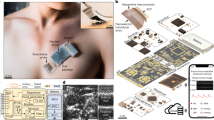
Abstract
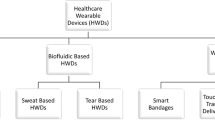
Wearable ultrasound technology refers to ultrasound devices designed with compact form factors that do not restrict the mobility or routine functions of the wearer. These devices are intended to provide continuous monitoring of internal tissue structures and offer therapeutic intervention without manual operation. Wearable ultrasound technology has potential applications in the management of chronic diseases, acute conditions during surgeries and emergencies, and post-operative care. This technology can provide clinicians and patients with data and insights, such as patterns of physiological variations over time and critical periods of disease progression, that are hardly attainable using conventional handheld ultrasound devices. In this Review, we discuss recent advances in wearable ultrasound technology, focusing on material selection, mechanical design, the integration of wearable systems, and exemplary medical applications. Additionally, we provide a framework for expanding the adoption of wearable ultrasound technology, particularly in low-resource settings, by exploring barriers in technology transfer. Finally, we identify critical challenges from scientific, engineering and clinical perspectives to advance wearable ultrasound technology to the next stages of development.
Key points
-
Wearable ultrasound technology enables hands-free, operator-independent and continuous operation.
-
The integration of miniaturized back-end circuits, autonomous signal processing algorithms and multimodal sensing systems is intended to enhance diagnosis accuracy, user experience and patient outcomes.
-
Wearable ultrasound technology has shown potential in a wide range of use cases, although most of the results are yet to be validated against gold standards in well-controlled clinical studies.
-
To enable clinical translation, it is necessary to carry out controlled clinical studies, establish safety protocols for therapeutic intervention, and integrate wearable data with electronic health records.
-
Future advancements should focus on improving imaging resolution, realizing efficient 3D imaging, integrating control electronics with low size, weight and power consumption, creating breathable device packaging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Miller, D. L. et al. Overview of therapeutic ultrasound applications and safety considerations. J. Med. Ultrasound 31, 623–634 (2012).
Li, K., Xu, Y. & Meng, M. Q.-H. An overview of systems and techniques for autonomous robotic ultrasound acquisitions. IEEE Trans. Med. Robot. Bionics 3, 510–524 (2021).
Steinhubl, S. R., Muse, E. D. & Topol, E. J. The emerging field of mobile health. Sci. Transl. Med. 7, 283rv283 (2015).
Majumder, S., Mondal, T. & Deen, M. J. Wearable sensors for remote health monitoring. Sensors 17, 130 (2017).
Li, Z., Tian, X., Qiu, C.-W. & Ho, J. S. Metasurfaces for bioelectronics and healthcare. Nat. Electron. 4, 382–391 (2021).
Jiang, L. et al. Flexible ultrasound-induced retinal stimulating piezo-arrays for biomimetic visual prostheses. Nat. Commun. 13, 3853 (2022).
Fu, B., Pu, C., Guo, L., Xu, H. & Peng, C. A wearable ultrasound transducer array for neuromodulation applications in the treatment of diabetic foot disease. In 2023 IEEE International Ultrasonics Symposium (IUS) 1–4 (IEEE, 2023).
Moon, S. et al. A Flexible Ultrasound transducer with tunable focusing for non-invasive brain stimulation. In 2023 IEEE International Ultrasonics Symposium (IUS) 1–4 (IEEE, 2023).
Sonmezoglu, S., Shen, K., Carmena, J. M. & Maharbiz, M. M. in Handbook of Neuroengineering (ed. Thakor, N. V.) 623–650 (Springer, 2023).
Yu, C. C. et al. A conformable ultrasound patch for cavitation-enhanced transdermal cosmeceutical delivery. Adv. Mater. 35, e2300066 (2023).
Li, S. et al. Stretchable electronic facial masks for sonophoresis. ACS Nano 16, 5961–5974 (2022).
Ngo, O. et al. Development of low frequency (20–100 kHz) clinically viable ultrasound applicator for chronic wound treatment. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 66, 572–580 (2019).
Best, T. M., Moore, B., Jarit, P., Moorman, C. T. & Lewis, G. K. Sustained acoustic medicine: wearable, long duration ultrasonic therapy for the treatment of tendinopathy. Phys. Sportsmed. 43, 366–374 (2015).
Song, Y., Min, J. & Gao, W. Wearable and implantable electronics: moving toward precision therapy. ACS Nano 13, 12280–12286 (2019).
Lin, M., Hu, H., Zhou, S. & Xu, S. Soft wearable devices for deep-tissue sensing. Nat. Rev. Mater. 7, 850–869 (2022).
Bollella, P., Sharma, S., Cass, A. E. G. & Antiochia, R. Minimally‐invasive microneedle‐based biosensor array for simultaneous lactate and glucose monitoring in artificial interstitial fluid. Electroanalysis 31, 374–382 (2019).
Krieger, K. J. et al. Development and evaluation of 3D-printed dry microneedle electrodes for surface electromyography. Adv. Mater. Technol. 5, 2000518 (2020).
Lee, G. H. et al. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 5, 149–165 (2020).
Huang, H., Wu, R. S., Lin, M. & Xu, S. Emerging wearable ultrasound technology. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 71, 713–729 (2023).
Zhang, L., Du, W., Kim, J. H., Yu, C. C. & Dagdeviren, C. An emerging era: conformable ultrasound electronics. Adv. Mater. 36, e2307664 (2023).
Li, Y. et al. Progress in wearable acoustical sensors for diagnostic applications. Biosens. Bioelectron. 237, 115509 (2023).
Wang, C. & Zhao, X. See how your body works in real time — wearable ultrasound is on its way. Nature 630, 817–819 (2024).
Du, W. et al. Conformable ultrasound breast patch for deep tissue scanning and imaging. Sci. Adv. 9, eadh5325 (2023).
Zhang, L. et al. A conformable phased-array ultrasound patch for bladder volume monitoring. Nat. Electron. 7, 77–90 (2023).
van Neer, P. et al. Flexible large-area ultrasound arrays for medical applications made using embossed polymer structures. Nat. Commun. 15, 2802 (2024).
Keller, K., Leitner, C., Baumgartner, C., Benini, L. & Greco, F. Fully printed flexible ultrasound transducer for medical applications. Adv. Mater. Technol. 8, 2300577 (2023).
Fukada, E. History and recent progress in piezoelectric polymers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 47, 1277–1290 (2000).
Pang, D. C. & Chang, C. M. Development of a novel transparent flexible capacitive micromachined ultrasonic transducer. Sensors 17, 1443 (2017).
Dang, Z.-M. Dielectric Polymer Materials for High-Density Energy Storage (William Andrew, 2018).
Kim, M., Kim, J. & Cao, W. Aspect ratio dependence of electromechanical coupling coefficient of piezoelectric resonators. Appl. Phys. Lett. 87, 132901 (2005).
Mattoon, J. S., Sellon, R. K. & Berry, C. R. Small Animal Diagnostic Ultrasound E-Book (Elsevier Health Sciences, 2020).
Chan, V. & Perlas, A. in Atlas of Ultrasound-Guided Procedures in Interventional Pain Management (ed. Narouze, S. N.) 13–19 (2011).
Rathod, V. T. A review of acoustic impedance matching techniques for piezoelectric sensors and transducers. Sensors 20, 4051 (2020).
Wu, D.-W. et al. Very high frequency (beyond 100 MHz) PZT kerfless linear arrays. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 56, 2304–2310 (2009).
Kim, T., Kim, J. & Jiang, X. Transit time difference flowmeter for intravenous flow rate measurement using 1–3 piezoelectric composite transducers. IEEE Sens. J. 17, 5741–5748 (2017).
Pashaei, V. et al. Flexible body-conformal ultrasound patches for image-guided neuromodulation. IEEE Trans. Biomed. Circuits Syst. 14, 305–318 (2020).
Zhu, K. et al. Increasing performances of 1–3 piezocomposite ultrasonic transducer by alternating current poling method. Micromachines 13, 1715 (2022).
Chen, Y. et al. High-frequency PIN–PMN–PT single crystal ultrasonic transducer for imaging applications. Appl. Phys. A 108, 987–991 (2012).
Zhang, S. et al. Recent developments on high Curie temperature PIN–PMN–PT ferroelectric crystals. J. Cryst. Growth 318, 846–850 (2011).
Tittmann, B. R., Parks, D. A. & Zhang, S. O. High temperature piezoelectrics — a comparison. In Proc. 13th International Symposium on Nondestructive Characterization of Materials (NDCM-XIII) 20–24 (2013).
Jaffe, H. Piezoelectric ceramics. J. Am. Ceram. Soc. 41, 494–498 (1958).
Lin, Y. et al. Studies on the electrostatic effects of stretched PVDF films and nanofibers. Nanoscale Res. Lett. 16, 79 (2021).
Persano, L. et al. High performance piezoelectric devices based on aligned arrays of nanofibers of poly(vinylidenefluoride-co-trifluoroethylene). Nat. Commun. 4, 1633 (2013).
Liu, Y. et al. Fabrication and optical properties of transparent P (VDF-TrFE) ultrathin films. Nanomaterials 12, 588 (2022).
Dallaev, R. et al. Brief review of PVDF properties and applications potential. Polymers 14, 4793 (2022).
Mao, D., Gnade, B. E. & Quevedo-Lopez, M. A. in Ferroelectrics: Physical Effects (ed. Lallart, M.) 23 (InTech, 2011).
Uchino, K. Advanced Piezoelectric Materials: Science and Technology (Woodhead, 2017).
Foster, F. S., Harasiewicz, K. A. & Sherar, M. D. A history of medical and biological imaging with polyvinylidene fluoride (PVDF) transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 47, 1363–1371 (2000).
Carey, S. et al. PVdF array characterisation for high frequency ultrasonic imaging. In IEEE Ultrasonics Symposium 2024 1930–1933 (IEEE, 2004).
Habib, A., Wagle, S., Decharat, A. & Melandsø, F. Evaluation of adhesive-free focused high-frequency PVDF copolymer transducers fabricated on spherical cavities. SMS 29, 045026 (2020).
Kim, H. S. et al. Dominant role of Young’s modulus for electric power generation in PVDF–BaTiO3 composite-based piezoelectric nanogenerator. Nanomaterials 8, 777 (2018).
Akasheh, F., Myers, T., Fraser, J. D., Bose, S. & Bandyopadhyay, A. Development of piezoelectric micromachined ultrasonic transducers. Sens. Actuat. A 111, 275–287 (2004).
Wang, T., Kobayashi, T. & Lee, C. Highly sensitive piezoelectric micromachined ultrasonic transducer operated in air. Micro Nano Lett. 11, 558–562 (2016).
Ledesma, E., Zamora, I., Uranga, A., Torres, F. & Barniol, N. Enhancing AlN PMUTs’ acoustic responsivity within a MEMS-on-CMOS process. Sensors 21, 8447 (2021).
Birjis, Y. et al. Piezoelectric micromachined ultrasonic transducers (PMUTs): performance metrics, advancements, and applications. Sensors 22, 9151 (2022).
Liu, W., Zhu, C. & Wu, D. Flexible piezoelectric micro ultrasonic transducer array integrated on various flexible substrates. Sens. Actuat. A 317, X–y (2021).
Qiu, Y. et al. Piezoelectric micromachined ultrasound transducer (PMUT) arrays for integrated sensing, actuation and imaging. Sensors 15, 8020–8041 (2015).
Li, J., Ren, W., Fan, G. & Wang, C. Design and fabrication of piezoelectric micromachined ultrasound transducer (pMUT) with partially-etched ZnO film. Sensors 17, 1381 (2017).
Huang, Y. et al. Collapsed regime operation of capacitive micromachined ultrasonic transducers based on wafer-bonding technique. In IEEE Symposium on Ultrasonics 2003 1161–1164 (IEEE, 2003).
Lee, B. C., Nikoozadeh, A., Park, K. K. & Khuri-Yakub, B. T. High-efficiency output pressure performance using capacitive micromachined ultrasonic transducers with substrate-embedded springs. Sensors 18, 2520 (2018).
Caliano, G., Matrone, G. & Savoia, A. S. Biasing of capacitive micromachined ultrasonic transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 64, 402–413 (2016).
Qin, Y., Sun, W. & Yeow, J. T. A robust control approach for MEMS capacitive micromachined ultrasonic transducer. Trans. Inst. Meas. Control. 41, 107–116 (2019).
Hu, H. et al. A wearable cardiac ultrasound imager. Nature 613, 667–675 (2023).
Shung, K. K. Diagnostic Ultrasound: Imaging and Blood Flow Measurements (CRC Press, 2005).
Szabo, T. L. Diagnostic Ultrasound Imaging: Inside Out (Academic, 2004).
Lee, W. & Roh, Y. Ultrasonic transducers for medical diagnostic imaging. Biomed. Eng. Lett. 7, 91–97 (2017).
Bhuyan, A. et al. Miniaturized, wearable, ultrasound probe for on-demand ultrasound screening. In 2011 IEEE International Ultrasonics Symposium 1060–1063 (IEEE, 2011).
Lin, M. et al. A fully integrated wearable ultrasound system to monitor deep tissues in moving subjects. Nat. Biotechnol. 42, 448–457 (2024).
Kenny, J. S. et al. A novel, hands-free ultrasound patch for continuous monitoring of quantitative Doppler in the carotid artery. Sci. Rep. 11, 7780 (2021).
Ritter, T. A., Shrout, T. R., Tutwiler, R. & Shung, K. K. A 30-MHz piezo-composite ultrasound array for medical imaging applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 49, 217–230 (2002).
Zhou, Q., Lam, K. H., Zheng, H., Qiu, W. & Shung, K. K. Piezoelectric single crystal ultrasonic transducers for biomedical applications. Prog. Mater. Sci. 66, 87–111 (2014).
Zhou, S. et al. Transcranial volumetric imaging using a conformal ultrasound patch. Nature 629, 810–818 (2024).
Wang, C. et al. Bioadhesive ultrasound for long-term continuous imaging of diverse organs. Science 377, 517–523 (2022).
Elloian, J. et al. Flexible ultrasound transceiver array for non-invasive surface-conformable imaging enabled by geometric phase correction. Sci. Rep. 12, 16184 (2022).
Hu, H. et al. Stretchable ultrasonic arrays for the three-dimensional mapping of the modulus of deep tissue. Nat. Biomed. Eng. 7, 1321–1334 (2023).
Bélanger, M. C. & Marois, Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low‐density polyethylene and polydimethylsiloxane: a review. J. Biomed. Mater. Res. 58, 467–477 (2001).
Ota, H. et al. R2R-based continuous production of patterned and multilayered elastic substrates with liquid metal wiring for stretchable electronics. Adv. Mater. Technol. 9, 1–14 (2024).
Chen, C. & Pertijs, M. A. Integrated transceivers for emerging medical ultrasound imaging devices: a review. Open J. Solid State Circuits Soc. 1, 104–114 (2021).
Zhang, Y. & Demosthenous, A. Integrated circuits for medical ultrasound applications: imaging and beyond. TBioCAS 15, 838–858 (2021).
Hettiarachchi, N., Ju, Z. & Liu, H. A new wearable ultrasound muscle activity sensing system for dexterous prosthetic control. In 2015 IEEE International Conference on Systems, Man, and Cybernetics 1415–1420 (IEEE, 2015).
Yang, X., Chen, Z., Hettiarachchi, N., Yan, J. & Liu, H. A wearable ultrasound system for sensing muscular morphological deformations. IEEE Trans. Syst. Man. Cybern. 51, 3370–3379 (2021).
Zhang, Y. et al. A four-channel analog front-end ASIC for wearable A-mode ultrasound hand kinematic tracking applications. In 2023 IEEE Biomedical Circuits and Systems Conference (BioCAS) 1–4 (IEEE, 2023).
Ye, X., Jiang, X., Wang, S. & Chen, J. A low-intensity pulsed ultrasound interface ASIC for wearable medical therapeutic device applications. Electronics 13, 1154 (2024).
Seok, C., Yamaner, F. Y., Sahin, M. & Oralkan, Ö. A wearable ultrasonic neurostimulator — Part I: A 1D CMUT phased array system for chronic implantation in small animals. IEEE Trans. Biomed. Circuits Syst. 15, 692–704 (2021).
Seok, C., Adelegan, O. J., Biliroğlu, A. Ö., Yamaner, F. Y. & Oralkan, Ö. A wearable ultrasonic neurostimulator — Part II: A 2D CMUT phased array system with a flip-chip bonded ASIC. IEEE Trans. Biomed. Circuits Syst. 15, 705–718 (2021).
Luo, Y. et al. Technology roadmap for flexible sensors. ACS Nano 17, 5211–5295 (2023).
Song, I. et al. Design and implementation of a new wireless carotid neckband Doppler system with wearable ultrasound sensors: preliminary results. Appl. Sci. 9, 2202 (2019).
Hoskins, P. R., Martin, K. & Thrush, A. Diagnostic Ultrasound: Physics and Equipment (CRC, 2019).
Kwinten, W. M. J., van Leuteren, P. G., van Duren-van Iersel, M., Dik, P. & Jira, P. E. SENS-U: continuous home monitoring of natural nocturnal bladder filling in children with nocturnal enuresis — a feasibility study. J. Pediatr. Urol. 16, 196 e191–196 e196 (2020).
Niu, H. et al. Design of an ultrasound bladder volume measurement and alarm system. In 5th International Conference on Bioinformatics and Biomedical Engineering 1–4 (IEEE, 2011).
Sempionatto, J. R. et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 5, 737–748 (2021).
Wang, C. et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2, 687–695 (2018).
Zhou, S. et al. Clinical validation of a wearable ultrasound sensor of blood pressure. Nat. Biomed. Eng. 9, 865–881 (2024).
Shahshahani, A., Laverdiere, C., Bhadra, S. & Zilic, Z. Ultrasound sensors for diaphragm motion tracking: an application in non-invasive respiratory monitoring. Sensors 18, 2617 (2018).
Yang, X., Sun, X., Zhou, D., Li, Y. & Liu, H. Towards wearable A-mode ultrasound sensing for real-time finger motion recognition. IEEE Trans. Neural. Syst. Rehabil. Eng. 26, 1199–1208 (2018).
Molyneux, P., Ellis, R. F. & Carroll, M. Reliability of a two-probe ultrasound imaging procedure to measure strain in the Achilles tendon. J. Foot Ankle Res. 12, 49 (2019).
AlMohimeed, I., Turkistani, H. & Ono, Y. Development of wearable and flexible ultrasonic sensor for skeletal muscle monitoring. In 2013 IEEE International Ultrasonics Symposium (IUS) 1137–1140 (IEEE, 2013).
Gao, X. et al. A wearable echomyography system based on a single transducer. Nat. Electron. 7, 1035–1046 (2024).
Ding, X. et al. A high-sensitivity bowel sound electronic monitor based on piezoelectric micromachined ultrasonic transducers. Micromachines 13, 2221 (2022).
Thijssen, J., Van Wijk, M. & Cuypers, M. Performance testing of medical echo/Doppler equipment. Eur. J. Ultrasound 15, 151–164 (2002).
Demi, L. Practical guide to ultrasound beam forming: beam pattern and image reconstruction analysis. Appl. Sci. 8, 1544 (2018).
Gennisson, J.-L., Deffieux, T., Fink, M. & Tanter, M. Ultrasound elastography: principles and techniques. Diagn. Interv. Imaging 94, 487–495 (2013).
Liu, H.-C. et al. Wearable bioadhesive ultrasound shear wave elastography. Sci. Adv. 10, eadk8426 (2024).
Wang, F. et al. Flexible Doppler ultrasound device for the monitoring of blood flow velocity. Sci. Adv. 7, eabi9283 (2021).
Chang, L.-W., Hsu, K.-H. & Li, P.-C. Graphics processing unit-based high-frame-rate color Doppler ultrasound processing. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 56, 1856–1860 (2009).
Evans, D. H., Jensen, J. A. & Nielsen, M. B. Ultrasonic colour Doppler imaging. Interface Focus 1, 490–502 (2011).
Wang, C. et al. Continuous monitoring of deep-tissue haemodynamics with stretchable ultrasonic phased arrays. Nat. Biomed. Eng. 5, 749–758 (2021).
Rubin, J. M., Bude, R. O., Carson, P. L., Bree, R. L. & Adler, R. S. Power Doppler US: a potentially useful alternative to mean frequency-based color Doppler US. Radiology 190, 853–856 (1994).
Bercoff, J. et al. Ultrafast compound Doppler imaging: providing full blood flow characterization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 58, 134–147 (2011).
Blans, M. J., Bosch, F. H. & van der Hoeven, J. G. The use of an external ultrasound fixator (Probefix) on intensive care patients: a feasibility study. Ultrasound J. 11, 26 (2019).
Shomaji, S., Basak, A., Mandai, S., Karam, R. & Bhunia, S. A wearable carotid ultrasound assembly for early detection of cardiovascular diseases. In 2016 IEEE Healthcare Innovation Point-of-Care Technologies Conference (HI-POCT) 17–20 (IEEE, 2016).
Shomaji, S. et al. Early detection of cardiovascular diseases using wearable ultrasound device. IEEE Consum. Electron. Mag. 8, 12–21 (2019).
Kenny, J. S., Munding, C. E., Eibl, A. M. & Eibl, J. K. Wearable ultrasound and provocative hemodynamics: a view of the future. Crit. Care 26, 329 (2022).
Weber, S. et al. Continuous wrist blood pressure measurement with ultrasound. Biomed. Tech. (Berl.) https://doi.org/10.1515/bmt-2013-4124 (2013).
Peng, C., Chen, M., Sim, H. K., Zhu, Y. & Jiang, X. Noninvasive and nonocclusive blood pressure monitoring via a flexible piezo-composite ultrasonic sensor. IEEE Sens. J. 21, 2642–2650 (2021).
Steinberg, S., Huang, A., Ono, Y. & Rajan, S. Continuous artery monitoring using a flexible and wearable single-element ultrasonic sensor. IEEE Instrum. Meas. Mag. 25, 6–11 (2022).
Xu, L. et al. Online continuous measurement of arterial pulse pressure and pressure waveform using ultrasound. Measurement 220, 113379 (2023).
Goncalves Seabra, A. C., Silva, A. F. D., Stieglitz, T. & Amado-Rey, A. B. In silico blood pressure models comparison. IEEE Sens. J. 22, 23486–23493 (2022).
Kenny, J. S. et al. A wireless wearable Doppler ultrasound detects changing stroke volume: proof-of-principle comparison with trans-esophageal echocardiography during coronary bypass surgery. Bioengineering 8, 203 (2021).
Frey, S., Vostrikov, S., Benini, L. & Cossettini, A. WULPUS: a Wearable Ultra Low-Power Ultrasound probe for multi-day monitoring of carotid artery and muscle activity. In 2022 IEEE International Ultrasonics Symposium (IUS) 1–4 (IEEE, 2022).
Khan, D. Z. et al. Robotic semi-automated transcranial Doppler assessment of cerebrovascular autoregulation in post-concussion syndrome: methodological considerations. Neurotrauma Rep. 1, 218–231 (2020).
Krings, E. J. et al. Design of a wearable ultrasound patch with soft and conformal matching layer. Front. Med. Eng. 86731, V001T004A011 (2023).
Li, L., Long, F. L. J., Lim, I., Sun, T. & Ren, H. An overhead collapsible origami-based mount for medical applications. Robotics 12, 21 (2023).
Purkayastha, S. & Sorond, F. Transcranial Doppler ultrasound: technique and application. Semin. Neurol. 32, 411–420 (2012).
Newell, D. W., Aaslid, R., Stooss, R. & Reulen, H. J. The relationship of blood flow velocity fluctuations to intracranial pressure B waves. J. Neurosurg. 76, 415–421 (1992).
Heres, H. M. et al. Image acquisition stability of fixated musculoskeletal sonography in an exercise setting: a quantitative analysis and comparison with freehand acquisition. J. Med. Ultrason. 47, 47–56 (2020).
Qu, M. et al. Continuously monitoring of muscle fatigue based on a wearable micromachined ultrasonic transducer probe. Sens. Actuat. A 365, 114892 (2024).
Brandenburg, J. E. et al. Ultrasound elastography: the new frontier in direct measurement of muscle stiffness. Arch. Phys. M. 95, 2207–2219 (2014).
Majdi, J. A., Acuña, S. A., Chitnis, P. V. & Sikdar, S. Toward a wearable monitor of local muscle fatigue during electrical muscle stimulation using tissue Doppler imaging. Wearable Technol. 3, e16 (2022).
Protopappas, V. C. et al. An ultrasound wearable system for the monitoring and acceleration of fracture healing in long bones. IEEE Trans. Biomed. Eng. 52, 1597–1608 (2005).
Sun, S. et al. MEMS ultrasonic transducers for safe, low-power and portable eye-blinking monitoring. Microsyst. Nanoeng. 8, 63 (2022).
Mendez, J. et al. Continuous A-mode ultrasound-based prediction of transfemoral amputee prosthesis kinematics across different ambulation tasks. IEEE Trans. Biomed. Eng. 71, 56–67 (2024).
Nazari, V. & Zheng, Y. P. Controlling upper limb prostheses using sonomyography (SMG): a review. Sensors 23, 1885 (2023).
Iravantchi, Y., Goel, M. & Harrison, C. BeamBand: Hand gesture sensing with ultrasonic beamforming. In Proc. 2019 CHI Conference on Human Factors in Computing Systems 1–10 (ACM, 2019).
Shi, J., Chang, Q. & Zheng, Y.-P. Feasibility of controlling prosthetic hand using sonomyography signal in real time: preliminary study. JRRD 47, 87–98 (2010).
Sgambato, B. G. et al. High performance wearable ultrasound as a human-machine interface for wrist and hand kinematic tracking. IEEE Trans. Biomed. Eng. 71, 484–493 (2024).
Godih, A. et al. Design and creation of a wearable circular ultrasonic device for a soft screening and diagnosis of breast abnormalities. J. Cancer Sci. Clin. Ther. 3, 251–265 (2019).
van Leuteren, P. G., Klijn, A. J., de Jong, T. P. & Dik, P. SENS-U: validation of a wearable ultrasonic bladder monitor in children during urodynamic studies. J. Pediatr. Urol. 14, 569.e561–569.e566 (2018).
Pu, C., Fu, B., Guo, L., Xu, H. & Peng, C. A stretchable and wearable ultrasonic transducer array for bladder volume monitoring application. IEEE Sens. J. 24, 15875–15883 (2024).
Weng, C. K., Chen, J. W., Lee, P. Y. & Huang, C. C. Implementation of a wearable ultrasound device for the overnight monitoring of tongue base deformation during obstructive sleep apnea events. Ultrasound Med. Biol. 43, 1639–1650 (2017).
Chen, A. et al. Machine-learning enabled wireless wearable sensors to study individuality of respiratory behaviors. Biosens. Bioelectron. 173, 112799 (2021).
Marugan-Rubio, D. et al. Concurrent validity and reliability of manual versus specific device transcostal measurements for breathing diaphragm thickness by ultrasonography in lumbopelvic pain athletes. Sensors 21, 4329 (2021).
Roham, M. et al. A mobile wearable wireless fetal heart monitoring system. In 2011 5th International Symposium on Medical Information and Communication Technology 135–138 (IEEE, 2011).
Nguyen, K. et al. Wearable fetal monitoring solution for improved mobility during labor & delivery. In 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 4397–4400 (IEEE, 2018).
Ryu, D. et al. Comprehensive pregnancy monitoring with a network of wireless, soft, and flexible sensors in high- and low-resource health settings. Proc. Natl Acad. Sci. USA 118, e2100466118 (2021).
Donovan, C. M. & Brooks, G. A. Endurance training affects lactate clearance, not lactate production. Am. J. Physiol. Endocrinol. Med. 244, E83–E92 (1983).
Cornelissen, V. A. & Smart, N. A. Exercise training for blood pressure: a systematic review and meta‐analysis. J. Am. Heart Assoc. 2, e004473 (2013).
Guo, J.-Y. et al. A comparative evaluation of sonomyography, electromyography, force, and wrist angle in a discrete tracking task. Ultrasound Med. Biol. 37, 884–891 (2011).
Lou, C., Yang, S., Ji, Z., Chen, Q. & Xing, D. Ultrashort microwave-induced thermoacoustic imaging: a breakthrough in excitation efficiency and spatial resolution. Phys. Rev. Lett. 109, 218101 (2012).
Choi, S., Park, E.-Y., Park, S., Kim, J. H. & Kim, C. Synchrotron X-ray induced acoustic imaging. Sci. Rep. 11, 4047 (2021).
Park, J. et al. Clinical translation of photoacoustic imaging. Nat. Rev. Bioeng. 3, 193–212 (2024).
Gao, X. et al. A photoacoustic patch for three-dimensional imaging of hemoglobin and core temperature. Nat. Commun. 13, 7757 (2022).
Park, J. et al. Opto-ultrasound biosensor for wearable and mobile devices: realization with a transparent ultrasound transducer. Biomed. Opt. Express 13, 4684–4692 (2022).
Park, B. et al. 3D wide‐field multispectral photoacoustic imaging of human melanomas in vivo: a pilot study. J. Eur. Acad. Dermatol. Venereol. 35, 669–676 (2021).
Feng, T. et al. Detection of collagen by multi-wavelength photoacoustic analysis as a biomarker for bone health assessment. Photoacoustics 24, 100296 (2021).
Jin, H. et al. A flexible optoacoustic blood ‘stethoscope’ for noninvasive multiparametric cardiovascular monitoring. Nat. Commun. 14, 4692 (2023).
Liu, Y.-H. et al. Transparent flexible piezoelectric ultrasound transducer for photoacoustic imaging system. IEEE Sens. J. 22, 2070–2077 (2022).
Zakrzewski, A. M., Huang, A. Y., Zubajlo, R. & Anthony, B. W. Real-time blood pressure estimation from force-measured ultrasound. IEEE Trans. Biomed. Eng. 65, 2405–2416 (2018).
Jaffe, A. T., Zubajlo, R. E., Daniel, L. & Anthony, B. W. Automated force-coupled ultrasound method for calibration-free carotid artery blood pressure estimation. Ultrasound Med. Biol. 48, 1806–1821 (2022).
Jaffe, A., Goryachev, I., Sodini, C. & Anthony, B. W. Central venous pressure estimation with force-coupled ultrasound of the internal jugular vein. Sci. Rep. 13, 1500 (2023).
Soto, J. T. et al. Multimodal deep learning enhances diagnostic precision in left ventricular hypertrophy. Eur. Heart J. Digit. Health 3, 380–389 (2022).
Petrozziello, A., Redman, C. W., Papageorghiou, A. T., Jordanov, I. & Georgieva, A. Multimodal convolutional neural networks to detect fetal compromise during labor and delivery. IEEE Access 7, 112026–112036 (2019).
Atrey, K., Singh, B. K. & Bodhey, N. K. Multimodal classification of breast cancer using feature level fusion of mammogram and ultrasound images in machine learning paradigm. Multimed. Tools Appl. 83, 21347–21368 (2024).
Izadifar, Z., Babyn, P. & Chapman, D. Mechanical and biological effects of ultrasound: a review of present knowledge. Ultrasound Med. Biol. 43, 1085–1104 (2017).
Manasseh, R. in Handbook of Ultrasonics and Sonochemistry (eds Ashokkumar, M. et al.) 33–68 (Springer, 2016).
Sundaram, J., Mellein, B. R. & Mitragotri, S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys. J. 84, 3087–3101 (2003).
Wu, J. Acoustic streaming and its applications. Fluids 3, 108 (2018).
Pitt, W. G., Husseini, G. A. & Staples, B. J. Ultrasonic drug delivery — a general review. Expert. Opin. Drug. Deliv. 1, 37–56 (2004).
Quarato, C. M. I. et al. A review on biological effects of ultrasounds: key messages for clinicians. Diagnostics 13, 855 (2023).
Ter Haar, G. HIFU tissue ablation: concept and devices. Therapeutic Ultrasound 880, 3–20 (2016).
Jang, H. J., Lee, J.-Y., Lee, D.-H., Kim, W.-H. & Hwang, J. H. Current and future clinical applications of high-intensity focused ultrasound (HIFU) for pancreatic cancer. Gut Liver 4, S57–S61 (2010).
Plaksin, M., Shapira, E., Kimmel, E. & Shoham, S. Thermal transients excite neurons through universal intramembrane mechanoelectrical effects. Phys. Rev. X 8, 011043 (2018).
Ye, J. et al. Ultrasonic control of neural activity through activation of the mechanosensitive channel MscL. Nano Lett. 18, 4148–4155 (2018).
Kim, E. et al. Wearable transcranial ultrasound system for remote stimulation of freely moving animal. IEEE Trans. Biomed. Eng. 68, 2195–2202 (2021).
Wasilczuk, K. M. et al. Modulating the inflammatory reflex in rats using low-intensity focused ultrasound stimulation of the vagus nerve. Ultrasound Med. Biol. 45, 481–489 (2019).
Herlihy, R. A. et al. Investigation of non-invasive focused ultrasound efficacy on depressive-like behavior in hemiparkinsonian rats. Exp. Brain Res. 242, 321–336 (2024).
Ji, N. et al. Blood pressure modulation with low-intensity focused ultrasound stimulation to the vagus nerve: a pilot animal study. Front. Neurosci. 14, 586424 (2020).
Mitragotri, S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 4, 255–260 (2005).
Rossmann, C., McCrackin, M. A., Armeson, K. E. & Haemmerich, D. Temperature sensitive liposomes combined with thermal ablation: effects of duration and timing of heating in mathematical models and in vivo. PLoS One 12, e0179131 (2017).
Yadollahpour, A., Mostafa, J., Samaneh, R. & Zohreh, R. Ultrasound therapy for wound healing: a review of current techniques and mechanisms of action. J. Pure Appl. Microbiol. 8, 4071–4085 (2014).
Samuels, J. A. et al. Low-frequency (<100 kHz), low-intensity (<100 mW/cm2) ultrasound to treat venous ulcers: a human study and in vitro experiments. J. Acoust. Soc. Am. 134, 1541–1547 (2013).
Liu, H. et al. Bone fracture healing under the intervention of a stretchable ultrasound array. ACS Nano 18, 19549–19560 (2024).
Hemery, X., Ohl, X., Saddiki, R., Barresi, L. & Dehoux, E. Low-intensity pulsed ultrasound for non-union treatment: a 14-case series evaluation. Orthop. Traumatol. Surg. Res. 97, 51–57 (2011).
Harrison, A. & Alt, V. Low-intensity pulsed ultrasound (LIPUS) for stimulation of bone healing — a narrative review. Injury 52, S91–S96 (2021).
Harrison, A., Lin, S., Pounder, N. & Mikuni-Takagaki, Y. Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair. Ultrasonics 70, 45–52 (2016).
Schandelmaier, S. et al. Low intensity pulsed ultrasound for bone healing: systematic review of randomized controlled trials. Br. Med. J. 356, j656 (2017).
Inoue, S. et al. Effects of ultrasound, radial extracorporeal shock waves, and electrical stimulation on rat bone defect healing. Ann. NY Acad. Sci. 1497, 3–14 (2021).
Hannemann, P., Mommers, E., Schots, J., Brink, P. & Poeze, M. The effects of low-intensity pulsed ultrasound and pulsed electromagnetic fields bone growth stimulation in acute fractures: a systematic review and meta-analysis of randomized controlled trials. Arch. Orthop. Trauma Surg. 134, 1093–1106 (2014).
Lewis, G. K. Jr, Langer, M. D., Henderson, C. R. Jr & Ortiz, R. Design and evaluation of a wearable self-applied therapeutic ultrasound device for chronic myofascial pain. Ultrasound Med. Biol. 39, 1429–1439 (2013).
Cheng, J. L. & MacDonald, M. J. Effect of heat stress on vascular outcomes in humans. J. Appl. Physiol. 126, 771–781 (2019).
Aiyer, R. et al. Therapeutic ultrasound for chronic pain management in joints: a systematic review. Pain Med. 21, 1437–1448 (2020).
Uddin, S. M. Z., Komatsu, D. E., Motyka, T. & Petterson, S. Low-intensity continuous ultrasound therapies — a systematic review of current state-of-the-art and future perspectives. J. Clin. Med. 10, 2698 (2021).
Jiang, L. et al. Flexible piezoelectric ultrasonic energy harvester array for bio-implantable wireless generator. Nano Energy 56, 216–224 (2019).
Morales González, R., Marzo, A., Freeman, E., Frier, W. & Georgiou, O. Ultrapower: Powering tangible & wearable devices with focused ultrasound. In Proc. 15th International Conference on Tangible, Embedded, and Embodied Interaction 1–13 (2021).
Li, S. et al. Ultrasound-driven piezoelectric current activates spinal cord neurocircuits and restores locomotion in rats with spinal cord injury. Bioelectron. Med. 6, 13 (2020).
Alam, M. et al. Development of a battery-free ultrasonically powered functional electrical stimulator for movement restoration after paralyzing spinal cord injury. J. Neuroeng. Rehabil. 16, 36 (2019).
Jin, P. et al. A flexible, stretchable system for simultaneous acoustic energy transfer and communication. Sci. Adv. 7, eabg2507 (2021).
Wei, X. & Liu, J. Power sources and electrical recharging strategies for implantable medical devices. FEPE China 2, 1–13 (2008).
Ho, J. S. et al. Wireless power transfer to deep-tissue microimplants. Proc. Natl Acad. Sci. USA 111, 7974–7979 (2014).
Food and Drug Administration. Marketing Clearance of Diagnostic Ultrasound Systems and Transducers: Guidance for Industry and Food and Drug Administration Staff (2023).
Duck, F. A. Medical and non-medical protection standards for ultrasound and infrasound. Prog. Biophys. Mol. Biol. 93, 176–191 (2007).
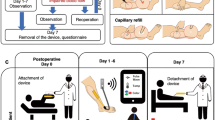
Song, P. et al. Clinical, safety and engineering perspectives on wearable ultrasound technology: a review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 71, 730–744 (2023).
Abramowicz, J. S. et al. AIUM official statement for recommended maximum scanning times for displayed thermal index values. J. Med. Ultrasound 42, E74–E75 (2023).
British Medical Ultrasound Society Guidelines for the safe use of diagnostic ultrasound equipment. Ultrasound 18, 52–59 (2010).
Mariappan, Y. K., Glaser, K. J. & Ehman, R. L. Magnetic resonance elastography: a review. Clin. Anat. 23, 497–511 (2010).
Elkins, C. J. & Alley, M. T. Magnetic resonance velocimetry: applications of magnetic resonance imaging in the measurement of fluid motion. Exp. Fluids 43, 823–858 (2007).
International Electrotechnical Commission. IEC 60601-2-37 Medical Electrical Equipment – Part 2 – 37: Particular Requirements for the Basic Safety and Essential Performance of Ultrasonic Medical Diagnostic and Monitoring Equipment, edition 2.1 (2015).
International Electrotechnical Commission. IEC 62359:2010/AMD1:2017 Ultrasonics – Field Characterization – Test Methods for the Determination of Thermal and Mechanical Indices Related To Medical Diagnostic Ultrasonic Fields, edition 2.1 (2017).
Bigelow, T. A. et al. The thermal index: its strengths, weaknesses, and proposed improvements. J. Med. Ultrasound 30, 714–734 (2011).
Odéen, H., Almquist, S., de Bever, J., Christensen, D. A. & Parker, D. L. MR thermometry for focused ultrasound monitoring utilizing model predictive filtering and ultrasound beam modeling. J. Ther. Ultrasound 4, 1–13 (2016).
Odéen, H. & Parker, D. L. Magnetic resonance thermometry and its biological applications — physical principles and practical considerations. Prog. Nucl. Magn. Reson. Spectrosc. 110, 34–61 (2019).
Banerjee, S., Hemphill, T. & Longstreet, P. Wearable devices and healthcare: data sharing and privacy. Inform. Soc. 34, 49–57 (2018).
Pandit, J. A., Pawelek, J. B., Leff, B. & Topol, E. J. The hospital at home in the USA: current status and future prospects. NPJ Digit. Med. 7, 48 (2024).
Xu, S., Kim, J., Walter, J. R., Ghaffari, R. & Rogers, J. A. Translational gaps and opportunities for medical wearables in digital health. Sci. Transl. Med. 14, eabn6036 (2022).
Ali, R. et al. Aberration correction in diagnostic ultrasound: a review of the prior field and current directions. Z. Med. Phys. 33, 267–291 (2023).
Lane, C. J. The inspection of curved components using flexible ultrasonic arrays and shape sensing fibres. Case Stud. Nondestr.Test. Eval. 1, 13–18 (2014).
Noda, T., Tomii, N., Nakagawa, K., Azuma, T. & Sakuma, I. Shape estimation algorithm for ultrasound imaging by flexible array transducer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 67, 2345–2353 (2020).
Mallart, R. & Fink, M. The van Cittert–Zernike theorem in pulse echo measurements. J. Am. Stat. Assoc. 90, 2718–2727 (1991).
Nock, L., Trahey, G. E. & Smith, S. W. Phase aberration correction in medical ultrasound using speckle brightness as a quality factor. J. Acoust. Soc. Am. 85, 1819–1833 (1989).
Mallart, R. & Fink, M. Adaptive focusing in scattering media through sound-speed inhomogeneities: the van Cittert Zernike approach and focusing criterion. J. Am. Stat. Assoc. 96, 3721–3732 (1994).
Nock, L., Trahey, G. E. & Smith, S. W. Phase aberration correction in medical ultrasound using speckle brightness as a quality factor. J. Am. Stat. Assoc. 85, 1819–1833 (1989).
Errico, C. et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 527, 499–502 (2015).
Opacic, T. et al. Motion model ultrasound localization microscopy for preclinical and clinical multiparametric tumor characterization. Nat. Commun. 9, 1527 (2018).
Christensen-Jeffries, K. et al. Super-resolution ultrasound imaging. Ultrasound Med. Biol. 46, 865–891 (2020).
Ilovitsh, T., Ilovitsh, A., Foiret, J., Fite, B. Z. & Ferrara, K. W. Acoustical structured illumination for super-resolution ultrasound imaging. Commun. Biol. 1, 3 (2018).
Lin, J. & Ma, C. Far-field acoustic subwavelength imaging with blind structured illumination and joint-sparsity reconstruction. Phys. Rev. Appl. 17, 054030 (2022).
Chen, X. et al. Superresolution structured illumination microscopy reconstruction algorithms: a review. Light. Sci. Appl. 12, 172 (2023).
Nelson, T. R. & Pretorius, D. H. Three-dimensional ultrasound imaging. Ultrasound Med. Biol. 24, 1243–1270 (1998).
Harrington, T. & Liu, S.-N. in Review of Progress in Quantitative Nondestructive Evaluation: 8, Part A and B (eds Thompson, D. O. & Chimenti, D. E.) 1067–1074 (Springer, 1989).
Seo, C. H. & Yen, J. T. A 256 × 256 2-D array transducer with row-column addressing for 3-D rectilinear imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 56, 837–847 (2009).
Rasmussen, M. F. & Jensen, J. A. in Medical Imaging 2013: Ultrasonic Imaging, Tomography, and Therapy 73–83 (SPIE, 2013).
Ramalli, A., Boni, E., Savoia, A. S. & Tortoli, P. Density-tapered spiral arrays for ultrasound 3-D imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 62, 1580–1588 (2015).
Wei, L. et al. Sparse 2-D PZT-on-PCB arrays with density tapering. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 69, 2798–2809 (2022).
Rothberg, J. M. et al. Ultrasound-on-chip platform for medical imaging, analysis, and collective intelligence. Proc. Natl Acad. Sci. USA 118, e2019339118 (2021).
Yin, L. et al. A self-sustainable wearable multi-modular E-textile bioenergy microgrid system. Nat. Commun. 12, 1542 (2021).
Yan, Z. et al. Recent advances in breathable electronics. Nano Res. 16, 4130–4142 (2023).
Hu, H. J. et al. Stretchable ultrasonic transducer arrays for three-dimensional imaging on complex surfaces. Sci. Adv. 4, eaar3979 (2018).
Li, X. et al. Micromachined PIN–PMN–PT crystal composite transducer for high-frequency intravascular ultrasound (IVUS) imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 61, 1171–1178 (2014).
Lee, J.-H. et al. Flexible piezoelectric micromachined ultrasonic transducer (pMUT) for application in brain stimulation. Microsyst. Technol. 23, 2321–2328 (2016).
Lin, D.-S., Zhuang, X., Wong, S. H., Kupnik, M. & Khuri-Yakub, B. T. Encapsulation of capacitive micromachined ultrasonic transducers using viscoelastic polymer. J. Microelectromech. Syst. 19, 1341–1351 (2010).
AlMohimeed, I. & Ono, Y. Ultrasound measurement of skeletal muscle contractile parameters using flexible and wearable single-element ultrasonic sensor. Sensors 20, 3616 (2020).
Tran, K. et al. Lung sliding detection in M-mode using wearable ultrasonic sensor: an in-vivo feasibility study. In 2023 IEEE International Ultrasonics Symposium (IUS) (IEEE, 2023).
Frija, G. et al. How to improve access to medical imaging in low- and middle-income countries? EClinicalMedicine 38, 101034 (2021).
Mariani, G. et al. Improving women’s health in low-income and middle-income countries. Part II: The needs of diagnostic imaging. Nucl. Med. Commun. 38, 1024–1028 (2017).
Frija, G., Salama, D. H., Kawooya, M. G. & Allen, B. A paradigm shift in point-of-care imaging in low-income and middle-income countries. EClinicalMedicine 62, 102114 (2023).
Maru, D. S.-R. et al. Turning a blind eye: the mobilization of radiology services in resource-poor regions. Glob. Health 6, 18 (2010).
Robertson, T. E. et al. Remote tele-mentored ultrasound for non-physician learners using FaceTime: a feasibility study in a low-income country. J. Crit. Care 40, 145–148 (2017).
Acknowledgements
The authors are grateful for financial support from the National Institutes of Health (1R21EB025521-01, 1R21EB027303-01A1, 3R21EB027303-02S1, 1R01EB033464-01 and 1R01HL171652-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
S.Z., G.P. and M.L. contributed equally to the literature review, figure design and manuscript writing. All authors contributed to writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks ChengKuo Lee, Francesco Greco, Laura Ferrari and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Ceramic counterparts (Young’s modulus ~50 GPa): https://support.piezo.com/article/62-material-properties
Drexel University: https://drexel.edu/news/archive/2016/november/ultrasound-wound-healing-device
Electromechanical coupling coefficient (0.17–0.58): https://www.ctscorp.com/Product-Series/3203HD.htm
Mobile phone integrated probes: https://www.butterflynetwork.com/teleguidance
PIN–PMN-PT: https://www.trstechnologies.com/Materials/High-Performance-PMN-PT-Piezoelectric-Single-Crystal
Vibration amplitude: https://www.americanpiezo.com/knowledge-center/piezo-theory/piezoelectric-constants/#:~:Text=For%20a%20thin%20disc%20of
ZetrOZ Systems LLC: https://samrecover.com/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, S., Park, G., Lin, M. et al. Wearable ultrasound technology. Nat Rev Bioeng 3, 835–854 (2025). https://doi.org/10.1038/s44222-025-00329-y
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44222-025-00329-y