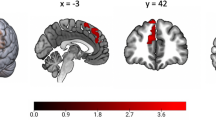
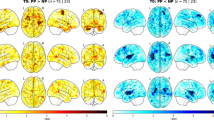
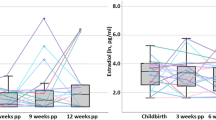
Abstract
The first 6 weeks postpartum are characterized by major changes in the bodies of cisgender women and an increased vulnerability to psychiatric disorders such as postpartum depression (PPD). This Perspective addresses the debate over the onset of PPD in the first 6 weeks postpartum and its probable relationship to physiological adaptation processes. Fluctuations in hormone levels during pregnancy and childbirth trigger simultaneous changes in brain structure and function, which are particularly dynamic in the first 6 weeks postpartum. At the same time, rapid hormone withdrawal coincides with mood disorders such as ‘baby blues’ or PPD. Understanding the covariance between the temporal trajectories of hormonal adaptations, time-dependent neuroplasticity and the onset of mood disorders may shed valuable light on the highly sensitive time frame of the first 6 weeks postpartum, which, in addition to being a critical period of transition, may prove to be crucial for the onset of PPD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Blackman, A. et al. Severe maternal morbidity and mental health hospitalizations or emergency department visits. JAMA Netw. Open 7, e247983 (2024).
Trost, S. L. et al. Preventing pregnancy-related mental health deaths: insights from 14 US maternal mortality review committees, 2008–17. Health Aff. 40, 1551–1559 (2021).
Xiao, M. et al. Trajectories of perinatal suicidal ideation from early pregnancy to six weeks postpartum and their influencing factors: a prospective longitudinal study. Psychiatry Res. 328, 115467 (2023).
Trautmann-Villalba, P. & Hornstein, C. Tötung des eigenen kindes in der postpartalzeit. Nervenarzt 78, 1290–1295 (2007).
Bai, Y. et al. Prevalence of postpartum depression based on diagnostic interviews: a systematic review and meta-analysis. Depress. Anxiety 2023, 8403222 (2023).
Stein, A. et al. Effects of perinatal mental disorders on the fetus and child. Lancet 384, 1800–1819 (2014).
Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Association, 2013); https://doi.org/10.1176/appi.books.9780890425596.744053
International Classification of Diseases Eleventh Revision (ICD-11) (World Health Organization, 2022).
Radoš, S. N. et al. Diagnosis of peripartum depression disorder: a state-of-the-art approach from the COST Action Riseup-PPD. Compr. Psychiatry 130, 152456 (2024).
Munk-Olsen, T. et al. Postpartum and non-postpartum depression: a population-based matched case-control study comparing polygenic risk scores for severe mental disorders. Transl. Psychiatry 13, 346 (2023).
Sharma, V. & Mazmanian, D. The DSM-5 peripartum specifier: prospects and pitfalls. Arch. Womens Ment. Health 17, 171–173 (2014).
Lindsay, J. R. & Nieman, L. K. The hypothalamic–pituitary–adrenal axis in pregnancy: challenges in disease detection and treatment. Endocr. Rev. 26, 775–799 (2005).
Galea, L. A. M. & Frokjaer, V. G. Perinatal depression: embracing variability toward better treatment and outcomes. Neuron 102, 13–16 (2019).
Kepley, J. M., Bates, K. & Mohiuddin, S. S. Physiology, Maternal Changes (StatPearls, 2023).
Bloch, M., Daly, R. C. & Rubinow, D. R. Endocrine factors in the etiology of postpartum depression. Compr. Psychiatry 44, 234–246 (2003).
Kennerley, H. & Gath, D. Maternity blues. I. Detection and measurement by questionnaire. Br. J. Psychiatry 155, 356–362 (1989).
Chechko, N., Losse, E., Frodl, T. & Nehls, S. Baby blues, premenstrual syndrome and postpartum affective disorders: intersection of risk factors and reciprocal influences. BJPsych Open 10, e3 (2024).
Rezaie-Keikhaie, K. et al. Systematic review and meta-analysis of the prevalence of the maternity blues in the postpartum period. J. Obstet. Gynecol. Neonatal Nurs. 49, 127–136 (2020).
Reck, C., Stehle, E., Reinig, K. & Mundt, C. Maternity blues as a predictor of DSM-IV depression and anxiety disorders in the first three months postpartum. J. Affect. Disord. 113, 77–87 (2009).
Luciano, M. et al. The transition from maternity blues to full-blown perinatal depression: results from a longitudinal study. Front. Psychiatry 12, 703180 (2021).
Glangeaud-Freudenthal, N. M. C., Crost, M. & Kaminski, M. Severe post-delivery blues: associated factors. Arch. Womens Ment. Health 2, 37–44 (1999).
Gotlib, I. H. et al. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport 16, 1731–1734 (2005).
Chechko, N., Stickel, S. & Votinov, M. Neural responses to monetary incentives in postpartum women affected by baby blues. Psychoneuroendocrinology 148, 105991 (2023).
Henshaw, C., Foreman, D. & Cox, J. Postnatal blues: a risk factor for postnatal depression. J. Psychosom. Obstet. Gynecol. 25, 267–272 (2004).
Bloch, M., Rotenberg, N., Koren, D. & Klein, E. Risk factors for early postpartum depressive symptoms. Gen. Hosp. Psychiatry 28, 3–8 (2006).
Turkmen, S., Backstrom, T., Wahlstrom, G., Andreen, L. & Johansson, I. M. Tolerance to allopregnanolone with focus on the GABA-A receptor. Br. J. Pharmacol. 162, 311–327 (2011).
Sundström-Poromaa, I., Comasco, E., Sumner, R. & Luders, E. Progesterone—friend or foe? Front. Neuroendocrinol. 59, 100856 (2020).
Deligiannidis, K. M. et al. Effect of zuranolone vs placebo in postpartum depression: a randomized control trial. JAMA Psychiatry 78, 951–959 (2021).
Meltzer-Brody, S. & Kanes, S. J. Allopregnanolone in postpartum depression: role in pathophysiology and treatment. Neurobiol. Stress 12, 100212 (2020).
Hantsoo, L. & Epperson, C. N. Allopregnanolone in premenstrual dysphoric disorder (PMDD): evidence for dysregulated sensitivity to GABA-A receptor modulating neuroactive steroids across the menstrual cycle. Neurobiol. Stress 12, 100213 (2020).
Perry, A., Gordon-Smith, K., Jones, L. & Jones, I. Phenomenology, epidemiology and aetiology of postpartum psychosis: a review. Brain Sci. 11, 47 (2021).
Hedges, V. L. et al. Estrogen withdrawal increases postpartum anxiety via oxytocin plasticity in the paraventricular hypothalamus and dorsal raphe nucleus. Biol. Psychiatry 89, 929–938 (2021).
Osborne, L. M., Betz, J. F., Yenokyan, G., Standeven, L. R. & Payne, J. L. The role of allopregnanolone in pregnancy in predicting postpartum anxiety symptoms. Front. Psychol. 10, 438990 (2019).
Nehls, S., Losse, E., Enzensberger, C., Frodl, T. & Chechko, N. Time-sensitive changes in the maternal brain and their influence on mother–child attachment. Transl. Psychiatry 14, 84 (2024).
Lotter, L. D., Nehls, S., Losse, E., Dukart, J. & Chechko, N. Temporal dissociation between local and global functional adaptations of the maternal brain to childbirth: a longitudinal assessment. Neuropsychopharmacology https://doi.org/10.1038/s41386-024-01880-9 (2024).
Sacher, J., Chechko, N., Dannlowski, U., Walter, M. & Derntl, B. The peripartum human brain: current understanding and future perspectives. Front. Neuroendocrinol. 59, 100859 (2020).
Oatridge, A. et al. Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. Am. J. Neuroradiol. 23, 19–26 (2002).
Hoekzema, E. et al. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 20, 287–296 (2017).
Hoekzema, E. et al. Mapping the effects of pregnancy on resting state brain activity, white matter microstructure, neural metabolite concentrations and grey matter architecture. Nat. Commun. 13, 6931 (2022).
Paternina-Die, M. et al. Women’s neuroplasticity during gestation, childbirth and postpartum. Nat. Neurosci. 27, 319–327 (2024).
Chechko, N. et al. The expectant brain—pregnancy leads to changes in brain morphology in the early postpartum period. Cereb. Cortex https://doi.org/10.1093/cercor/bhab463 (2021).
Rocchetti, M. et al. Neurofunctional maps of the ‘maternal brain’ and the effects of oxytocin: a multimodal voxel‐based meta‐analysis. Psychiatry Clin. Neurosci. 68, 733–751 (2014).
Kim, P., Strathearn, L. & Swain, J. E. The maternal brain and its plasticity in humans. Horm. Behav. 77, 113–123 (2016).
Barba-Müller, E., Craddock, S., Carmona, S. & Hoekzema, E. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch. Womens Ment. Health 22, 289–299 (2018).
Ben Shalom, D. The amygdala–insula–medial prefrontal cortex–lateral prefrontal cortex pathway and its disorders. Front. Neuroanat. 16, 1028546 (2022).
Chattarji, S., Tomar, A., Suvrathan, A., Ghosh, S. & Rahman, M. M. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat. Neurosci. 18, 1364–1375 (2015).
Barth, C., Villringer, A. & Sacher, J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 9, 37 (2015).
Green, A. D. & Galea, L. A. M. Adult hippocampal cell proliferation is suppressed with estrogen withdrawal after a hormone-simulated pregnancy. Horm. Behav. 54, 203–211 (2008).
Kinsley, C. H. et al. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm. Behav. 49, 131–142 (2006).
Pawluski, J. L. & Galea, L. A. M. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience 149, 53–67 (2007).
Servin-Barthet, C. et al. The transition to motherhood: linking hormones, brain and behaviour. Nat. Rev. Neurosci. 24, 605–619 (2023).
Carmona, S. et al. Pregnancy and adolescence entail similar neuroanatomical adaptations: a comparative analysis of cerebral morphometric changes. Hum. Brain Mapp. 40, 2143–2152 (2019).
Martínez-García, M., Paternina-Die, M., Desco, M., Vilarroya, O. & Carmona, S. Characterizing the brain structural adaptations across the motherhood transition. Front. Glob. Womens Health 2, 742775 (2021).
Etkin, A., Egner, T. & Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 15, 85–93 (2011).
Price, J. L. & Drevets, W. C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 16, 61–71 (2012).
Hahn, L. et al. Early identification of postpartum depression using demographic, clinical, and digital phenotyping. Transl. Psychiatry 11, 121 (2021).
Gidén, K., Vinnerljung, L., Iliadis, S. I., Fransson, E. & Skalkidou, A. Feeling better?—Identification, interventions, and remission among women with early postpartum depressive symptoms in Sweden: a nested cohort study. Eur. Psychiatry 67, e14 (2024).
Bäckström, T. et al. Allopregnanolone and mood disorders. Prog. Neurobiol. 113, 88–94 (2014).
Bixo, M., Andersson, A., Winblad, B., Purdy, R. H. & Bäckström, T. Progesterone, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 764, 173–178 (1997).
Bethea, C. L., Reddy, A. P., Tokuyama, Y., Henderson, J. A. & Lima, F. B. Protective actions of ovarian hormones in the serotonin system of macaques. Front. Neuroendocrinol. 30, 212–238 (2009).
Li, Y., Raaby, K. F., Sánchez, C. & Gulinello, M. Serotonergic receptor mechanisms underlying antidepressant-like action in the progesterone withdrawal model of hormonally induced depression in rats. Behav. Brain Res. 256, 520–528 (2013).
Kim, H. J., You, M. J., Sung, S., Rim, C. & Kwon, M. S. Possible involvement of microglial P2RY12 and peripheral IL-10 in postpartum depression. Front. Cell. Neurosci. 17, 1162966 (2023).
Tan, X. et al. Inhibition of autophagy in microglia alters depressive-like behavior via BDNF pathway in postpartum depression. Front. Psychiatry 9, 411696 (2018).
Zhai, D. S. et al. TOM40 mediates the effect of TSPO on postpartum depression partially through regulating calcium homeostasis in microglia. J. Affect. Disord. 348, 283–296 (2024).
Stickel, S. et al. Neural correlates of depression in women across the reproductive lifespan—an fMRI review. J. Affect. Disord. 246, 556–570 (2019).
Deecher, D., Andree, T. H., Sloan, D. & Schechter, L. E. From menarche to menopause: exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology https://doi.org/10.1016/j.psyneuen.2007.10.006 (2008).
Steiner, M., Dunn, E. & Born, L. Hormones and mood: from menarche to menopause and beyond. J. Affect. Disord. 74, 67–83 (2003).
Li, Y. et al. Abnormalities of cortical structures in patients with postpartum depression: a surface-based morphometry study. Behav. Brain Res. 410, 113340 (2021).
Chen, C. et al. Aberrant structural and functional alterations in postpartum depression: a combined voxel-based morphometry and resting-state functional connectivity study. Front. Neurosci. 17, 1138561 (2023).
Sacher, J. et al. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J. Affect. Disord. 140, 142–148 (2012).
Kim, P. et al. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav. Neurosci. 124, 695–700 (2010).
Lisofsky, N., Gallinat, J., Lindenberger, U. & Kühn, S. Postpartal neural plasticity of the maternal brain: early renormalization of pregnancy-related decreases? Neurosignals 27, 12–24 (2019).
Zhang, K., Wang, M., Zhang, J., Du, X. & Chen, Z. Brain structural plasticity associated with maternal caregiving in mothers: a voxel-and surface-based morphometry study. Neurodegener. Dis. 19, 192–203 (2020).
Martínez-García, M. et al. Do pregnancy-induced brain changes reverse? The brain of a mother six years after parturition. Brain Sci. 11, 168 (2021).
Morawetz, C., Bode, S., Derntl, B. & Heekeren, H. R. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: a meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 72, 111–128 (2017).
Acknowledgements
This research is supported by the Deutsche Forschungsgemeinschaft (DFG; grant numbers 410314797 and 512021469).
Author information
Authors and Affiliations
Contributions
N.C. and S.N. contributed equally to the conceptualization, drafting, writing, and critical revision of this perspective. Both authors approved the final version of the manuscript for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Alexander Dufford and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chechko, N., Nehls, S. Maternal neuroplasticity and mental health during the transition to motherhood. Nat. Mental Health 3, 396–401 (2025). https://doi.org/10.1038/s44220-025-00399-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44220-025-00399-2