primarily consumes brown algae Lobophora variegata and Sargassum spp. and Siganids were observed to preferentially consume brown algae in autumn and red algae in winter113,114. However, in conditions of low algal density (such as Gökova Bay), Siganus spp. exhibit non-selective feeding behaviour, consuming available algae indiscriminately115 highlighting this significant challenge for the recovery of native macroalgae in the eastern Mediterranean.
Reductions in herbivorous grazing pressure through more highly assisted restoration could support ecosystem recovery (e.g. targeted fishing of IAS outside NFZ and promotion of herbivorous fish species within the supply chain). Alternatively, restoration of other native macroalgae species of different thallus types, such as coralline algae with calcareous structures not subject to the same grazing impacts from Signaus spp., could become an important restoration focus. However, these calcareous macroalgae are highly vulnerable to climate impacts and may not support the species dependent on Cystoseira canopies116. Incidental observations during monitoring suggested some erect macroalgae survived at depths outside of Siganus spp. grazing pressures (less than 5 cm and greater than 30 m where temperature is lower). Siganus spp. are eurythermic, tolerating a wide temperature range which has facilitated their spread from the Red Sea117, but greater depths within the photic zone may be below their observed tolerable temperature range118. Increases in seawater temperature at greater depths may be negatively affecting macroalgal reproduction and growth rates119,120, consistent with temperature data and prolonged marine heatwaves observed34,118. Analyzing the species composition and potential for macroalgae regeneration at greater or lesser depths than our study could improve understanding of restoration potential50,111 beyond the impacts from major herbivorous IAS grazing.
Knowledge on historical composition of macroalgae in Gökova Bay is limited, presenting challenges for measuring restoration success50, assessing vulnerability of macroalgal assemblages and associated organisms, and understanding future scenarios. Low nutrient availability in the south eastern Aegean means resilience of macroalgae is already likely to be low121 and predicting thresholds for macrophyte recovery is complex, non-linear and highly site specific121. NFZ could become important refuge for native species (e.g. through eliminating pressures from bottom contact fishing gears or reducing IAS proportions) but the macroalgae monitoring suggests the conditions provided by NFZ are not yet sufficient to enable macroalgae regeneration. Quantifying the level of reduction of IAS herbivores (or duration of exclusion) to allow macroalgae regeneration of Cystoseira spp. in the specific conditions of Gökova Bay may be complex122, but could be a useful next step to inform restoration potential and guide more highly-assisted macroalgal restoration activities.
Restoration of foundation species can return lost ecosystem services, and restored habitats can increase the abundance of commercially valuable species123,124. The benefits of well-enforced MPAs for fishers have been demonstrated both in the Mediterranean125 and globally through movement of juvenile adults and larval dispersal, resulting in increased CPUE for fishers126,127. Networks including NFZ, such as that examined here, are expected to deliver scaled ecological and economic benefits beyond their boundaries127,128 so our analyses examined the catch of local fisheries functioning within Gökova Bay but beyond NFZ boundaries. Results from local small-scale fisheries potentially reflect broader ecological challenges and trends in the eastern Mediterranean14. The significant annual decline observed in CPUE of SSF using nets is characteristic of wider overfishing and habitat degradation51. The decline in the CPUE of non-key species using nets may be consistent with research showing non-target species in heavily fished areas decline more rapidly as ecosystem resilience diminishes129. This may also reflect ecological damage caused by IAS such as the silver-cheeked toadfish (Lagocephalus sceleratus), first recorded in the region in 2003130 and linked to declines in fish biomass from disruption to local food webs and economic loss experienced by local fisheries from reduced CPUE131. A minor annual increase in CPUE of key species, such as shrimp, more effectively targeted by trammel nets, suggests some resilience or effective management practices for these species. Rising costs (e.g. fuel, netting, rigging) or alternative income opportunities (e.g. tourism) could be reducing soak times and/or deterring fishers from maintaining and replacing nets, potentially leading to reduced fishing hours per day of fishing and a lower CPUE.
In contrast, the annual increase in CPUE for longlines suggests more efficient techniques have been adopted by SSF or increases in longline-targeted species. NFZs can lead to biomass recovery for higher trophic level predators and commercially valuable species, and longlines can be efficient in targeting these species42. While NFZs may offer localised benefits for key species and possible spill-over effects for SSF132, they may not address broader threats degrading ecosystems outside protected areas133. The increase in SSF longline CPUE for key and non-key species could suggest this fishing gear is more resilient to changes in fish distribution and abundance than nets. Reduction in the use of nets due to adoption of longlines may help safeguard some species vulnerable to net by-catch and entanglement134,135 (e.g. Mediterranean monk seals in Gökova Bay73), but other species may be at greater risk from longlines. By-catch is not well documented in the eastern Mediterranean136,137 and we do not have information on the unintended catch from small-scale fishing in Gökova Bay so a more comprehensive understanding is needed across species and gear types.
CPUE for nets and longlines peaked in autumn, particularly for key species, potentially highlighting optimal fishing due to species migrations or breeding cycles129. The seasonal variation and trends observed align with studies documenting higher fish availability during specific periods due to natural cycles and environmental factors63, although soak times may also vary seasonally. Rising sea surface temperatures are linked to shifts in species distribution and proliferation of NIS in the Mediterranean, which can disrupt traditional fishing patterns and decrease predictability133. Climate change is therefore likely to continue to impact both distribution and composition of catch, and could impact SSF engagement and support for restoration interventions unless they are fully engaged in adaptation planning138. In light of high costs of fishing, SSF in Gökova are thought to be balancing fishing with income from tourism. Flexible fishing practices to align fishing effort with periods of higher fish availability and key seasonal peaks could improve efficiency and sustainability of fish stocks while shortening soak times of fishing gears could mitigate by-catch139 and harm to non-key species. Trends in CPUE of increased yields close to the MPA boundaries42 for some gears and some species suggest less-assisted restoration activities related to NFZ enforcement may be providing some fisheries benefits in Gökova Bay. However, the mechanism by which NFZ export fishable biomass towards adjacent areas140 remains undocumented in Gökova Bay and was beyond the scope of this study.
Pressures from fishing, disturbance and habitat loss can have profound impacts on abundance of higher trophic level predators leading to trophic cascades141 from the decline of their key function within the ecosystem. The loss of this natural control can simplify food-webs and impact the abundance of prey species including NIS142, impacting habitats and exacerbating overgrazing by herbivores, although effects may not be predictable or direct143. Monitoring threatened higher trophic level predators’ presence and habitat preferences is therefore important to understand the health and functioning of the ecosystem in light of restoration efforts in Gökova Bay, and ensure most appropriate interventions. Although mobile, these species rely on key habitats144,145, use important breeding areas and potentially act as controls on prey species including NIS146,147,148,149,150 in priority habitats. Work previously defined cave use duration and preferences of M. monachus54 to understand sites of local importance, and demonstrated M. monachus using a coastal cave dry-ledge construction within the wider MPA73 complementing NFZ establishment. Here, we aimed to understand the preferences of C. plumbeus within Boncuk ISRA (Karacasöğüt NFZ).
Results show variation in sandbar sharks’ temperature preferences. Peaks in observations during periods with higher seawater temperatures (>20°C)91,151 are likely linked to reproductive cycles and migratory patterns which bring C. plumbeus to coastal areas152. This is aligned with research suggesting C. plumbeus only becomes active at temperatures of 18.1 °C or above148,153. This apparent preference for warm waters supports previous research on the sensitivity of C. plumbeus to changes in water temperature148, attributed to different factors including enhanced metabolic rates and increased prey availability154. Future monitoring will focus on more consistent data collection across season to. increase data gathering in autumn and winter (when shark presence is expected to be lowest according to temperature analyses) alongside analyses of observations during high, stable temperatures which are expected to exceed the optimal range, as sharks seek temperate conditions and migrate to cooler, deeper waters148,149 or alternative areas.
Our observations stress the potential impacts of climate change on C. plumbeus distribution155,156 and as global temperatures rise, precise sea temperature monitoring is essential to confirm thresholds or other abiotic parameters influencing C. plumbeus behaviour. Our results may have implications for understanding C. plumbeus interactions with tourism and fisheries sectors, and for refinement of marine spatial planning in Gökova Bay. Reports of shark interactions with leisure boats, tourist activities157 and other vessels158 are emerging, and strengthening seasonal protection of Gökova SEPA (in particular ISRAs and suspected nursery areas like Boncuk Bay) during peaks in shark activity may help mitigate negative impacts. Ongoing camera monitoring at Boncuk Bay will show us whether spatial restrictions are responsive to climate impacts144. Labour intensive manual analyses of video data limits the feasibility of long-term monitoring and AI solutions159 are needed to ensure sustainability of this technique. In future, AI technologies may also identify unique individuals, to understand C. plumbeus populations. Tagging152 to understand specific movements and migrations of this mobile species within and beyond Gökova Bay during different conditions could further inform conservation, restoration and adaptive management.
Climate considerations are important to our understanding of restoration potential and progress in Gökova Bay but evidence is lacking globally to decipher and predict complex impacts and interactions within MPAs18. Results suggest some direct influence from increased temperature (e.g. C. plumbeus160, spread of IAS) alongside other potential climate impacts not well-documented here (e.g. macroalgae and interactions with IAS), and it is increasingly urgent to study these in the eastern Mediterranean to inform restoration, as seas warm comparatively fast161. Well-enforced NFZ may support ecosystem resilience and livelihoods but considering climate resilience within marine spatial planning, threat reduction, MPA enforcement, management planning and monitoring (and in design of more highly-assisted restoration interventions) will help restoration to respond to change.
While Türkiye is not bound by the EU Nature Restoration Law it has ambitious protection and restoration targets and plans162, and is committed to restore 30% of degraded coastal and marine ecosystems under the Global Biodiversity Framework162. In the case study presented the approach has evolved to work at multiple scales (NFZs, FRAs, MPAs, SEPAs) to enable multi-habitat, multi-species restoration through natural recovery. Lower intensity interventions (e.g. SEPAs) pre-date and have in-practice facilitated introduction of more localised, more assisted enforcement activities (e.g. NFZ) and experimental restoration for specific habitats and species of perceived importance for ecosystem integrity (Fig. 3). This provides an example of the conceptual and practical integration of more traditional protection measures as restoration163 within a degraded ecosystem, consistent with Chazdon et al.69 who highlight that removing human sources of degradation is essential for recovery, regardless of the further interventions required to assist or extend ecosystem response. Multiple synergistic restoration actions have been undertaken in Gökova Bay, implemented with accumulating scale and intensity over time and space demonstrating the potential and need for increasingly intense assisted restoration in a coastal seascape. The step-wise evolution of interventions has led to increased clarity and targets for specific species or habitats, demonstrating a progression towards increasingly assisted regeneration. Through our observations of natural regeneration we find evidence that ecosystems in Gökova Bay are responding (e.g. fish biomass) to restoration interventions, despite no reference ecosystem or seascape-scale targets and without a significant focus on highly assisted restoration activities.
The complexity of this work supports the need to move beyond the dichotomy of active versus passive restoration and think across a continuum of interventions across habitats, species and scales, for most effective and achievable marine restoration6. Social and economic benefits of marine restoration are rarely measured164 and in Gökova Bay, a holistic monitoring programme is in place to understand aspects of ecosystem integrity, social implications of MPAs and wider seascape restoration. While we found evidence of increased fish catch for key species in small-scale longline fisheries, an integrated analysis was not feasible, and social-economic data available was not easily integrated (Fig. 3) due to multiple factors (e.g. uncontrolled environmental variables across a large heterogeneous ecosystem, operational challenges of economic instability, complexity of retrospectively aligning independent spatial sampling strategies of data). While progress is being measured towards multiple synergistic goals that consider the broader socio-ecological system165, these could not yet be reliably synthesized and therefore the full picture of benefits across multiple interconnected habitats in the region remains to be comprehensively understood. It remains hard to unpick change in system health, and even harder to attribute change despite broad indicators. Without key environmental analyses as-yet missing (including comprehensive benthic habitat mapping and connectivity assessment) our experience highlights the challenges in evidencing large-scale ecological change.
Marine restoration can continue to learn from terrestrial experience and ensure greater integration of socio-ecological factors that enable recovery5,164,166,167. Though clear targets were not established at the outset, this should not prevent progress and ambition, and existing frameworks and views of stakeholders (predominantly local fishers) guided direction of travel compared to historical knowledge. Project targets in more recent years were used to establish the existing condition of metrics, alongside current literature and frameworks to guide MPA ambitions and implementation99,168. The approach from Gökova Bay has become a focal point in Türkiye and an exemplar in the wider Mediterranean and is being replicated along the adjacent coastline168, underscoring the value of targeted multi-habitat interventions to catalyse broader regional restoration efforts.
Data Availability
Data is available upon request for non-commercial research and educational purposes.
References
United Nations. The UN Sustainable Development Goals. https://www.globalgoals.org/goals/14-life-below-water/ (2015).
Convention on Biological Diversity. Kunming-Montreal Global Biodiversity Framework. Decision Adopted by the Conference of the Parties to the Convention on Biological Diversity. https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-04-en.pdf (2022).
Obura, D. The Kunming-Montreal global biodiversity framework: business as usual or a turning point? One Earth. 6, 77–80 (2023).
Bell-James, J. et al. The Global Biodiversity Framework’s ecosystem restoration target requires more clarity and careful legal interpretation. Nat. Ecol. Evol. 8, 840–841, https://doi.org/10.1038/s41559-024-02389-6 (2024).
Kenny, I. et al. Aligning social and ecological goals for successful marine restoration. Biol. Conserv. 288, 110357, https://doi.org/10.1016/j.biocon.2023.110357 (2023).
Krishnan, A. & Osuri, A. M. Beyond the passive–active dichotomy: aligning research with the intervention continuum framework of ecological restoration. Restor. Ecol. 31, 13828, https://doi.org/10.1111/rec.13828 (2023).
Coll, M. et al. The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS ONE 5, e11842 (2010).
Boudouresque, C. F. Marine biodiversity of the Mediterranean: status of species, populations and communities. Sci. Rep. Port.-Cros Natl. Park. 20, 97–146 (2004).
Abdulla, A., Gomei, M., Hyrenbach, D., Notarbartolo-di-Sciara, G. & Agardy, T. Challenges facing a network of representative marine protected areas in the Mediterranean: prioritizing the protection of underrepresented habitats. ICES J. Mar. Sci. 66, 22–28 (2009).
Jordà, G., Marbà, N. & Duarte, C. Mediterranean seagrass vulnerable to regional climate warming. Nat. Clim. Change 2, 821–824, https://doi.org/10.1038/nclimate1533 (2012).
Albano, P. G. et al. Native biodiversity collapse in the eastern Mediterranean. Proc. R. Sci. 288, 20202469, https://doi.org/10.1098/rspb.2020.2469 (2021).
Fortibuoni, T. et al. Analysis of long-term changes in a Mediterranean marine ecosystem based on fishery landings. Front. Mar. Sci. 4, 33, https://doi.org/10.3389/fmars.2017.00033 (2017).
Coll, M., Lotze, H. K. & Romanuk, T. N. Structural degradation in Mediterranean Sea food webs: testing ecological hypotheses using stochastic and mass-balance modelling. Ecosystems 11, 939–960 (2008).
Demirel, N., Zengin, M. & Ulman, A. First large-scale eastern Mediterranean and Black Sea stock assessment reveals a dramatic decline. Front. Mar. Sci. 7, 103, https://doi.org/10.3389/fmars.2020.00103 (2020).
FAO. The State of Mediterranean and Black Sea Fisheries 2023 – Special edition. General Fisheries Commission for the Mediterranean. https://openknowledge.fao.org/handle/20.500.14283/cc8888en (2023).
Mejjad, N., Rossi, A. & Pavel, A. B. The coastal tourism industry in the Mediterranean: a critical review of the socio-economic and environmental pressures & impacts. Tour. Manag. Perspect. 44, 101007 (2022).
McCarthy, A. H. et al. Destructive fishing: An expert-driven definition and exploration of this quasi-concept. Conserv. Lett. e13015. https://doi.org/10.1111/conl.13015 (2024).
Claudet, J. & Fraschetti, S. Human-driven impacts on marine habitats: a regional meta-analysis in the Mediterranean Sea. Biol. Conserv. 143, 2195–2206, https://doi.org/10.1016/j.biocon.2010.06.004 (2010).
Biton-Porsmoguer, S. & Lloret, J. Potential impacts of bottom trawling on species of skates (Rajiformes: Rajidae): The case of the Gulf of Cádiz and the Western Mediterranean. Cybium 44, 255–263, https://doi.org/10.26028/cybium/2020-443-006 (2020).
Bevilacqua, S. et al. The status of coastal benthic ecosystems in the Mediterranean Sea: evidence from ecological indicators. Front. Mar. Sci. 7, 475, https://doi.org/10.3389/fmars.2020.00475 (2020).
Sangün, L., Güney, O. I. & Berk, A. Economic efficiency performance of small-scale fisheries in the East Mediterranean coast of Turkey. N. Medit. 17, 71–80 (2018).
Aydın, C., Kaykaç, H. & Tosunoğlu, Z. Reflection of changed catch composition on characteristics of fishing gear; Gökova Bay case study. Ege J. Fish. Aquat. Sci. Aquat. Sci. 35, 251–260 (2018).
Tosunoğlu, Z. & Ünal, V. The effect of landing decrease on fishing gears: A case of Gökçeada fishery. COMU J. Mar. Sci. Fish. 4, 11–19 (2021).
Galil, B. S., Nehring, S. & Panov, V. Waterways as invasion highways–Impact of climate change and globalization. Biological invasions, 193, (ed. Nentwig, W.) 59-74 (Heidelberg, 2007).
Çinar, M. E. et al. Current status (as of end of 2020) of marine alien species in Turkey. PLoS ONE 16, e0251086, https://doi.org/10.1371/journal.pone.0251086 (2021).
Galil, B. S. A Sea, a Canal, a Disaster: The Suez Canal and the Transformation of the Mediterranean Biota. The Suez Canal: Past Lessons and Future Challenges. (ed. Lutmar, C. & Rubinovitz, Z.). https://doi.org/10.1007/978-3-031-15670-0_10 (Cham, 2023).
Zenetos, A. et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 13, 328–352 (2012).
Kourantidou, M. et al. Economic costs of invasive alien species in the Mediterranean basin. NeoBiota 67, 427–458, https://doi.org/10.3897/neobiota.67.58926 (2021).
Lejeusne, C., Chevaldonné, P., Pergent-Martini, C., Boudouresque, C. F. & Pérez, T. Climate change effects on a miniature ocean: the highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 25, 250–260, https://doi.org/10.1016/j.tree.2009.10.009 (2010).
Raitsos, D. E. et al. Global climate change amplifies the entry of tropical species into the Eastern Mediterranean Sea. Limnol. Oceanogr. 55, 1478–1484, https://doi.org/10.4319/lo.2010.55.4.1478 (2010).
Cos, J. et al. The Mediterranean climate change hotspot in the CMIP5 and CMIP6 projections. Earth Syst. Dynam. 13, 321–340, https://doi.org/10.5194/esd-13-321-2022 (2022).
Lionello, P. & Scarascia, L. The relation between climate change in the Mediterranean region and global warming. Reg. Environ. Change 18, 1481–1493, https://doi.org/10.1007/s10113-018-1290-1 (2018).
Ozer, T., Gertman, I., Kress, N., Silverman, J. & Herut, B. Interannual thermohaline (1979–2014) and nutrient (2002–2014) dynamics in the Levantine surface and intermediate water masses, SE Mediterranean Sea. Glob. Planet. Chang. 151, 60–67, https://doi.org/10.3389/fmars.2020.00300 (2017).
Hobday, A. J. et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 141, 227–238, https://doi.org/10.1016/j.pocean.2015.12.014 (2016).
Garrabou, J. et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob. Change Biol. 15, 1090–1103, https://doi.org/10.1111/j.1365-2486.2008.01823.x (2009).
Frölicher, T. L., Fischer, E. M. & Gruber, N. Marine heatwaves under global warming. Nature 560, 360–364, https://doi.org/10.1038/s41586-018-0383-9 (2018).
Litsi-Mizan, V. et al. Decline of seagrass (Posidonia oceanica) production over two decades in the face of warming of the Eastern Mediterranean Sea. N. Phytol. 239, 2126–2137, https://doi.org/10.1111/nph.19084 (2023).
Okuş, E. et al. Gökova Özel Çevre Koruma Bölgesinin Kıyı ve Deniz Alanlarının Biyolojik Çeşitliliğinin Tespiti Projesi Final Raporu. TC. Çevre ve Orman Bakanlığı Özel Çevre Koruma Kurumu Başkanlığı. İstanbul Üniversitesi Deniz Bilimleri ve İşletmeciliği Enstitüsü (İstanbul, 2006).
Çoker, T. & Akyol, O. An evaluation on the fish diversity of Gökova Bay (Aegean Sea). Ege J. Fish. Aquat. Sci. 31, 161–166, https://doi.org/10.12714/egejfas.2014.31.3.08 (2014).
Kıraç, C. O. et al. Gökova Özel Çevre Koruma Bölgesi Kıyı ve Deniz Alanları Bütünleşik Yönetim Planlaması. Türkiye Kıyıları Ulusal Konferansı (KAY Türk Milli Komitesi), 29 (Trabzon, 2010).
Eronat, C. & Sayin, E. Temporal evolution of the water characteristics in the bays along the eastern coast of the Aegean Sea: Saros, İzmir, and Gökova bays. Turk. J. Earth Sci. 23, 53–66 (2014).
Ünal, V. & Kizilkaya, Z. A Long and Participatory Process towards Successful Fishery Management of Gökova Bay, Turkey. Catastr. Recovery Stories Fish. Manag. Success 509–532 (2019).
Duman, M. et al. Mapping Posidonia Oceanica (Linnaeus) meadows in the Eastern Aegean Sea coastal areas of Turkey: Evaluation of habitat maps produced using the acoustic ground-discrimination systems. Int. J. Environ. Geoinform. 6, 67–75, https://doi.org/10.30897/ijegeo.544695 (2019).
Alan, V. & Akçalı, B. Monitoring of Posidonia oceanica seagrass meadows on the south-west Coast of Türkiye by the balisage method. 6th National Marine Sciences Conference (Rize, 2024).
Akçalı, B., Kaboğlu, G. & Güçlüsoy, H. A review on Posidonia oceanica (Linnaeus) Delile coverage along the Turkish coasts until 2019. J. Black Sea/Meditter. Environ. 25, 115–124 (2019).
Mcleod, E. et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, 552–560, https://doi.org/10.1890/110004 (2011).
Alan, V. et al. Blue carbon: Studies on determination of organic carbon (OC) stocks in Posidonia oceanica sediments in south-western coasts of Türkiye. 6th National Marine Sciences Conference (Rize, 2024).
Kennedy, H. et al. Seagrass sediments as a global carbon sink: Isotopic constraints. Glob. Biogeochem. 24, 4, https://doi.org/10.1029/2010GB003848 (2010).
Traganos, D. et al. Spatially explicit seagrass extent mapping across the entire mediterranean. Front. Mar. Sci. 9, 871799, https://doi.org/10.3389/fmars.2022.871799 (2022).
Cebrian, E. et al. A roadmap for the restoration of Mediterranean macroalgal forests. Front. Mar. Sci. 8, 709219, https://doi.org/10.3389/fmars.2021.709219 (2021).
Sala, E. et al. The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PloS one 7, e32742, https://doi.org/10.1371/journal.pone.0032742 (2012).
Monfort, T. et al. The three-dimensional structure of Mediterranean shallow rocky reefs: Use of photogrammetry-based descriptors to assess its influence on associated teleost assemblages. Front. Mar. Sci. 8, 639309, https://doi.org/10.3390/jmse11040759 (2021).
Kıraç, C. and N. Veryeri. Putting PEEN to practice in marine and coastal areas: a demonstration project ensuring the eco-logical resilience, coherence and sustainable future of Gökova Bay SEPA in Turkey. (SAD-AFAG, Ankara. 2010).
Ünal, V., Tıraşın, E. M. & Tosunoğlu, Z. Transition towards an ecosystem approach to fisheries in the Mediterranean Sea–Lessons learned through selected case studies Chapter 9: An ecosystem approach to fisheries management for small-scale fisheries in Gökova marine protected area, Turkey: Challenges encountered during the transition process. FAO Fisheries and Aquaculture Technical Paper 681, (ed. Vasconcellos, M., Ünal, V.) 117-136 (Rome, 2022).
Saydam, E. & Güçlüsoy, H. Revealing the Mediterranean monk seal (Monachus monachus)’s cave preference in Gökova Bay on the southwest coast of Türkiye. Sustainability 15, 12017, https://doi.org/10.3390/su151512017 (2023).
Karamanlidis, A. A., Adamantopoulou, S., Tounta, E. & Dendrinos, D. Monachus monachus eastern Mediterranean subpopulation. The IUCN Red List of Threatened Species. 2019-1 https://doi.org/10.2305/IUCN.UK.2019-1.RLTS.T120868935A120869697.en (2019).
IUCN SSC Shark Specialist Group. Boncuk Bay ISRA Factsheet. Dubai: IUCN SSC Shark Specialist Group (Dubai, 2023).
Rigby, C. L. et al. Carcharhinus plumbeus. The IUCN Red List of Threatened Species (2021): e.T3853A2874370. https://doi.org/10.2305/IUCN.UK.2021-2.RLTS.T3853A2874370.en.
Garbutt, A. et al. Seascape Scale Restoration: Restoring our coastal habitats for nature and people. Blue Marine Foundation Report (London, 2024).
Preston, J. et al. Seascape connectivity: evidence, knowledge gaps and implications for coastal ecosystem restoration practice and policy. Invited submission for the Special Collection: Bridging Land and Seascape Restoration for Ecoscape Recovery. Ocean. Sustain. (in review).
Güçlüsoy, H. Marine and coastal protected areas of Turkish Aegean coasts. The Aegean Sea marine biodiversity, fisheries, conservation and governance. Turkish Marine Research Foundation. (ed. Katağan T., Tokaç, A., Beşiktepe, Ş. & Öztürk, B.) 669-684 (İstanbul, 2015).
Coşkun, B. & Yıldırım Pank, A. The Legal Ground of Special Environmental Protection Areas and The Latest Developments on This Issue. Eurasia. Acad. Sci., Eurasia. Educ. Lit. J. 12, 53–56, https://doi.org/10.17740/eas.edu.2020-V12-05 (2020).
Sala, E., Kizilkaya, Z., Yildirim, D. & Ballesteros, E. Alien marine fishes deplete algal biomass in the eastern Mediterranean. PloS One 6, e17356. https://doi.org/10.1371/journal.pone.0017356 (2011).
Vergés, A. et al. Tropical rabbitfish and the deforestation of a warming temperate sea. J. Ecol. 102, 1518–1527, https://doi.org/10.1111/1365-2745.12324 (2014).
Bermejo, R. et al. Marine forests of the Mediterranean-Atlantic Cystoseira tamariscifolia complex show a southern Iberian genetic hotspot and no reproductive isolation in parapatry. Sci. rep. 8, 10427, https://doi.org/10.1038/s41598-018-28811-1 (2018).
Gann, G. D. et al. International principles and standards for the practice of ecological restoration. Second edition. Restor. Ecol. 27, S1–S46. https://doi.org/10.1111/rec.13035 (2019).
Morey, G. et al. Weight–length relationships of littoral to lower slope fishes from the western Mediterranean. Fish. Res. 62, 89–96, https://doi.org/10.1016/S0165-7836(02)00250-3 (2003).
Keenleyside, K. A., Dudley, N., Cairns, S., Hall C. M. & Stolton S. Ecological restoration for protected areas: Principles, guidelines and best practices. (IUCN, 2012).
Chazdon, R. L. et al. The intervention continuum in restoration ecology: rethinking the active–passive dichotomy. Restor. Ecol. e13535. https://doi.org/10.1111/rec.13535 (2021).
Anıl, Ö., Alan, V. & Olguner, M. T. Marine litter: Distribution and composition along the southwestern coast of Turkey. 6th National Marine Sciences Conference (Rize, 2024).
Brown, J. & Macfadyen, G. Ghost fishing in European waters: Impacts and management responses. Mar. Pol. 31, 488–504, https://doi.org/10.1016/j.marpol.2006.10.007 (2007).
Chapman, J. K., Anderson, L. G., Gough, C. L. & Harris, A. R. Working up an appetite for lionfish: a market-based approach to manage the invasion of Pterois volitans in Belize. Mar. Pol. 73, 256–262, https://doi.org/10.1016/j.marpol.2016.07.023 (2016).
Saydam, E., Güçlüsoy, H. & Kızılkaya, Z. A. A novel approach for Mediterranean monk seal conservation: an artificial ledge in a marine cave. Oryx 57, 149–151 (2023).
TVKGM. “Gökova ÖÇKB Yönetim Planı”. 180 p. https://webdosya.csb.gov.tr/db/tabiat/editordosya/DigitalKopya24mart2020.pdf (2019)
Atkinson, J. & Bonser, S. P. “Active” and “passive” ecological restoration strategies in meta-analysis. Restor. Ecol. 28, 1032–1035, https://doi.org/10.1111/rec.13229 (2020).
Salomidi, M. et al. Setting an ecological baseline prior to the bottom-up establishment of a marine protected area in Santorini Island, Aegean Sea. Mediterr. Mar. Sci. 17, 720–737, https://doi.org/10.12681/mms.1802 (2016).
Froese, R. & Pauly, D. FishBase. http://www.fishbase.org (2011).
Richardson, D. M., Pyšek, P. & Carlton, J. T. A compendium of essential concepts and terminology in biological invasions. In Fifty Years of Invasion Ecology: The Legacy of Charles Elton (ed. D. M. Richardson), 409–420 (Oxford,2011).
Çinar, M. E., Arianoutsou, M., Zenetos, A. & Golani, D. Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review. Aquat. Invasions 9, 391–423, https://doi.org/10.3391/ai.2014.9.4.01 (2014).
Papke, L. E. & Wooldridge, J. M. Econometric methods for fractional response variables with an application to 401 (K) plan participation rates. J. Appl. Econom. 11, 619–632 (1996).
Baum, C. F. Stata tip 63: Modeling proportions. Stata J. 8, 299–303, https://doi.org/10.1177/1536867X08008002 (2008).
Mullahy, J. Multivariate fractional regression estimation of econometric share models. J. Econom. Methods 4, 71–100, https://doi.org/10.1515/jem-2012-0006 (2015).
SMART, Version 1.0.478 (mobile application), Version 7.5.6 (desktop). Available online: https://smartconservationtools.org/en-us/.
Olguner, M. T. & Deval, M. C. Catch and selectivity of 40 and 44 mm trammel nets in small-scale fisheries in the Antalya Bay, Eastern Mediterranean. Ege J. Fish. Aquat. Sci. Aquat. Sci. 30, 167–173, https://doi.org/10.12714/egejfas.2013.30.4.04 (2013).
Deniz izleme kılavuzları. T. C. Çevre ve Şehircilik Bakanlığı, ÇED, İzin ve Denetim Genel Müdürlüğü (Gebze, 2018).
Zeki, S. Research on Upper Infralittoral Zone Macroalgae in the South of Gökova Specially Protected Area. M.Sc. thesis Institute of Marine Sciences and Management, Istanbul University (2006).
Taşkın, E. A new non-destructive method for the assessment of the ecological status of coastal waters by using marine macrophytes. J. Black Sea/Meditter. Environ. 24, 48–58 (2020).
Orfanidis, S., Panayotidis, P. & Ugland, K. Ecological Evaluation Index continuous formula (EEI-c) application: A step forward for functional groups, the formula and reference condition values. Mediterr. Mar. Sci. 12, 199–231, https://doi.org/10.12681/mms.60 (2011).
Rodríguez-Prieto, C., Ballesteros, E., Boisset, F. & Afonso-Carrillo, J. Guía de las macroalgas y fanerógamas marinas del Mediterráneo occidental 1–656 (Ediciones Omega SA Barcelona, 2013).
Gerakaris, V. & Tsiamis, K. Technical assistance for capacity building on water quality monitoring, coastal & transitional BQes sampling and analysis procedures. Institute of Marine Biological Resources and Inland Waters (Greece, 2014).
Barash, A. et al. Some like it hot: Investigating thermoregulatory behavior of carcharhinid sharks in a natural environment with artificially elevated temperatures. Fishes 8, 428, https://doi.org/10.3390/fishes8090428 (2023).
Suding, K. N. Toward an era of restoration in ecology: successes, failures, and opportunities ahead. Annu. Rev. Ecol. Evol. Syst. 42, 465–487, https://doi.org/10.1146/annurev-ecolsys-102710-145115 (2011).
Borja, A. Grand challenges in marine ecosystems ecology. Front. Mar. Sci. 1, 1, https://doi.org/10.3389/fmars.2014.00001 (2014).
Pansini, A., Bosch-Belmar, M., Berlino, M., Sarà, G. & Ceccherelli, G. Collating evidence on the restoration efforts of the seagrass Posidonia oceanica: current knowledge and gaps. Sci. Total Environ. 851, 158320, https://doi.org/10.1016/j.scitotenv.2022.158320 (2022).
Campbell, S. J. et al. Weak compliance undermines the success of no-take zones in a large government-controlled marine protected area. PloS one 7, e50074, https://doi.org/10.1371/journal.pone.0050074 (2012).
Sini, M. et al. Small-scale coastal fishing shapes the structure of shallow rocky reef fish in the Aegean Sea. Front. Mar. Sci. 6, 599, https://doi.org/10.3389/fmars.2019.00599 (2019).
Quimbayo, J. P. et al. The COVID-19 pandemic has altered illegal fishing activities inside and outside a marine protected area. Curr. Biol. 32, R765–R766, https://doi.org/10.1016/j.cub.2022.06.030 (2022).
Reis-Filho, J. A. et al. The challenges and opportunities of using small drones to monitor fishing activities in a marine protected area. Fish. Manag. Ecol. 29, 745–752, https://doi.org/10.1111/fme.12557 (2022).
Grorud-Colvert, K. et al. The MPA Guide: A framework to achieve global goals for the ocean. Science 373, eabf0861, https://doi.org/10.1126/science.abf0861 (2021).
Tsirintanis, K. et al. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 17, 308–352, https://doi.org/10.3391/ai.2022.17.3.01 (2022).
Tsirintanis, K., Sini, M., Ragkousis, M., Zenetos, A. & Katsanevakis, S. Cumulative negative impacts of invasive alien species on marine ecosystems of the aegean sea. Biology 12, 933, https://doi.org/10.3390/biology12070933 (2023).
Fanelli, E., Azzurro, E., Bariche, M., Cartes, J. E. & Maynou, F. Depicting the novel Eastern Mediterranean food web: a stable isotopes study following Lessepsian fish invasion. Biol. Invasions 17, 2163–2178, https://doi.org/10.1007/s10530-015-0868-5 (2015).
Giakoumi, S. & Pey, A. Assessing the effects of marine protected areas on biological invasions: a global review. Front. Environ. Sci. 4, 49, https://doi.org/10.3389/fmars.2017.00049 (2017).
Ardura, A., Juanes, F., Planes, S. & Garcia-Vazquez, E. Rate of biological invasions is lower in coastal marine protected areas. Sci. Rep. 6, 33013, https://doi.org/10.1038/srep33013 (2016).
Giakoumi, S. et al. Exploring the Relationships between Marine Protected Areas and Invasive Fish in the World’s Most Invaded Sea. Ecol. Appl. 29, e01809, https://doi.org/10.1002/eap.1809 (2018).
García-Rubies, A., Hereu, B. & Zabala, M. Long-term recovery patterns and limited spillover of large predatory fish in a Mediterranean MPA. PLoS One 8, e73922, https://doi.org/10.1371/journal.pone.0073922 (2013).
Molloy, P. P., McLean, I. B. & Côté, I. M. Effects of marine reserve age on fish populations: a global meta-analysis. J. Appl. Ecol. 46, 743–751, https://doi.org/10.1111/j.1365-2664.2009.01662.x (2009).
Vafidis, D., Antoniadou, C., Voulgaris, K., Varkoulis, A. & Apostologamvrou, C. Abundance and population characteristics of the invasive sea urchin Diadema setosum (Leske, 1778) in the south Aegean Sea (eastern Mediterranean). J. Biol. Res. -Thessalon. 28, 1–14, https://doi.org/10.1186/s40709-021-00142-9 (2021).
Dinçtürk, E., Öndes, F., Alan, V. & Dön, E. Mass Mortality of the Invasive Sea Urchin Diadema setosum in Türkiye, Eastern Mediterranean Possibly Reveals Vibrio Bacteria Infection. Mar. Ecol. e12837. https://doi.org/10.1111/maec.12837 (2024).
Zulkifly, S. B. et al. The genus Cladophora Kützing (Ulvophyceae) as a globally distributed ecological engineer. J. Phycol. 49, 1–17, https://doi.org/10.1111/jpy.12025 (2013).
Savin, A. et al. Assessment of macroalgal communities on shallow rocky reefs in the Aegean Sea indicates an impoverished ecological status. Mediterr. Mar. Sci. 24, 241–258, https://doi.org/10.12681/mms.31034 (2023).
Lundberg, B. & Golani, D. Diet adaptation of Lessepsian migrant rabbitfishes, Siganus luridus and S. rivulatus, to the algal resources of the Mediterranean coast of Israel. Mar. Ecol. 16, 73–89, https://doi.org/10.1111/j.1439-0485.1995.tb00395.x (1995).
Shakman, E., Boedeker, C., Bariche, M. & Kinzelbach, R. Food and feeding habits of the Lessepsian migrants Siganus luridus Rüppell, 1828 and Siganus rivulatus Forsska°l, 1775 (Teleostei: Siganidae) in the southern Mediterranean (Libyan coast). J. Biol. Res. Thessalon. 12, 115–124 (2009).
Al-Rzagy, S., Abomies, E. & Shackman, E. Feeding habits of non-indigenous fishes Siganus luridus, Siganus rivulatus and native fish Sarpa salpa in western coast of Libya. Libyan J.Ecol. Environ. Sci.Technol. 5, 55–64 (2023).
Lundberg, B., Ogorek, R., Galil, B. & Goren, M. Algal food of Siganid fishes in Shiqmona, Israel. Isr. J. Zool. 45, 314 (1999).
Rindi, F. et al. Coralline algae in a changing Mediterranean Sea: How can we predict their future, if we do not know their present. Front. Environ. Sci. 6, 723, https://doi.org/10.3389/fmars.2019.00723 (2019).
Saoud, I. P., Mohanna, C. & Ghanawi, J. Effects of temperature on survival and growth of juvenile spinefoot rabbitfish (Siganus rivulatus). Aquac. Res. 39, 491–497, https://doi.org/10.1111/j.1365-2109.2007.01903.x (2008).
Bengil, F. et al. Descriptive capability of datasets as proxy of sea water temperature in coastal systems: An evaluation from the Aegean Sea. Turk. J. Fish. Aquat. Sci. 21, 627–635, https://doi.org/10.4194/1303-2712-v21_12_05 (2021).
Andrews, S., Bennett, S. & Wernberg, T. Reproductive seasonality and early life temperature sensitivity reflect vulnerability of a seaweed undergoing range reduction. Mar. Ecol. Prog. Ser. 495, 119–129, https://doi.org/10.3354/meps10567 (2014).
Muth, A. F., Graham, M. H., Lane, C. E. & Harley, C. D. Recruitment tolerance to increased temperature present across multiple kelp clades. Ecology 100, 1–7, https://doi.org/10.1002/ecy.2594 (2019).
Boada, J. et al. Immanent conditions determine imminent collapses: nutrient regimes define the resilience of macroalgal communities. Proc. R. Soc. B 284, 20162814, https://doi.org/10.1098/rspb.2016.2814 (2017).
Dimitriadis, C. et al. Evaluating the long term effectiveness of a Mediterranean marine protected area to tackle the effects of invasive and range expanding herbivorous fish on rocky reefs. Mar. Environ. Res. 193, 106293, https://doi.org/10.1016/j.marenvres.2023.106293 (2024).
Smith, R. S., Cheng, S. L. & Castorani, M. C. Meta-analysis of ecosystem services associated with oyster restoration. Conserv. Biol. 37, e13966, https://doi.org/10.1111/cobi.13966 (2023).
Orth, R. J. et al. Restoration of seagrass habitat leads to rapid recovery of coastal ecosystem services. Sci. Adv. 6, eabc6434, https://doi.org/10.1126/sciadv.abc6434 (2020).
Forcada, A. et al. Effects of habitat on spillover from marine protected areas to artisanal fisheries. Mar. Ecol. Prog. Ser. 379, 197–211, https://doi.org/10.3354/meps07892 (2009).
Ban, N. C. et al. Well-being outcomes of marine protected areas. Nat. Sustain. 2, 524–532, https://doi.org/10.1038/s41893-019-0306-2 (2019).
Harrison, H. B. et al. Larval export from marine reserves and the recruitment benefit for fish and fisheries. Curr. Biol. 22, 1023–1028, https://doi.org/10.1016/j.cub.2012.04.008 (2012).
Russ, G. R. & Alcala, A. C. Do marine reserves export adult fish biomass? Evidence from Apo Island, central Philippines. Mar. Ecol. Prog. Ser. 132, 1–9, https://doi.org/10.3354/meps132001 (1996).
Cochrane, K., De Young, C., Soto, D., Bahri, T. Climate change implications for fisheries and aquaculture. FAO Fisheries and aquaculture technical paper, 530. https://doi.org/10.1002/9781119154051.ch3 (2009).
Akyol, O. & Ünal, V. Long journey of Lagocephalus sceleratus (Gmelin, 1789) throughout the Mediterranean Sea. Nat. Eng. Sci. 2, 41–47, https://doi.org/10.28978/nesciences.369534 (2017).
Ünal, V. & Bodur, H. G. The socio-economic impacts of the silver-cheeked toadfish on small-scale fishers: A comparative study from the Turkish coast. Ege J. Fish. Aquat. Sci. 34, 119–127, https://doi.org/10.12714/egejfas.2017.34.2.01 (2017).
Colléter, M. et al. Fishing inside or outside? A case studies analysis of potential spillover effect from marine protected areas, using food web models. J. Mar. Syst. 139, 383–395, https://doi.org/10.1016/j.jmarsys.2014.07.023 (2014).
Skliris, N. et al. Decadal scale variability of sea surface temperature in the Mediterranean Sea in relation to atmospheric variability. Ocean Dyn. 62, 13–30, https://doi.org/10.1007/s10236-011-0493-5 (2012).
Güçlüsoy, H. Damage by monk seals to gear of the artisanal fishery in the Foça Monk Seal Pilot Conservation Area. Turk. Fish. Res. 90, 70–77, https://doi.org/10.1016/j.fishres.2007.09.012 (2008).
Karamanlidis, A. A., Adamantopoulou, S., Kallianiotis, A. A., Tounta, E. & Dendrinos, P. An interview-based approach assessing interactions between seals and small-scale fisheries informs the conservation strategy of the endangered Mediterranean monk seal. Aquat. Conserv. Mar. Freshw. Ecosyst. 30, 928–936, https://doi.org/10.1002/aqc.3307 (2020).
Kleitou, P., Antoniou, C., Giovos, I. & Kletou, D. How accurately are we describing the longline bycatch? The case of the ‘rare’ shark Alopias superciliosus in eastern Mediterranean. Int. J. Fish. Aquat. Stud. 5, 375–378 (2017).
Erguden, D., Kabasakal, H. & Ayas, D. Fisheries bycatch and conservation priorities of young sharks (Chondrichthyes: Elasmobranchii) in the Eastern Mediterranean. Zool. Middle East 68, 135–144, https://doi.org/10.1080/09397140.2022.2051916 (2022).
Harper, S. J. et al. Commercial fisher perceptions illuminate a need for social justice considerations in navigating climate change impacts on fisheries systems. Ecol. Soc. 28. https://doi.org/10.5751/ES-14142-280221 (2023).
Foster, D. G., Pulver, J. R., Scott-Denton, E. & Bergmann, C. Minimizing bycatch and improving efficiency in the commercial bottom longline fishery in the Eastern Gulf of Mexico. Fish. Res. 196, 117–125, https://doi.org/10.1016/j.fishres.2017.08.007 (2017).
Di Lorenzo, M., Claudet, J. & Guidetti, P. Spillover from marine protected areas to adjacent fisheries has an ecological and a fishery component. J. Nat. Conser. 32, 62–66, https://doi.org/10.1016/j.jnc.2016.04.004 (2016).
Pinnegar, J. K. et al. Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Environ. Conserv. 27, 179–200, https://doi.org/10.1017/S0376892900000205 (2000).
Pauly, D. & Watson, R. Background and interpretation of the ‘Marine Trophic Index’as a measure of biodiversity. Philos. Trans. R. Soc. B 360, 415–423, https://doi.org/10.1098/rstb.2004.1597 (2005).
Pinnegar, J. K. & Polunin, N. V. Predicting indirect effects of fishing in Mediterranean rocky littoral communities using a dynamic simulation model. Ecol. Model. 172, 249–267, https://doi.org/10.1016/j.ecolmodel.2003.09.010 (2004).
Öztürk, B. Save the sandbar sharks of Boncuk Bay, Turkey. Proceedings of the International Workshop on Mediterranean Cartilaginous Fish with Emphasis on Southern and Eastern Mediterranean. Turkish Marine Research Foundation, 23, 42-47 (İstanbul, 2006).
Karamanlidis, A. A. Current status, biology, threats and conservation priorities of the vulnerable Mediterranean monk seal. Endanger. Species Res. 53, 341–361 (2024).
Filiz, H. Notes on the stomach contents of juvenile sandbar sharks from the south Aegean Sea, Turkey. 2nd International Congress on Applied Ichthyology & Aquatic Environment (Hydromedir2016), Book of Abstracts, 270–273 (Volos, 2016).
Ellis, J. K. & Musick, J. A. Ontogenetic changes in the diet of the sandbar shark, Carcharhinus plumbeus, in lower Chesapeake Bay and Virginia (USA) coastal waters. Environ. Biol. Fish. 80, 51–67, https://doi.org/10.1007/s10641-006-9116-2 (2007).
Saïdi, B., Bradai, M. N., Bouaïn, A. & Capapé, C. Feeding habits of the sandbar shark Carcharhinus plumbeus (Chondrichthyes: Carcharhinidae) from the Gulf of Gabès, Tunisia. Cah. Biol. Mar. 48, 139–144 (2007).
Karamanlidis, A. A. et al. Monachus monachus. The IUCN Red List of Threatened Species 2023: e.T13653A238637039 https://www.iucnredlist.org/species/13653/238637039 (2023).
Pierce, G. J. et al. Diet of the Monk seal (Monachus monachus) in Greek waters. Aquat. Mamm. 37, 284–297, https://doi.org/10.1578/AM.37.3.2011.284 (2011).
Filiz, H., Gülşahin, A., Cerim, H. & Sevingel, N. Kum Köpekbalığı (Carcharhinus plumbeus, Nardo, 1827) peşinde. Bilimsel Araştırma Projeleri Koordinasyon Birimi Sonuç (Kesin) Raporu, Proje no: BAP 13/07, Muğla, 49 sayfa (Muğla, 2014).
Altobelli, A. N. & Szedlmayer, S. T. Migration and residency of sandbar, Atlantic sharpnose, bull, and nurse sharks in the Northern Gulf of Mexico. North Am. J. Fish. Manag. 40, 1324–1343, https://doi.org/10.1002/nafm.10501 (2020).
Filiz, H. Year-Round Aggregation of Sandbar Shark, Carcharhinus plumbeus (Nardo, 1827), in Boncuk Cove in the southern Aegean Sea, Turkey (Carcharhiniformes: Carcharhinidae). Zool. Middle East 65, 35–39, https://doi.org/10.1080/09397140.2018.1540148 (2019).
Stillwell, C. E. & Kohler, N. E. Food habits of the sandbar shark Carcharhinus plumbeus off the U. S. northeast coast, with estimates of daily ration. Fish. Bull. 91, 138–150 (1993).
Conrath, C. L. & Musick, J. A. Investigations into depth and temperature habitat utilization and overwintering grounds of juvenile sandbar sharks, Carcharhinus plumbeus: the importance of near shore North Carolina waters. Environ. Biol. Fish. 82, 123–131, https://doi.org/10.1007/s10641-007-9263-0 (2008).
Crear, D. P., Latour, R. J., Friedrichs, M. A., St-Laurent, P. & Weng, K. C. Sensitivity of a shark nursery habitat to a changing climate. Mar. Ecol. Prog. Ser. 652, 123–136, https://doi.org/10.3354/meps13483 (2020).
Cattano, C. Sandbar shark aggregation in the central Mediterranean Sea and potential effects of tourism. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 1420–1428, https://doi.org/10.1002/aqc.3517 (2021).
Bilecenoğlu, M. Conservation and Monitoring Project of Sandbar Sharks (Carcharhinus plumbeus) in Boncuk Bay, Gökova Special Environmental Protection Area. Environmental Protection Agency for Special Areas, Republic of Turkey Ministry of Environment and Forestry (Ankara, 2008).
Jenrette, J. et al. Shark detection and classification with machine learning. Ecol. Inf. 69, 101673, https://doi.org/10.1016/j.ecoinf.2022.101673 (2022).
Crear, D. P. Predicting the impacts of climate change on the sandbar shark and cobia. Ph.D. thesis Virginia Institute of Marine Science, William & Mary. https://doi.org/10.25773/g2jk-bc64 (2020).
Garrabou, J. et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob. Chang. Biol. 28, 5708–5725, https://doi.org/10.1111/gcb.16301 (2022).
Convention on Global Biodiversity, Kunming-Montreal Global Biodiversity Framework. Target 2. https://www.cbd.int/gbf/targets/2 (2022).
Abelson, A. et al. Challenges for restoration of coastal marine ecosystems in the anthropocene. Front. Mar. Sci. 7, 544105, https://doi.org/10.3389/fmars.2020.544105 (2020).
Saunders, M. et al. Bright spots in coastal marine ecosystem restoration. Curr. Biol. 30, R1500–R1510, https://doi.org/10.1016/j.cub.2020.10.056 (2020).
Tedesco, A. M. et al. Beyond ecology: ecosystem restoration as a process for social-ecological transformation. Trends Ecol. Evol. 38, 643–653, https://doi.org/10.1016/j.tree.2023.02.007 (2023).
Waring, B. G. Grand challenges in ecosystem restoration. Front. Environ. Sci. 11, 1353829, https://doi.org/10.3389/fenvs.2023.1353829 (2024).
Fischer, J., Riechers, M., Loos, J., Martin-Lopez, B. & Temperton, V. M. Making the UN decade on ecosystem restoration a social-ecological endeavour. Trends Ecol. Evol. 36, 20–28, https://doi.org/10.1016/j.tree.2020.08.018 (2021).
Ünal, V., Yıldırım, D. & Tıraşın, E. M. Implementation of the ecosystem approach to fisheries for the small-scale fisheries in Gökova Bay, Turkey: baseline report. FAO Fisheries and Aquaculture Technical Paper 646 (Rome, 2019).
Acknowledgements
We thank Can Görgün for sharing historical knowledge on the Akyaka Fisheries Cooperative and on historical development of the NFZs, Özkan Anıl for marine ranger training and development, Harriet Branson for technical advice on SMART data processing, Çağdaş Yaşar for previous SMART data management, Dilara Arslan for historical data management within AKD and the dedicated rangers of Gökova Bay.We thank the colleagues within the Mediterranean Conservation Society (AKD) for their collaboration and support during this study, alongside Fauna & Flora and the Endangered Landscapes & Seascapes Programme (managed by the Cambridge Conservation Initiative in partnership with Arcadia) and Whitley Fund for Nature for their support.
Author information
Authors and Affiliations
Contributions
The paper was conceptualised between B.M.G., H.G., K.W. and E.S. Authors collecting data include Z.K. (Fish biomass), I.T. (Macroalgae, trial intervention), B.A., S.P., T.O. and V.A. (sandbar sharks), T.O. and Z.T. (cooperative fish catch landings) and TT (processing of data, including SMART). Maps and infographics were created by K.W. and B.M.G. Statistical analyses were completed by B.M.R. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
BMG was employed by Fauna & Flora during the research period (Jan 2019 - Dec 2023) and was after employed by the Endangered Landscapes & Seascapes Programme, a funder of this research (Jan 2024 - ongoing). This may constitute a non-financial competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kızılkaya, Z., Saydam, E., Walker, K. et al. Progress and challenges of multi-habitat marine restoration in the eastern Aegean Sea, Türkiye. npj Ocean Sustain 4, 12 (2025). https://doi.org/10.1038/s44183-025-00114-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44183-025-00114-9
This article is cited by
-
Assessing climate-driven shifts in Liquidambar orientalis using ensemble species distribution models in the Eastern Mediterranean Region
BMC Plant Biology (2025)
-
Bridging land and seascape restoration for ecoscape recovery
npj Ocean Sustainability (2025)